Current Issue
Display Method: |
2025, 37(6): 879-881.
doi: 10.21147/j.issn.1000-9604.2025.06.01
Abstract:
2025, 37(6): 882-899.
doi: 10.21147/j.issn.1000-9604.2025.06.02
Abstract:
Breast cancer represents a significant and growing public health challenge in China, marked by a rising incidence and distinct variations across age groups and geographical regions. This review synthesizes recent evidence regarding the epidemiology, early detection, and early treatment in the Chinese context. We outline current patterns of disease burden and the spectrum of risk factors—both modifiable and non-modifiable. We note ongoing shifts linked to reproductive trends, lifestyle changes, and an aging population. Screening practices are increasingly evolving towards stratified, risk-adapted pathways. These approaches often combine mammography with adjunct imaging modalities such as ultrasound, digital breast tomosynthesis, or magnetic resonance imaging for selected populations, while artificial intelligence is under active investigation to enhance image interpretation and streamline workflow. Contemporary early management strategies emphasize breast-conserving surgery and selective axillary surgery, alongside the expanded application of hypofractionated and precision-targeted radiotherapy. Systemic therapy is increasingly guided by tumor subtype. In the adjuvant setting, molecular profiling and multigene assays are now routinely utilized to tailor treatment intensity to individual tumor biology, facilitating both treatment escalation or de-escalation where appropriate. Concurrently, in the neoadjuvant setting, research efforts within China and globally are focused on evaluating novel therapeutic regimens and biomarker-driven strategies to improve pathologic complete response rates and inform subsequent postoperative care. A consolidated understanding of these evolving themes is crucial for shaping effective clinical practice and health policies, ultimately supporting the goals of earlier diagnosis and improved patient outcomes in China.
Breast cancer represents a significant and growing public health challenge in China, marked by a rising incidence and distinct variations across age groups and geographical regions. This review synthesizes recent evidence regarding the epidemiology, early detection, and early treatment in the Chinese context. We outline current patterns of disease burden and the spectrum of risk factors—both modifiable and non-modifiable. We note ongoing shifts linked to reproductive trends, lifestyle changes, and an aging population. Screening practices are increasingly evolving towards stratified, risk-adapted pathways. These approaches often combine mammography with adjunct imaging modalities such as ultrasound, digital breast tomosynthesis, or magnetic resonance imaging for selected populations, while artificial intelligence is under active investigation to enhance image interpretation and streamline workflow. Contemporary early management strategies emphasize breast-conserving surgery and selective axillary surgery, alongside the expanded application of hypofractionated and precision-targeted radiotherapy. Systemic therapy is increasingly guided by tumor subtype. In the adjuvant setting, molecular profiling and multigene assays are now routinely utilized to tailor treatment intensity to individual tumor biology, facilitating both treatment escalation or de-escalation where appropriate. Concurrently, in the neoadjuvant setting, research efforts within China and globally are focused on evaluating novel therapeutic regimens and biomarker-driven strategies to improve pathologic complete response rates and inform subsequent postoperative care. A consolidated understanding of these evolving themes is crucial for shaping effective clinical practice and health policies, ultimately supporting the goals of earlier diagnosis and improved patient outcomes in China.
2025, 37(6): 900-911.
doi: 10.21147/j.issn.1000-9604.2025.06.03
Abstract:
This comprehensive review integrates population-based registries, hospital databases and Global Burden of Disease data to describe the evolving leukemia burden in China from 2000 to 2022. The overall incidence has stabilized nationally, but the absolute number of cases continues to increase as the population ages. A bimodal age pattern persists, with acute leukemias clustering in young children and older adults, while chronic forms predominate in mid-to-late life, and males are consistently more affected by all subtypes. Rapid expansion of haploidentical hematopoietic stem cell transplantation has resulted in marked survival gains for both acute myeloid leukemia and acute lymphoblastic leukemia, and its seamless integration with molecularly targeted agents, venetoclax-based regimens and chimeric antigen receptor T-cell therapy has transformed acute leukemias into potentially curable diseases for an expanding proportion of patients. In parallel, universal access to tyrosine kinase inhibitors and standardized molecular monitoring have turned chronic myeloid leukemia into a manageable chronic condition, and survival of patients with chronic lymphocytic leukemia is improving as novel Bruton’s tyrosine kinase and BCL-2 inhibitors diffuse into clinical practice. Tobacco, obesity, benzene and radon remain the principal modifiable drivers of leukemogenesis. Strengthening data completeness, widening equitable access to precision therapies and controlling these environmental risks are essential to sustaining the observed continuous improvement in leukemia patient survival and ensuring that ever more Chinese patients achieve a cure or durable disease control in the decades ahead.
This comprehensive review integrates population-based registries, hospital databases and Global Burden of Disease data to describe the evolving leukemia burden in China from 2000 to 2022. The overall incidence has stabilized nationally, but the absolute number of cases continues to increase as the population ages. A bimodal age pattern persists, with acute leukemias clustering in young children and older adults, while chronic forms predominate in mid-to-late life, and males are consistently more affected by all subtypes. Rapid expansion of haploidentical hematopoietic stem cell transplantation has resulted in marked survival gains for both acute myeloid leukemia and acute lymphoblastic leukemia, and its seamless integration with molecularly targeted agents, venetoclax-based regimens and chimeric antigen receptor T-cell therapy has transformed acute leukemias into potentially curable diseases for an expanding proportion of patients. In parallel, universal access to tyrosine kinase inhibitors and standardized molecular monitoring have turned chronic myeloid leukemia into a manageable chronic condition, and survival of patients with chronic lymphocytic leukemia is improving as novel Bruton’s tyrosine kinase and BCL-2 inhibitors diffuse into clinical practice. Tobacco, obesity, benzene and radon remain the principal modifiable drivers of leukemogenesis. Strengthening data completeness, widening equitable access to precision therapies and controlling these environmental risks are essential to sustaining the observed continuous improvement in leukemia patient survival and ensuring that ever more Chinese patients achieve a cure or durable disease control in the decades ahead.
