Current Issue
Display Method: |
2026, 38(1): 1-26.
doi: 10.21147/j.issn.1000-9604.2026.01.01
Abstract:
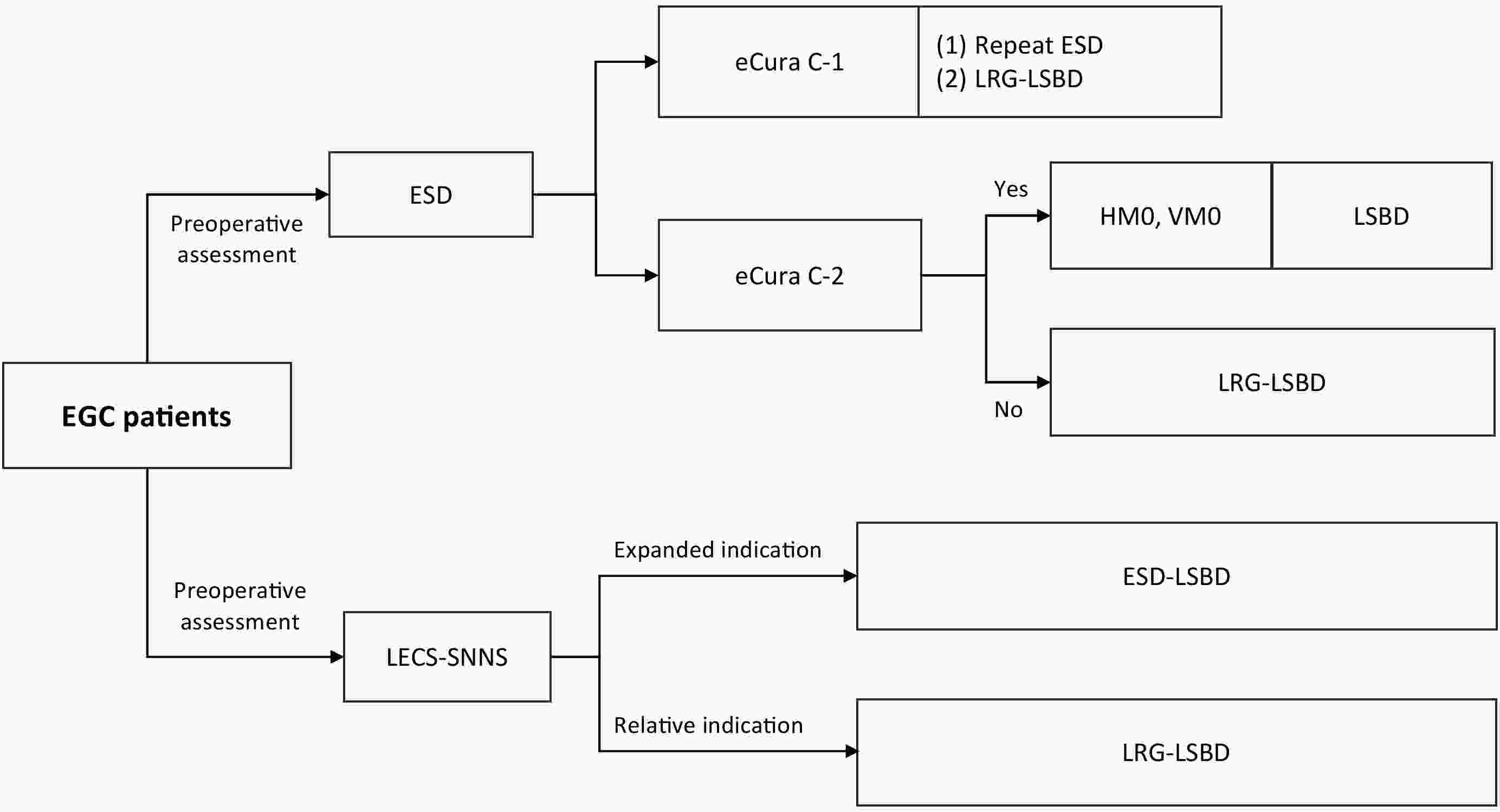
With the advancement of surgical techniques and enhanced management of early gastric cancer (EGC), minimally invasive function-preserving surgical approaches have emerged as a common goal for patients and clinicians. Laparoscopic-endoscopic cooperative surgery combined with sentinel lymph node navigation surgery (LECS-SNNS) has drawn increasing interest because of its dual benefits of minimal invasiveness and organ function preservation. However, robust evidence-based support for guiding clinical implementation remains limited. To address this gap, we systematically evaluated available studies on the clinical application of LECS-SNNS in EGC and integrated expert insights to formulate 20 recommendations. These included preoperative assessment, surgical techniques, intraoperative endoscopic procedures, pathological evaluation, postoperative care, and follow-up. This consensus aimed to provide comprehensive guidance for the standardized application of LECS-SNNS, thereby advancing precise, minimally invasive, and function-preserving treatment for EGC.