2025, 37(6): 912-928.
doi: 10.21147/j.issn.1000-9604.2025.06.04
Abstract:
Cancer is a leading cause of death in China, and its epidemiological profile has shifted markedly in recent years. This review summarizes contemporary trends in cancer incidence and mortality, delineates the major modifiable risk factors, and highlights recent national efforts to ease the burden of cancer. In 2022, China recorded 4.8 million new cancer cases (crude rate: 341.7 per 100,000) and 2.5 million cancer deaths (182.3 per 100,000). Lung, colorectal, thyroid, liver, stomach, and female breast cancers accounted for 65% of all diagnoses, while lung, liver, stomach, colorectal, and esophageal cancers constituted 67.5% of cancer deaths. Notable shifts in sex-specific rankings underscored the rising mortality burden of prostate, female breast, and cervical cancers. China has made measurable progress in cancer control. Between 2000 and 2018, the age-standardized mortality rate for all cancers declined by approximately 1.3% annually, and the age-standardized 5-year relative survival improved from 30.9% in 2003−2005 to 43.7% in 2019−2021. According to Global Burden of Disease (GBD) 2023, nearly half of cancer deaths and disability-adjusted life years (DALYs) were attributable to modifiable risk factors such as tobacco, air pollution, high alcohol use, dietary risks, and unsafe sex. In response, the government has implemented a suite of prevention-oriented policies, including expansion of human papillomavirus (HPV) vaccination, strengthened tobacco control, sustained air pollution reduction, enhanced health education, and broadened cancer screening coverage. Collectively, these initiatives demonstrate a sustained national commitment to reducing the cancer burden.
Cancer is a leading cause of death in China, and its epidemiological profile has shifted markedly in recent years. This review summarizes contemporary trends in cancer incidence and mortality, delineates the major modifiable risk factors, and highlights recent national efforts to ease the burden of cancer. In 2022, China recorded 4.8 million new cancer cases (crude rate: 341.7 per 100,000) and 2.5 million cancer deaths (182.3 per 100,000). Lung, colorectal, thyroid, liver, stomach, and female breast cancers accounted for 65% of all diagnoses, while lung, liver, stomach, colorectal, and esophageal cancers constituted 67.5% of cancer deaths. Notable shifts in sex-specific rankings underscored the rising mortality burden of prostate, female breast, and cervical cancers. China has made measurable progress in cancer control. Between 2000 and 2018, the age-standardized mortality rate for all cancers declined by approximately 1.3% annually, and the age-standardized 5-year relative survival improved from 30.9% in 2003−2005 to 43.7% in 2019−2021. According to Global Burden of Disease (GBD) 2023, nearly half of cancer deaths and disability-adjusted life years (DALYs) were attributable to modifiable risk factors such as tobacco, air pollution, high alcohol use, dietary risks, and unsafe sex. In response, the government has implemented a suite of prevention-oriented policies, including expansion of human papillomavirus (HPV) vaccination, strengthened tobacco control, sustained air pollution reduction, enhanced health education, and broadened cancer screening coverage. Collectively, these initiatives demonstrate a sustained national commitment to reducing the cancer burden.
2025, 37(6): 929-936.
doi: 10.21147/j.issn.1000-9604.2025.06.05
Abstract:
Esophageal cancer (EC) is one of the most common malignancies in China, accounting for over half of the world’s new cases and deaths, and posing a severe threat to national health. Currently, the prevention and control of EC primarily rely on secondary prevention through screening, early diagnosis and early treatment to reduce EC-specific mortality. Upper gastrointestinal endoscopy with Lugol’s iodine or optical staining followed by histopathological diagnosis of biopsy samples, serves as the gold standard for EC screening and has been widely implemented in numerous national public health programs for early cancer detection and treatment. Nevertheless, traditional pathology-centered diagnostic and surveillance strategies face significant challenges in current screening practices. Relying on large-scale population-based screening cohorts, including the Efficacy of Endoscopic Screening for Esophageal Cancer in China (ESECC) trial (ClinicalTrials.gov, identifier NCT01688908), a series of investigations into the diagnosis and surveillance of EC screening provided critical new insights and evidence for optimizing endoscopic screening strategies for EC in China. Summarizing relevant research findings from the above investigations, this article provides a systematic review of the epidemiology, current screening diagnostic and surveillance strategies, existing challenges, and key innovations related to EC. These insights offer valuable guidance for public health practice and clinical decision-making in the prevention and screening of EC and other cancers.
Esophageal cancer (EC) is one of the most common malignancies in China, accounting for over half of the world’s new cases and deaths, and posing a severe threat to national health. Currently, the prevention and control of EC primarily rely on secondary prevention through screening, early diagnosis and early treatment to reduce EC-specific mortality. Upper gastrointestinal endoscopy with Lugol’s iodine or optical staining followed by histopathological diagnosis of biopsy samples, serves as the gold standard for EC screening and has been widely implemented in numerous national public health programs for early cancer detection and treatment. Nevertheless, traditional pathology-centered diagnostic and surveillance strategies face significant challenges in current screening practices. Relying on large-scale population-based screening cohorts, including the Efficacy of Endoscopic Screening for Esophageal Cancer in China (ESECC) trial (ClinicalTrials.gov, identifier NCT01688908), a series of investigations into the diagnosis and surveillance of EC screening provided critical new insights and evidence for optimizing endoscopic screening strategies for EC in China. Summarizing relevant research findings from the above investigations, this article provides a systematic review of the epidemiology, current screening diagnostic and surveillance strategies, existing challenges, and key innovations related to EC. These insights offer valuable guidance for public health practice and clinical decision-making in the prevention and screening of EC and other cancers.
2025, 37(6): 937-948.
doi: 10.21147/j.issn.1000-9604.2025.06.06
Abstract:
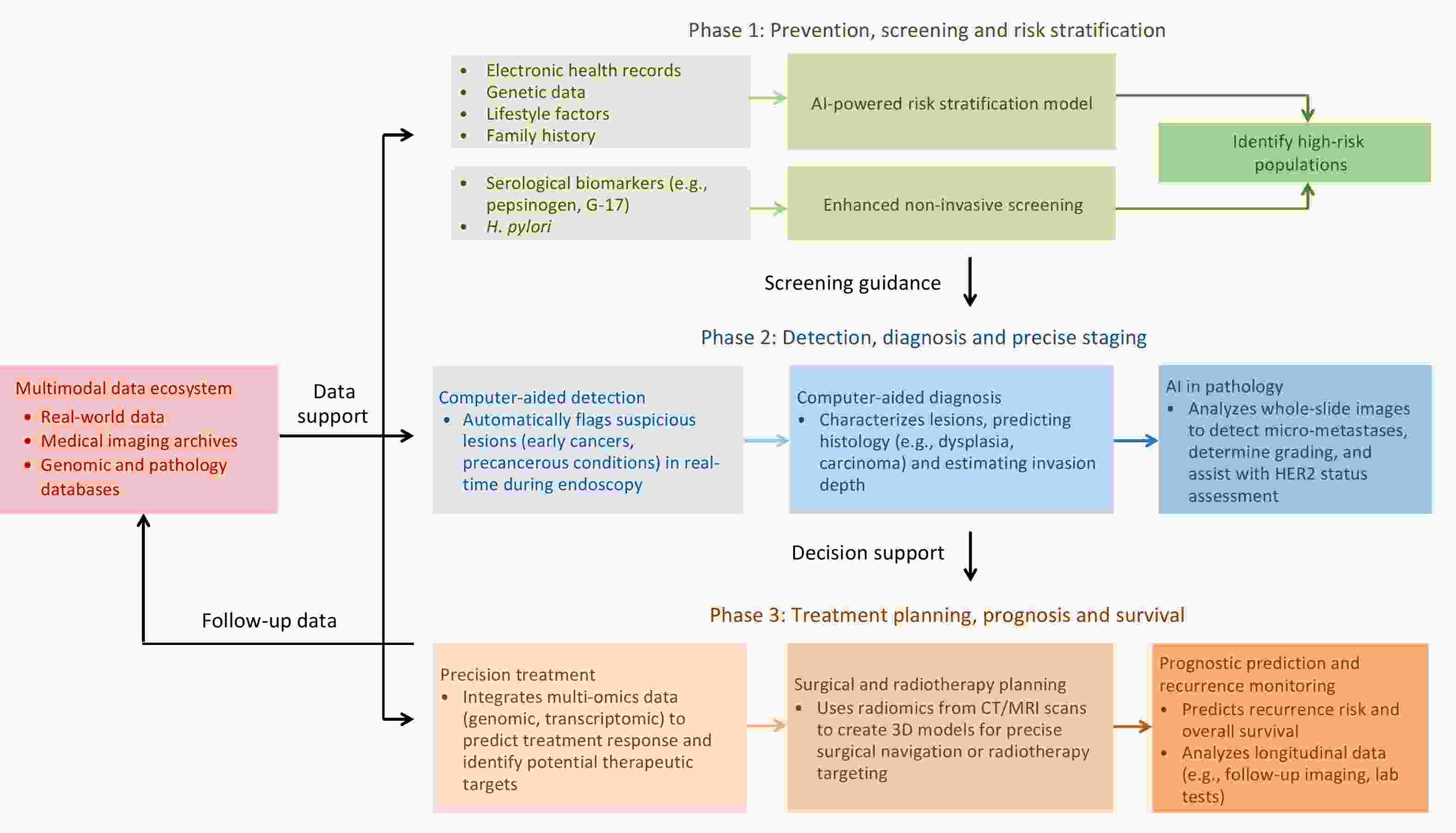
Gastric cancer (GC) remains a significant public health burden in China, accounting for a high proportion of global incidence and mortality. Generally, the incidence and mortality of GC demonstrate marked demographic and geographic disparities in China, with higher rates in males, older adults and rural residents. Helicobacter pylori (H. pylori) infection serves as the main risk factor and contributes to approximately three-quarters of GC cases in China. Large-scale randomized controlled intervention trials in high-risk areas have provided robust evidence to support H. pylori eradication as an effective primary prevention strategy. Other established risk factors include high-salt diet and inadequate consumption of fresh fruits and vegetables. For secondary prevention, several national screening programs utilizing endoscopic examination have been conducted in high-risk populations, which were proven effective in early detection and mortality reduction. However, GC prevention and control in China still face great challenges, including increasing antibiotic resistance, limited screening coverage, and regional disparities in healthcare resources. Further efforts are urgently needed to integrate personalized risk prediction, family-based H. pylori control, and optimized cost-effective screening strategies for precision prevention to finally reduce the burden of GC.
Gastric cancer (GC) remains a significant public health burden in China, accounting for a high proportion of global incidence and mortality. Generally, the incidence and mortality of GC demonstrate marked demographic and geographic disparities in China, with higher rates in males, older adults and rural residents. Helicobacter pylori (H. pylori) infection serves as the main risk factor and contributes to approximately three-quarters of GC cases in China. Large-scale randomized controlled intervention trials in high-risk areas have provided robust evidence to support H. pylori eradication as an effective primary prevention strategy. Other established risk factors include high-salt diet and inadequate consumption of fresh fruits and vegetables. For secondary prevention, several national screening programs utilizing endoscopic examination have been conducted in high-risk populations, which were proven effective in early detection and mortality reduction. However, GC prevention and control in China still face great challenges, including increasing antibiotic resistance, limited screening coverage, and regional disparities in healthcare resources. Further efforts are urgently needed to integrate personalized risk prediction, family-based H. pylori control, and optimized cost-effective screening strategies for precision prevention to finally reduce the burden of GC.
2025, 37(6): 949-961.
doi: 10.21147/j.issn.1000-9604.2025.06.07
Abstract:
The National Health Commission of the People’s Republic of China Guidelines for Diagnosis and Treatment of Colorectal Cancer (2025 edition), based on evidence-based medicine, integrates cutting-edge international advances with Chinese clinical practice, and supplements and completes the previous versions. This version of the guidelines, retains the core diagnostic and treatment framework, highlights new contents such as “Surgical treatment of anal canal cancer” and “New technologies and advances in diagnosis and treatment”, and systematically summarizes the core points in the surgical treatment, medical oncology treatment, radiation oncology treatment, imaging, and pathology treatment. It is designed to help clinicians quickly grasp the key points of the guidelines and promote the standardization, precision, and consistence of colorectal cancer diagnosis and treatment.
The National Health Commission of the People’s Republic of China Guidelines for Diagnosis and Treatment of Colorectal Cancer (2025 edition), based on evidence-based medicine, integrates cutting-edge international advances with Chinese clinical practice, and supplements and completes the previous versions. This version of the guidelines, retains the core diagnostic and treatment framework, highlights new contents such as “Surgical treatment of anal canal cancer” and “New technologies and advances in diagnosis and treatment”, and systematically summarizes the core points in the surgical treatment, medical oncology treatment, radiation oncology treatment, imaging, and pathology treatment. It is designed to help clinicians quickly grasp the key points of the guidelines and promote the standardization, precision, and consistence of colorectal cancer diagnosis and treatment.