With the advancement of surgical techniques and enhanced management of early gastric cancer (EGC), minimally invasive function-preserving surgical approaches have emerged as a common goal for patients and clinicians. Laparoscopic-endoscopic cooperative surgery combined with sentinel lymph node navigation surgery (LECS-SNNS) has drawn increasing interest because of its dual benefits of minimal invasiveness and organ function preservation. However, robust evidence-based support for guiding clinical implementation remains limited. To address this gap, we systematically evaluated available studies on the clinical application of LECS-SNNS in EGC and integrated expert insights to formulate 20 recommendations. These included preoperative assessment, surgical techniques, intraoperative endoscopic procedures, pathological evaluation, postoperative care, and follow-up. This consensus aimed to provide comprehensive guidance for the standardized application of LECS-SNNS, thereby advancing precise, minimally invasive, and function-preserving treatment for EGC.
2026, 38(1): 27-38.
doi: 10.21147/j.issn.1000-9604.2026.01.02
Abstract:
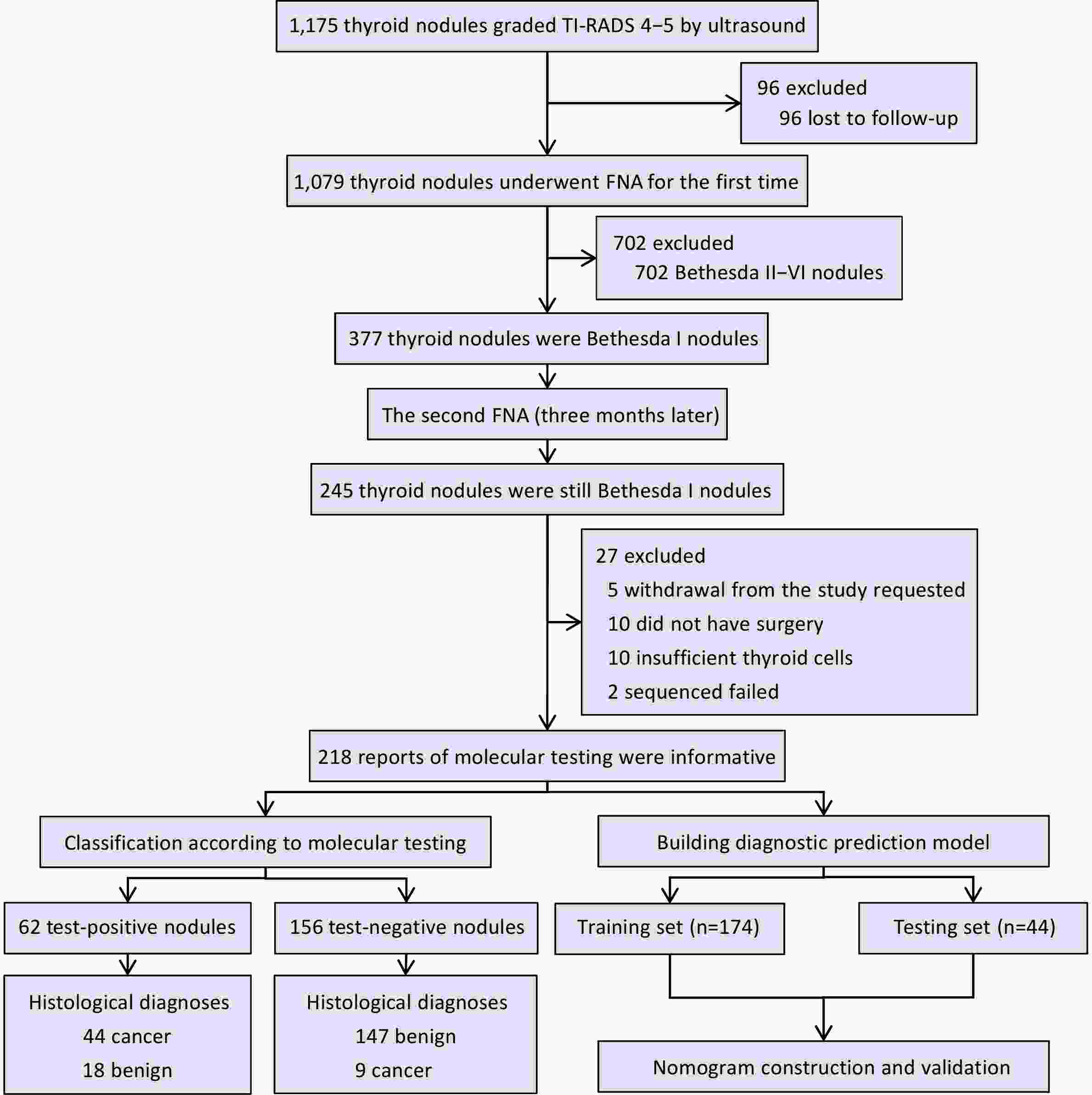
ObjectiveNon-diagnostic thyroid nodules (Bethesda I) account for 5%−20% of all thyroid nodules. Accurate differentiation of benign and malignant nodules can reduce unnecessary surgeries and repeat biopsies. Herein we evaluated the diagnostic efficacy of multigene testing in non-diagnostic thyroid nodules and developed a predictive model integrating molecular and clinical data. MethodsIn this prospective cohort study, 1,175 patients with thyroid nodules were evaluated for inclusion, of which 218 patients with Bethesda I nodules