2025, 37(6): 962-972.
doi: 10.21147/j.issn.1000-9604.2025.06.08
Abstract:
ObjectiveA subset of patients with human epidermal growth factor receptor 2 positive (HER2+) breast cancer shows insensitivity to neoadjuvant therapy (NAT), often evidenced by imaging results indicating stable disease (SD) or progressive disease (PD), which may reflect intrinsic resistance to treatment. We aimed to investigate the factors associated with NAT insensitivity and its prognostic value in HER2+ breast cancer. MethodsThis study included consecutive patients with HER2+ breast cancer who received NAT consisting of chemotherapy combined with anti-HER2 monoclonal antibodies. NAT insensitivity was defined as SD or PD on the basis of treatment response evaluations. Statistical analyses were conducted on the collected clinical data, and HER2 heterogeneity was subsequently assessed. ResultsA total of 541 patients were included in the study, among whom 63 (11.6%) were categorized as NAT-insensitive group and 478 (88.4%) as NAT-sensitive group. Hormone receptor (HR) status (P=0.033), HER2 status (P=0.036) and anti-HER2 therapy (P=0.007) were associated with NAT sensitivity. NAT-insensitive group had a significantly shorter event-free survival (EFS) (3-year: 69.4% vs. 94.3%; P<0.001) and remained an independent prognostic factor according to Cox models [hazard ratio (HR)=8.637; 95% confidence interval (95% CI), 3.091−24.136; P<0.001]. Exploratory analysis revealed a greater proportion of HER2 heterogeneity in the NAT-insensitive group (19.4% vs. 4.3%; P=0.035). ConclusionsHR positivity, HER2 2+/fluorescence in situ hybridization (FISH)+ status, and trastuzumab monotherapy are associated with NAT insensitivity, and NAT insensitivity independently indicates poor EFS. This study also highlights the need for prospective studies to clarify the role of HER2 heterogeneity and other mechanisms involved in predicting the response to NAT.
2025, 37(6): 973-983.
doi: 10.21147/j.issn.1000-9604.2025.06.09
Abstract:
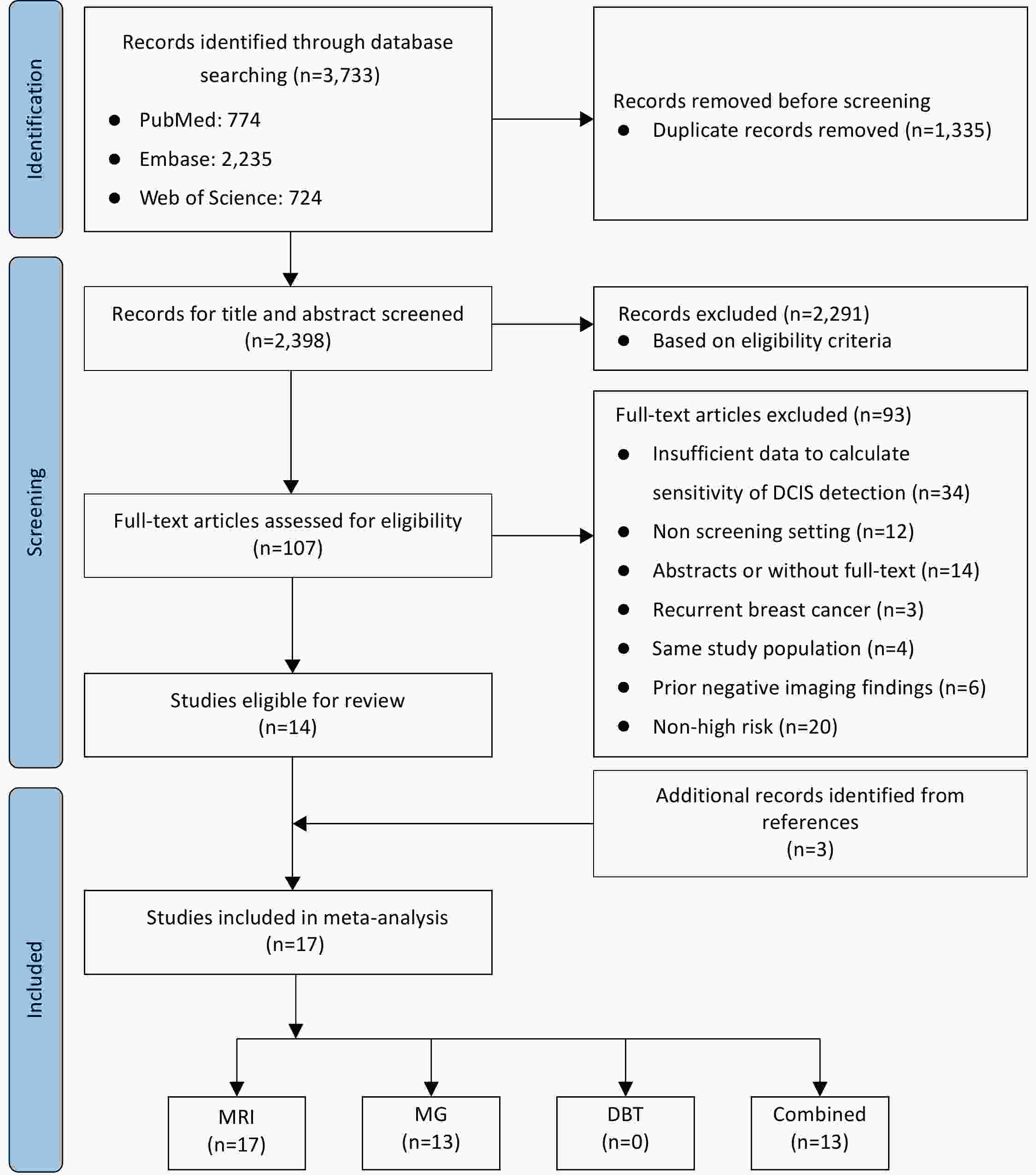
ObjectiveGiven the improved sensitivity of magnetic resonance imaging (MRI) for detecting ductal carcinoma in situ (DCIS), the omission of routine mammography (MG) or digital breast tomosynthesis (DBT) in high-risk breast cancer screening is under consideration. We aim to conduct a systematic review and meta-analysis to compare the screening sensitivity of MRI, MG and DBT for detecting DCIS in high-risk females. MethodsPubMed, Embase, and Web of Science were searched for studies reporting the sensitivity of detecting DCIS in high-risk females up to July 02, 2025. Study quality was assessed with quality assessment of diagnostic accuracy studies-2 (QUADAS-2). Pooled sensitivity was estimated using a random-effects model, overall and stratified by age (<40 and ≥40 years old) and BRCA status (BRCA1 and BRCA2). Meta-regression was used to compare modalities. ResultsSeventeen studies (18,348 participants, 211 with DCIS) were included. MRI showed significantly higher pooled sensitivity [85%, 95% confidence interval (95% CI): 74%−94%] than MG (36%, 95% CI: 23%−50%; P<0.001). No DBT data were available. Combined MRI and MG yielded the highest sensitivity (99%, 95% CI: 97%−100%), but offered no significant gain over MRI alone in females <40 years old (P=0.091) and in BRCA1 mutation carriers (P=0.143). ConclusionsMRI is more sensitive than MG for DCIS detection in high-risk females. In females <40 years old and BRCA1 mutation carriers, adding MG to MRI provides no additional diagnostic value. Considering the potential trade-offs, the routine use of MG in these subgroups should be carefully reconsidered.