met our inclusion criteria. The primary outcome was diagnostic accuracy of molecular testing, and the secondary outcome was the performance of a predictive model integrating molecular and clinical data. ResultsFinal histopathology identified 165 benign and 53 malignant nodules. Molecular testing detected 10 distinct point mutations and seven gene fusions. Among benign nodules, 147 tested negative and 18 tested positive, whereas 44 malignant nodules tested positive and nine tested negative. In nodules with ultrasound grades 4−5 and fine-needle aspiration cytology (FNAC) results categorized as non-diagnostic, molecular testing achieved sensitivity of 83.00%, specificity of 89.00%, positive predictive value (PPV) of 71.00%, negative predictive value (NPV) of 94.20%, and overall accuracy of 87.60%. The predictive model incorporated 18 clinical and 19 molecular features. Eleven non-zero predictors were selected via least absolute shrinkage and selection operator (LASSO), and the model achieved area under curve (AUC) of 0.95 in the training set and 0.96 in the testing set. Decision curve analysis indicated greater net benefit compared with conventional diagnostic approaches. ConclusionsMolecular testing significantly improved diagnostic accuracy for Bethesda I thyroid nodules. Integrating molecular and clinical data enabled the development of a robust predictive model, facilitating precise, individualized patient management and reducing the need for repeat FNAC and unnecessary surgeries.
2026, 38(1): 39-51.
doi: 10.21147/j.issn.1000-9604.2026.01.03
Abstract:
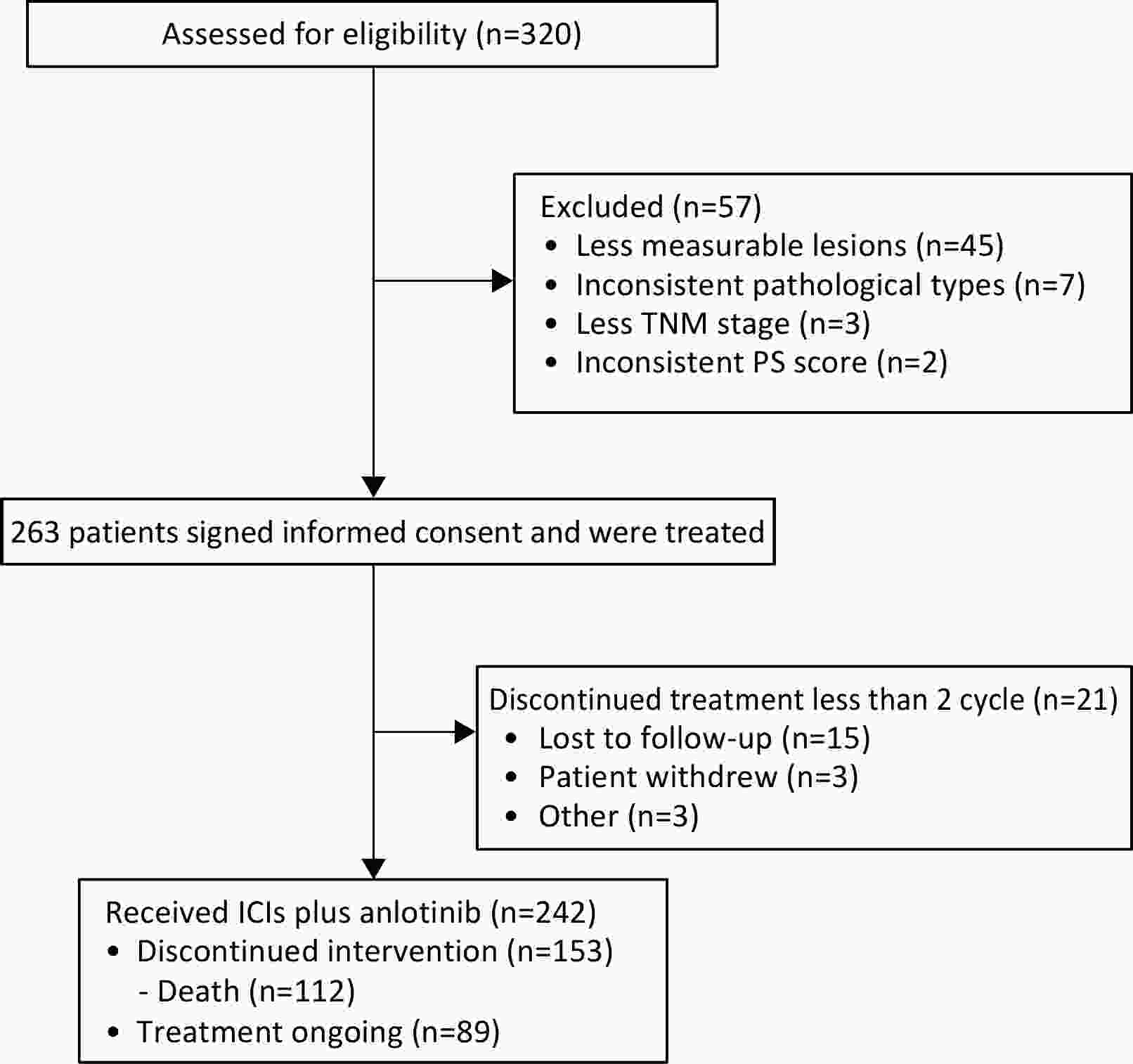