2025, 37(6): 984-999.
doi: 10.21147/j.issn.1000-9604.2025.06.10
Abstract:
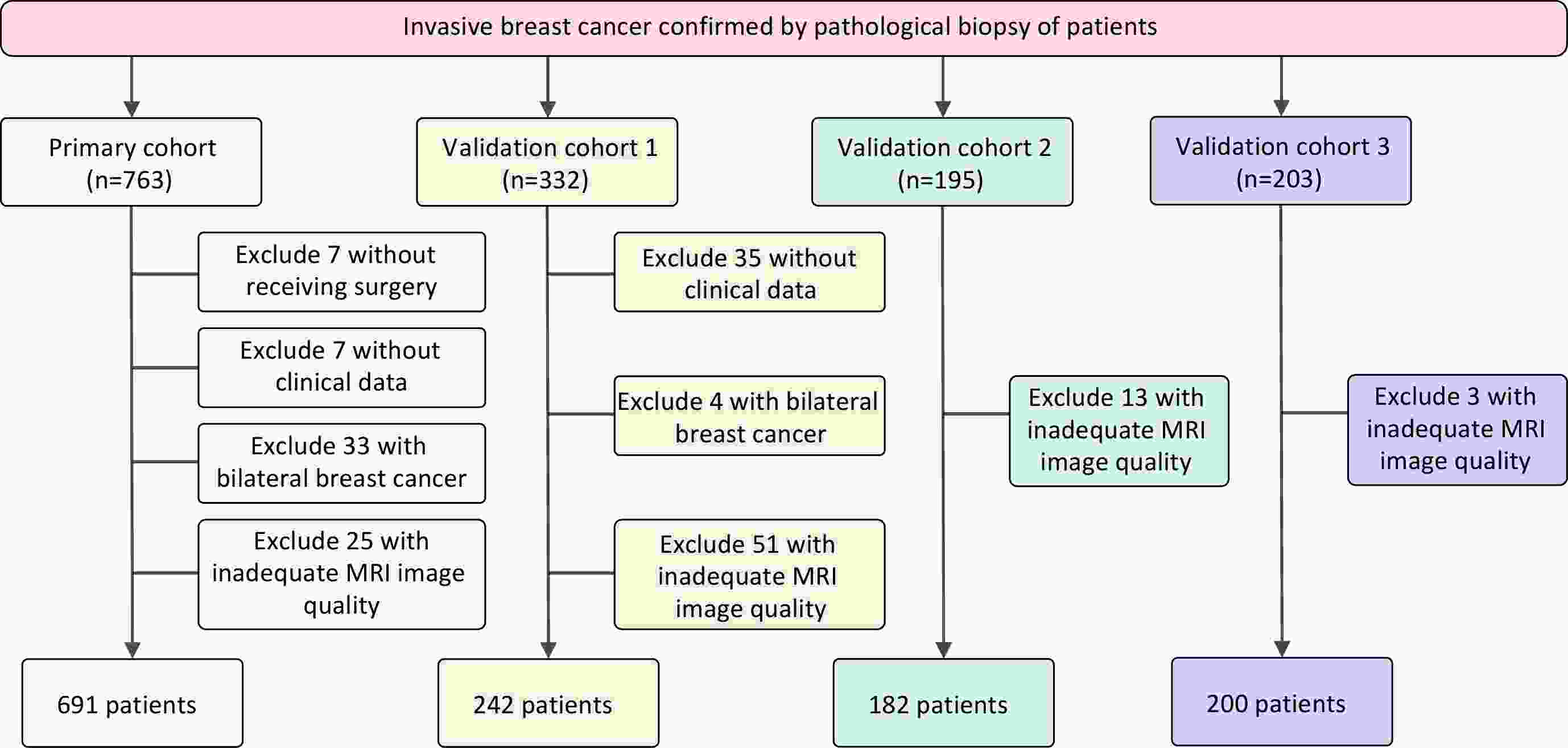
ObjectiveNeoadjuvant therapy (NAT) has become the standard treatment option for patients with locally advanced breast cancer. How to non-invasively screen out patients with pathological complete response (pCR) after NAT has become an urgent world-wide clinical problem. Our work aims to the assessment of neoadjuvant treatment response in breast cancer patients for higher accuracy prediction using innovative artificial intelligence system. MethodsIn this study, we retrospectively collected longitudinal (pre-NAT and post-NAT) multi-parametric magnetic resonance imaging (MRI) and clinicopathologic data of a total of 1,315 breast cancer patients (clinical stage I−III) who had undergone NAT followed by standard surgery and treated across 5 independent medical centers from January 2010 to January 2023. We used radiomics, 3D convolutional neural network technology and clinical data statistical analysis methods to extract and screen multimodal features, and then developed and validated a Clinical-Radiomics-Deep-Learning (CRDL) model to predict patients’ pCR outcomes based on multimodal fusion features. ResultsWe use the area under the receiver operating characteristic curve (AUC) in the primary cohort (PC) and 3 external validation cohorts (VC1−3) to evaluate the model performance. The results showed that the AUC in the PC composed of 2 medical centers was 0.947 [95% confidence interval (95% CI): 0.931−0.960], and the AUC values in VC1−3 were 0.857 (95% CI: 0.810−0.901), 0.883 (95% CI: 0.841−0.918) and 0.904 (95% CI: 0.860−0.941), respectively. ConclusionsThe CRDL model demonstrated high accuracy and robustness in predicting pCR to NAT using multimodal fusion data. This study provides a strong foundation for non-invasive assessment of pCR status in breast cancer patients following NAT and offers critical insights to guide clinical decision-making in post-NAT treatment planning.
2025, 37(6): 1000-1019.
doi: 10.21147/j.issn.1000-9604.2025.06.11
Abstract:
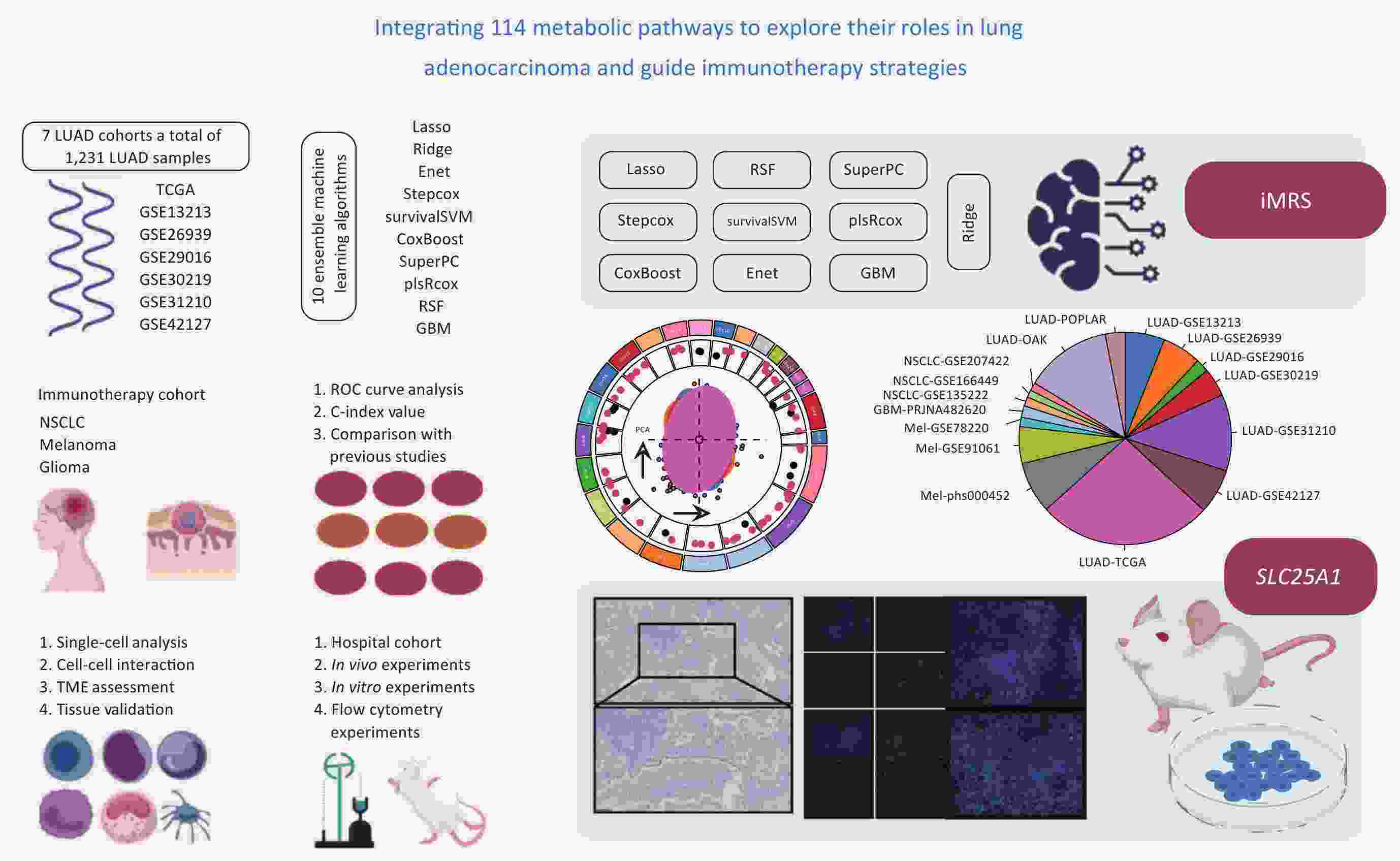
ObjectiveLung adenocarcinoma (LUAD) is the most common subtype of lung cancer. Despite significant advances in immunotherapy, treatment responses vary substantially among individuals. Metabolic reprogramming, as a hallmark of cancer, plays a crucial role in tumor progression and immune evasion. However, the interplay between metabolic features and tumor immune microenvironment in LUAD remains to be systematically elucidated. MethodsWe analyzed data from 1,231 LUAD patients across seven global cohorts and developed an integrated Metabolism-Related Signature (iMRS) using machine learning approaches based on 114 metabolic features. The signature’s ability to predict immunotherapy response was validated using 9 immunotherapy cohorts (n=712, including LUAD, melanoma, and glioma). An in-house LUAD tissue cohort (n=146) confirmed the prognostic significance of SLC25A1, a key gene within the signature, and its spatial relationship with immune cells. In vivo and in vitro experiments investigated SLC25A1’s role in cancer promotion, immune exclusion, and its impact on programmed cell death protein 1 (PD-1) therapy efficacy. ResultsiMRS demonstrated superior prognostic performance in LUAD patients, outperforming 129 published LUAD signatures. In immunotherapy cohorts, responders showed significantly lower iMRS scores. High iMRS was associated with reduced immune activity and “cold” tumor characteristics. SLC25A1 (correlation coefficient=0.54, P<0.05), a key gene in the signature, showed the highest expression in CD8 desert phenotype and correlated with poor prognosis. Multiplexed immunofluorescence revealed exclusion patterns between SLC25A1 and immune cells (CD4+ T cells and CD20+ B cells). SLC25A1 knockdown reduced lung metastasis and enhanced anti-PD-1 efficacy by increasing CD8+ T cell abundance and cytotoxicity [increased interferon-γ (IFN-γ)+/GZMB+ CD8+ T cells]. ConclusionsiMRS provides personalized immunotherapy prediction for LUAD patients. SLC25A1, identified as a novel immune-exclusion related oncogene, represents a promising therapeutic target for LUAD treatment.