ObjectiveChemotherapy-based regimens remain the standard first- and second-line treatment options for patients with driver gene-negative non-small cell lung cancer (NSCLC). However, in real-world settings, certain patients cannot tolerate chemotherapy or opt to decline it. Immune checkpoint inhibitors (ICIs) constitute the preferred chemotherapy-free alternative. To enhance patient prognosis, this study aimed to examine the efficacy of ICIs combined with anlotinib in real-world scenarios. MethodsThis prospective, multicenter, real-world study evaluated the efficacy and safety of ICIs combined with anlotinib in patients with advanced NSCLC. Patients undergoing first- or second-line treatment were enrolled. The primary endpoint was progression-free survival (PFS), while the secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety. ResultsIn total, 242 patients were enrolled from 28 centers. The median PFS for the entire cohort was 7.8 [95% confidence interval (95% CI), 7.0−9.5] months, OS events occurred in 112 (46.3%) patients, with a current median OS of 17.0 (95% CI, 15.1−19.4) months. The ORR and DCR were 36.0% (95% CI, 30.2%−42.2%) and 97.9% (95% CI, 95.3%−99.1%), respectively. The median PFS was 9.8 (95% CI, 7.4−12.5) months for first-line therapy and 6.9 (95% CI, 6.0−8.3) months for second-line therapy. Treatment-related adverse events (AEs) occurred in 198 (81.8%) patients, with grade 3−4 AEs reported in 22 (9.1%) patients. ConclusionsThis multicenter, real-world study demonstrates that the anlotinib-ICI combination regimen exhibits clinically meaningful efficacy and tolerability as a chemotherapy-free alternative for advanced NSCLC, offering viable evidence to guide treatment for patients who are unsuitable for conventional chemotherapy.
2026, 38(1): 52-66.