2025, 37(6): 1020-1033.
doi: 10.21147/j.issn.1000-9604.2025.06.12
Abstract:
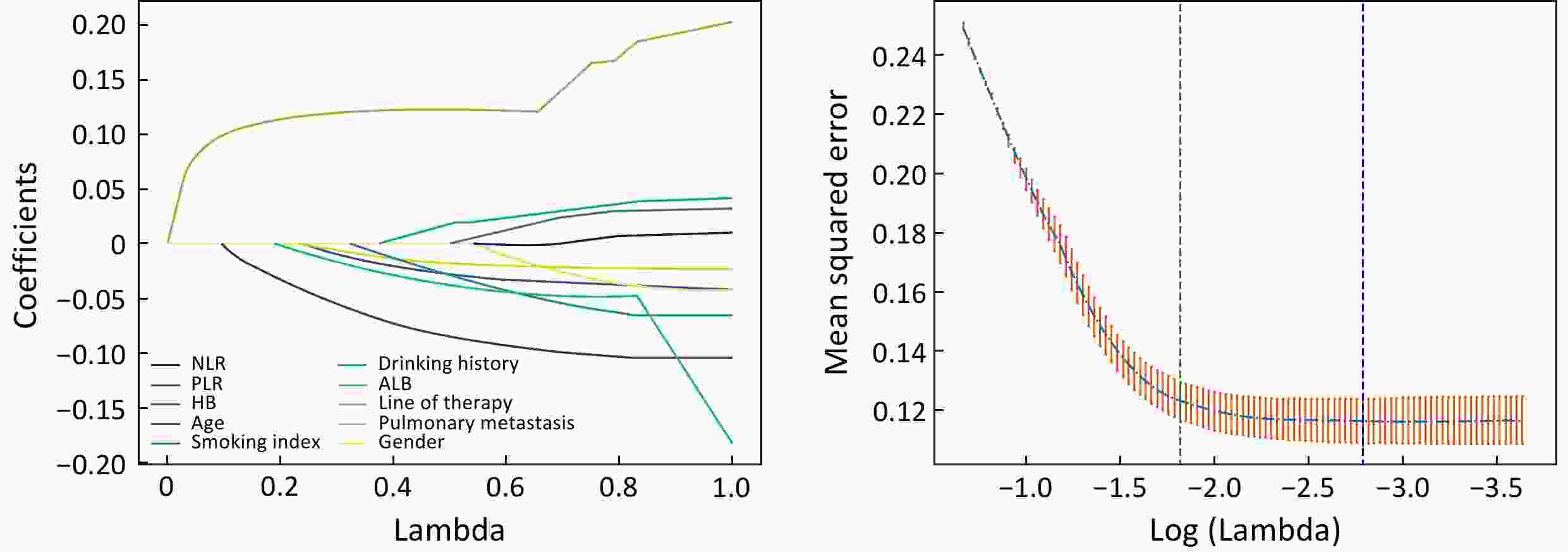
ObjectiveImmune checkpoint inhibitor-related pneumonitis (ICIP) is a common and potentially life-threatening adverse event with non-specific symptoms. It is of significance to identify high-risk population of ICIP. However, existing prediction models for ICIP are often limited by their reliance on clinically inaccessible variables and homogeneous methodologies, hindering their clinical utility. This study aimed to develop a clinical risk-prediction model for ICIP in patients with gastrointestinal (GI) cancer based on four machine learning (ML) methods. MethodsWe conducted a retrospective analysis of data from GI cancer patients who received immune checkpoint inhibitors (ICIs) between 2018 and 2022 in Beijing Cancer Hospital. For each patient, 36 clinical indicators associated with pneumonia risk were gathered. The dataset was split into training and testing sets in a ratio of 7:3. Variable selection was first performed using Least Absolute Shrinkage and Selection Operator (LASSO) regression. Subsequently, four ML algorithms: logistic regression (LR), random forest (RF), Support vector machine (SVM), and Adaptive Boosting (AdaBoost), were employed to develop and validate ICIP prediction models. The models’ performance was assessed using sensitivity, specificity, precision, F1-score, and the area under the receiver operating characteristic curve (AUC) value. The optimal cutoff point for the best model was determined and a web-based tool was developed based on it. ResultsWe collected medical data from 1,101 GI cancer patients. Ten predictive variables were identified as significant: gender, age, treatment line, smoking index, drinking history, lung metastasis, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, hemoglobin, and albumin. After constructing and comparing four ML models, the RF model demonstrated best performance with an AUC of 0.899. The web-based tool for ICIP risk prediction is available at https://healthy.aistarfish.com/business/pneumonia-prediction/#/home . ConclusionsWe analyzed 36 clinical predictors of ICIP in 1,101 patients treated with ICIs, and 10 variables were included. The smoking index, albumin and hemoglobin emerged as novel predictors specific to GI cancers. Among the models constructed using four ML methods, the RF model showed the best performance. Additionally, a web-based tool was developed to facilitate the early clinical identification of populations at high risk of ICIP. Future directions include external validation of the model to enhance clinical usability.
2025, 37(6): 1034-1057.
doi: 10.21147/j.issn.1000-9604.2025.06.13
Abstract:
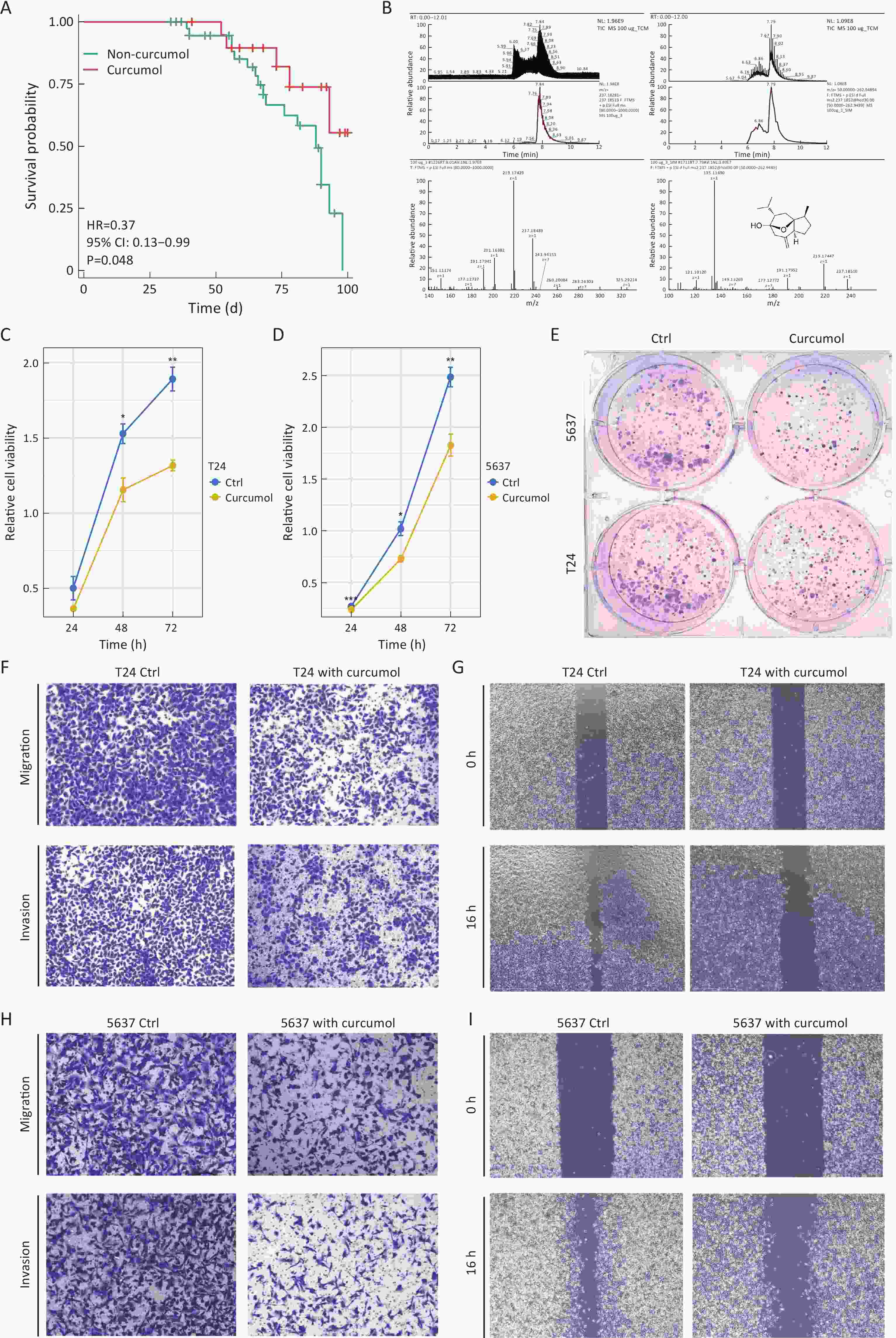
ObjectiveCisplatin-based chemotherapy is a cornerstone for bladder cancer treatment, but the development of resistance remains a major clinical challenge. Curcumol, a bioactive sesquiterpenoid derived from Curcumae Rhizoma, has shown anti-tumor potential. This study investigated the efficacy of curcumol in overcoming cisplatin resistance and elucidated its underlying molecular mechanisms in bladder cancer progression. MethodsClinical correlation was assessed in patients receiving neoadjuvant chemotherapy with or without Curcumae Rhizoma. The anti-tumor effects of curcumol were evaluated in both cisplatin-sensitive and cisplatin-resistant bladder cancer cells. Multi-omics approaches, including RNA sequencing, proteomics and metabolomics, were employed. Key mechanisms involving H3K9 lactylation (H3K9la) were explored via Western blotting, immunohistochemistry, and cleavage under targets and tagmentation (CUT&Tag) assays. The role of the identified target ORC6 was validated through genetic knockout and overexpression. Finally, ferroptosis was confirmed by measuring lipid peroxidation [malondialdehyde (MDA)], total iron levels, and ferroptosis-related protein markers in vitro. ResultsClinical data indicated that patients administered Curcumae Rhizoma exhibited enhanced responses to neoadjuvant chemotherapy. In addition, curcumol suppressed the proliferation, migration, and invasion of both bladder cancer cells and cisplatin-resistant cells. Mechanistically, proteomic analysis and non-targeted metabolomics revealed that curcumol suppresses glycolysis and lactate production. Subsequently, Western blotting analysis demonstrated a marked reduction in H3K9la levels in both T24 and 5637 cells following curcumol treatment. This decrease in H3K9la was also observed in patient tumor tissues via immunohistochemistry staining. CUT&Tag analysis identified that H3K9la is enriched with the highest number of reads at the ORC6 promoter region. Combined in vitro and in vivo experiments indicated that OCR6 exerted a tumor-promoting effect on bladder cancer. Its knockout induced G0/G1 phase arrest and enhanced apoptosis, while its expression contributed to cancer progression by enhancing invasive and migratory capabilities. Furthermore, ORC6 overexpression correlated with ferroptosis scores and ferroptosis-related genes. In vitro, OCR6 knockout promoted ferroptosis via DNA damage, characterized by elevated MDA content, decreased expression of core ferroptosis-related proteins (GPX4 and SLC7A11), increased percentage of γH2AX-positive cells and longer DNA tails. Finally, we performed rescue experiments using a ferroptosis inhibitor in ORC6 knockout cells, which indicated that ferroptosis inhibitor could weaken the effect of ORC6 knockout on the invasive, migratory, and proliferative capacities. ConclusionsOur findings demonstrated that curcumol effectively counteracted cisplatin resistance and inhibited bladder cancer progression by targeting the glycolysis-H3K9la-ORC6 axis to induce ferroptosis. This study established a critical link between metabolic reprogramming, histone lactylation, and ferroptosis, providing a novel therapeutic avenue for treating chemoresistant bladder cancer.
2025, 37(6): 1058-1061.
doi: 10.21147/j.issn.1000-9604.2025.06.14
Abstract:
2025, 37(6): 1062.
doi: 10.21147/j.issn.1000-9604.2025.06.15
Abstract:

 Abstract
Abstract FullText HTML
FullText HTML PDF 1308KB
PDF 1308KB