doi: 10.21147/j.issn.1000-9604.2026.01.04
Abstract:
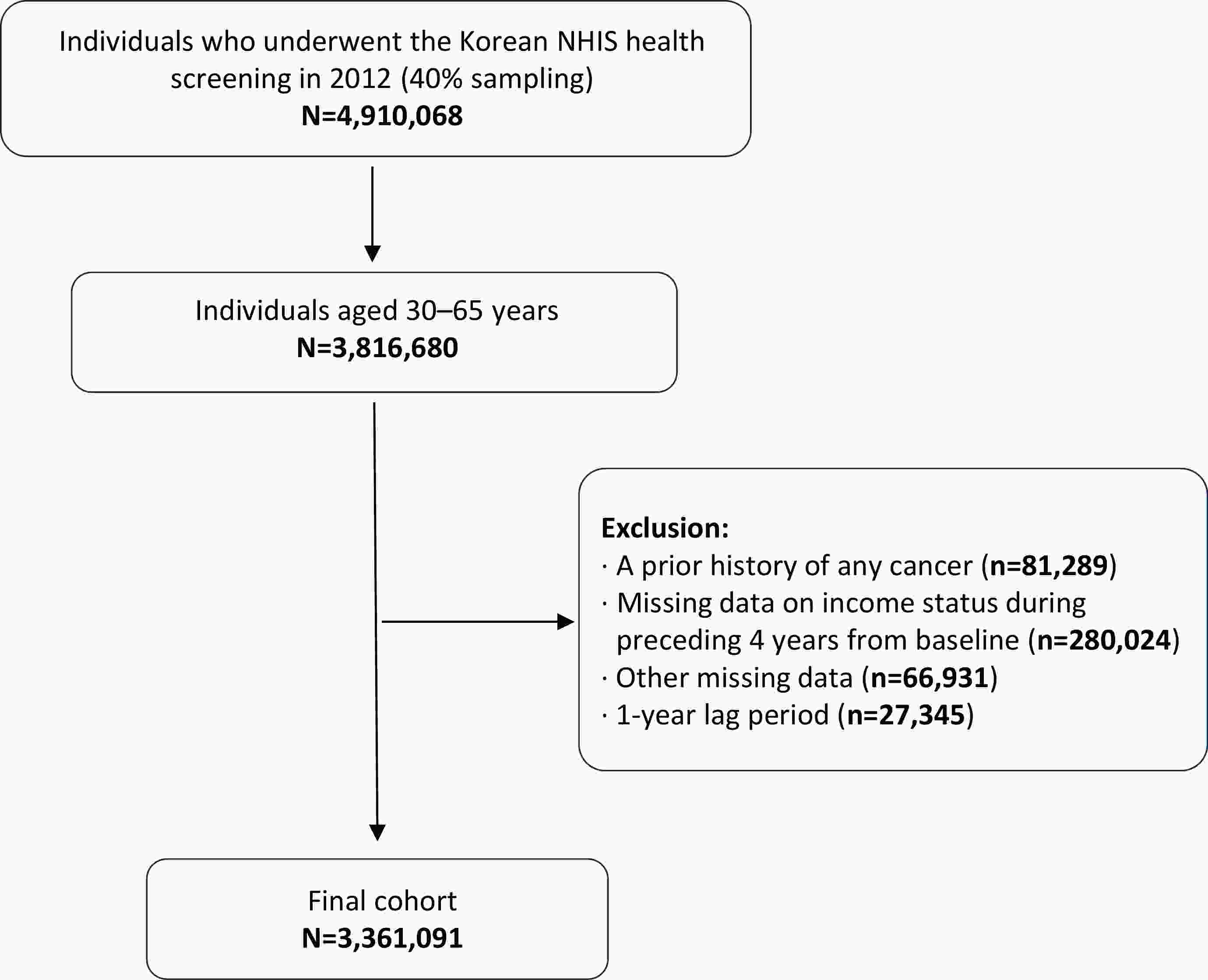
ObjectiveThis study aimed to evaluate the associations of baseline income, cumulative income exposure, and income volatility with the incidence of pancreatic and biliary tract cancers in a nationwide Korean cohort. MethodsWe analyzed 3,361,091 adults aged 30−65 years who underwent the 2012 National Health Insurance Service (NHIS) health screening. Income level was derived from insurance premium data assessed over the five years preceding baseline (2008−2012) and categorized into baseline income quartiles, cumulative exposure to low or high income, and income volatility based on annual percentage changes. Incident pancreatic and biliary tract cancers were identified using diagnostic codes and the copayment reduction registry. Associations were evaluated using Cox proportional hazards models with adjustment for demographic, lifestyle, and clinical covariates, and cumulative incidence was compared using Kaplan-Meier curves. ResultsDuring a median follow-up of 9.6 years, 14,469 pancreatic cancers and 6,647 biliary tract cancers were newly diagnosed. Lower baseline income was associated with a higher risk of pancreatic and biliary tract cancers, whereas sustained high-income exposure was associated with reduced risk. Cumulative low-income exposure showed a positive linear trend with pancreatic cancer incidence. Income volatility was modestly associated with pancreatic cancer and was positively associated with biliary tract cancer in the fully adjusted model. These associations were generally consistent across subgroups, with a stronger inverse association between prolonged high-income exposure and pancreatic cancer among individuals without diabetes. ConclusionsIncome level and income stability were significantly associated with the incidence of pancreatic and biliary tract cancers. Lower baseline income was associated with higher risk, whereas sustained high-income exposure was protective. Income volatility was associated with increased cancer risk, particularly for biliary tract cancer. These findings highlight the importance of incorporating income dynamics into cancer prevention strategies and addressing socioeconomic instability among vulnerable populations.
2026, 38(1): 67-82.
doi: 10.21147/j.issn.1000-9604.2026.01.05
Abstract:
ObjectiveWe investigated the clinical value of a novel circulating tumor cell (CTC) detection method—subtraction enrichment combined with immunostaining and fluorescence in situ hybridization (SE-iFISH)—in ovarian cancer (OC). This study evaluated the diagnostic and prognostic significance of chromosome 8 aneuploidy in CTCs and circulating tumor endothelial cells (CTECs) for preoperative diagnosis, treatment efficacy assessment, and recurrence monitoring. MethodsA total of 331 patients were enrolled, including 56 with newly diagnosed primary OC, 265 with benign ovarian tumors, and 10 with borderline tumors. Peripheral blood CTCs and CTECs were detected using SE-iFISH; their quantity and ploidy characteristics were analyzed in relation to clinical indicators. To assess dynamic CTC changes during disease progression and treatment response, 72 patients were followed longitudinally, of whom 19 experienced recurrence. ResultsThe CTC detection rate in OC patients was 92.9%, with significantly higher counts than that in the benign tumor group (median 5 vs. 2). Receiver operating characteristic analysis demonstrated good diagnostic performance for total CTCs [area under the curve (AUC)=0.699], with triploid CTCs achieving the highest efficacy (AUC=0.792), surpassing carbohydrate antigen 125 (CA125) (AUC=0.702). Postoperative follow-up showed that 70% of patients exhibited concurrent decreases in CTCs and CA125 levels, indicating disease improvement. In 30% of patients, CTC levels did not correlate with changes in CA125 levels. Individual case evidence suggests that CTC alterations may serve as an early indicator of recurrence or metastasis. Among the 19 recurrent cases, 73.7% showed elevated CTCs at recurrence that decreased following treatment. In four patients, CTCs reflected disease progression earlier than CA125, indicating higher sensitivity for recurrence monitoring. ConclusionsCTCs with chromosome 8 aneuploidy demonstrate significant clinical value in the preoperative diagnosis, treatment efficacy evaluation, and recurrence monitoring of OC. Dynamic CTC changes may serve as a more sensitive indicator than CA125 for disease surveillance, supporting the translational potential of CTC-based biomarkers in OC.
2026, 38(1): 83-99.
doi: 10.21147/j.issn.1000-9604.2026.01.06
Abstract:
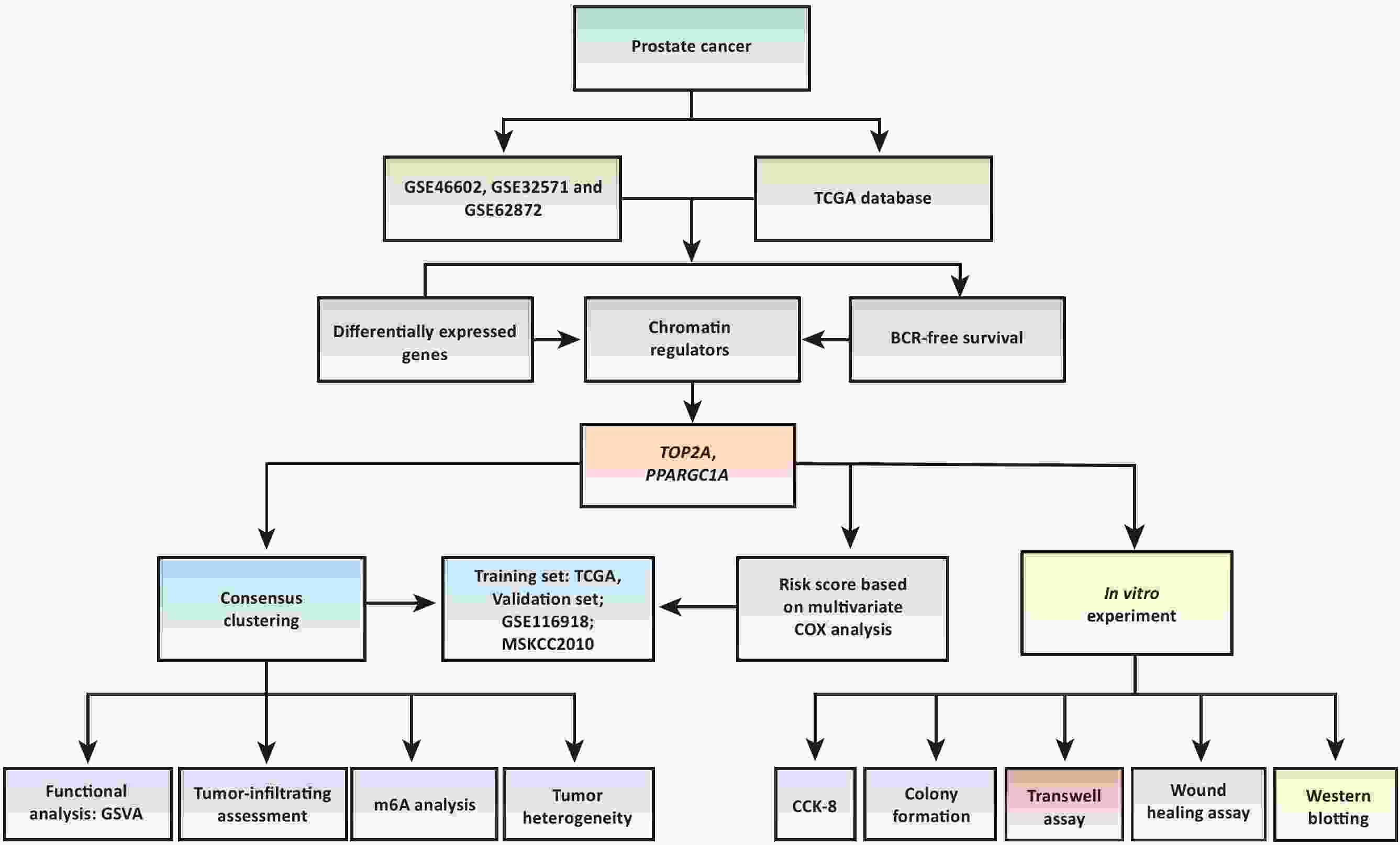
ObjectiveTo identify chromatin regulators (CRs)-based molecular subtypes and risk scores for accurately predicting biochemical recurrence (BCR) after radical prostatectomy (RAP) in prostate cancer (PCa) patients. MethodsDifferentially expressed genes (DEGs) between tumor and normal samples from The Cancer Genome Atlas (TCGA) and gene expression omnibus (GEO) databases were intersected with CR-related and prognostic genes. Consensus clustering, risk score analysis, functional analysis, immune microenvironment, m6A, and heterogeneity assessments were performed using R software. In vitro validation used DU145 and C42B PCa cell lines. Topoisomerase II alpha (TOP2A) was knocked down via siRNA. Assays included CCK-8 proliferation, colony formation, transwell migration/invasion, wound healing, and western blotting (WB) for pathway validation. ResultsTOP2A and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) defined molecular subtypes and a risk score in TCGA, validated in a GEO dataset. Cluster 2 exhibited significantly shorter BCR-free survival vs. cluster 1 in TCGA [hazard ratio (HR): 2.21; 95% confidence interval (95% CI): 1.32−3.73; P=0.003)], GEO (HR: 2.05; 95% CI: 1.05−4.02; P=0.010), and MSKCC2010 (HR: 5.93; 95% CI: 1.96−17.87; P<0.001). Similar survival differences were observed between high- and low-risk groups (defined by the median risk score). Cluster 2 showed greater tumor heterogeneity and higher m6A gene expression. Gene set variation analysis (GSVA) revealed downregulated cell-cycle pathways in cluster 2, alongside suppressed tumor-infiltrating immune cells. TOP2A knockdown significantly impaired PCa cell proliferation, colony formation, migration, and invasion. Mechanistically, it suppressed phosphoinositide 3-kinase (PI3K)/AKT serine/threonine kinase (AKT) pathway activation, reducing phosphorylated PI3K and AKT levels without altering total protein. ConclusionsTOP2A and PPARGC1A effectively stratify PCa subtypes for RAP patients. TOP2A drives malignant progression via the PI3K/AKT pathway.
2026, 38(1): 100-118.
doi: 10.21147/j.issn.1000-9604.2026.01.07
Abstract:
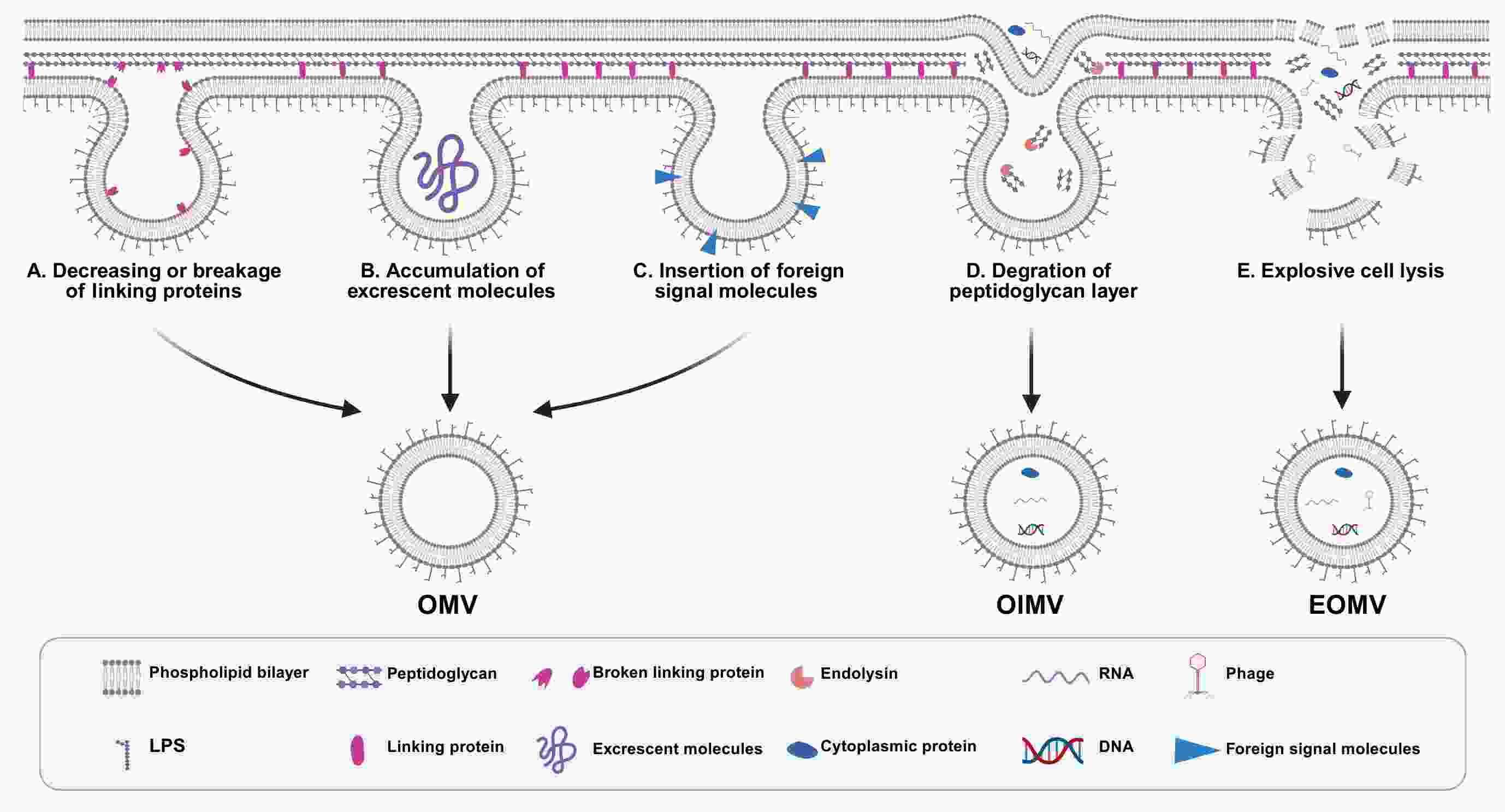
Bacterial outer membrane vesicles (OMVs) are spherical nanostructures that originate from Gram-negative bacteria. They are gaining attention as powerful tools in cancer diagnostics and therapy due to their unique biological properties. These vesicles, which range from 50 to 250 nm in size, carry molecular components from their parent bacteria, allowing them to play important roles in bacterial defense and microbial ecosystems. Their lipid bilayer structure facilitates targeted drug delivery, while their natural immunogenic properties hold promise for cancer immunotherapy by helping overcome immune evasion in the tumor microenvironment. Moreover, OMVs have potential as biomarkers in liquid biopsies, particularly for cancers associated with bacteria, such as gastric and colorectal cancers. Their ability to interact with the intratumoral microbiota further indicates their relevance in tumor pathogenesis. This review aims to provide a comprehensive overview of the fundamental biology of OMVs and their emerging applications in cancer therapy.
Bacterial outer membrane vesicles (OMVs) are spherical nanostructures that originate from Gram-negative bacteria. They are gaining attention as powerful tools in cancer diagnostics and therapy due to their unique biological properties. These vesicles, which range from 50 to 250 nm in size, carry molecular components from their parent bacteria, allowing them to play important roles in bacterial defense and microbial ecosystems. Their lipid bilayer structure facilitates targeted drug delivery, while their natural immunogenic properties hold promise for cancer immunotherapy by helping overcome immune evasion in the tumor microenvironment. Moreover, OMVs have potential as biomarkers in liquid biopsies, particularly for cancers associated with bacteria, such as gastric and colorectal cancers. Their ability to interact with the intratumoral microbiota further indicates their relevance in tumor pathogenesis. This review aims to provide a comprehensive overview of the fundamental biology of OMVs and their emerging applications in cancer therapy.

 Abstract
Abstract FullText HTML
FullText HTML PDF 10100KB
PDF 10100KB