2016 Vol.28(4)
Display Mode: |
2016, 28(4): 383-396.
doi: 10.21147/j.issn.1000-9604.2016.04.01
Abstract:
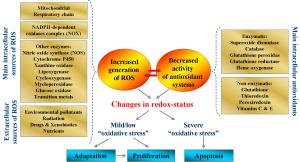
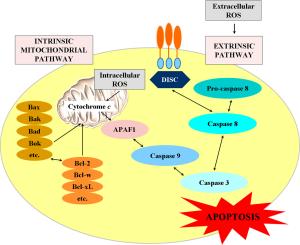
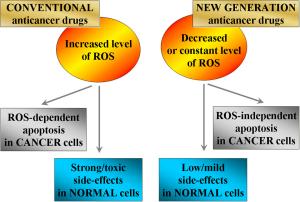
Many studies demonstrate that conventional anticancer drugs elevate intracellular level of reactive oxygen species (ROS) and alter redox-homeostasis of cancer cells. It is widely accepted that anticancer effect of these chemotherapeutics is due to induction of oxidative stress and ROS-mediated apoptosis in cancer. On the other hand, the harmful side effects of conventional anticancer chemotherapy are also due to increased production of ROS and disruption of redox-homeostasis of normal cells and tissues. This article describes the mechanisms for triggering and modulation of apoptosis through ROS-dependent and ROS-independent pathways. We try to answer the question: "Is it possible to induce highly specific apoptosis only in cancer cells, without overproduction of ROS, as well as without harmful effects on normal cells and tissues?" The review also suggests a new therapeutic strategy for selective killing of cancer cells, without significant impact on viability of normal cells and tissues, by combining anticancer drugs with redox-modulators, affecting specific signaling pathways and avoiding oxidative stress.
Many studies demonstrate that conventional anticancer drugs elevate intracellular level of reactive oxygen species (ROS) and alter redox-homeostasis of cancer cells. It is widely accepted that anticancer effect of these chemotherapeutics is due to induction of oxidative stress and ROS-mediated apoptosis in cancer. On the other hand, the harmful side effects of conventional anticancer chemotherapy are also due to increased production of ROS and disruption of redox-homeostasis of normal cells and tissues. This article describes the mechanisms for triggering and modulation of apoptosis through ROS-dependent and ROS-independent pathways. We try to answer the question: "Is it possible to induce highly specific apoptosis only in cancer cells, without overproduction of ROS, as well as without harmful effects on normal cells and tissues?" The review also suggests a new therapeutic strategy for selective killing of cancer cells, without significant impact on viability of normal cells and tissues, by combining anticancer drugs with redox-modulators, affecting specific signaling pathways and avoiding oxidative stress.
2016, 28(4): 397-403.
doi: 10.21147/j.issn.1000-9604.2016.04.02
Abstract:
Based upon studies from randomized clinical trials, the extended (D2) lymph node dissection is now recommended as a standard procedure for local advanced gastric cancer worldwide. However, the rational extent lymphadenectomy for local advanced gastric cancer has remained a topic of debate in the past decades. Due to the limitation of low metastatic rate in para-aortic nodes (PAN) in JCOG9501, the clinical benefit of D2+ para-aortic nodal dissection (PAND) for patients with stage T4 and/or stage N3 disease, which is very common in China and other countries except Japan and Korea, cannot be determined. Furthermore, the role of splenectomy for complete resection of No.10 and No.11 nodes has been controversial, and however, the final results from the randomized trial of JCOG0110 have yet to be completed. Gastric cancer with the No.14 and No.13 lymph node metastasis is defined as M1 stage in the current version of the Japanese classification. We propose that D2+No.14v and +No.13 lymphadenectomy may be an option in a potentially curative gastrectomy for tumors with apparent metastasis to the No.6 nodes or infiltrate to duodenum. The examined lymph node and extranodal metastasis are significantly associated with the survival of gastric cancer patients.
Based upon studies from randomized clinical trials, the extended (D2) lymph node dissection is now recommended as a standard procedure for local advanced gastric cancer worldwide. However, the rational extent lymphadenectomy for local advanced gastric cancer has remained a topic of debate in the past decades. Due to the limitation of low metastatic rate in para-aortic nodes (PAN) in JCOG9501, the clinical benefit of D2+ para-aortic nodal dissection (PAND) for patients with stage T4 and/or stage N3 disease, which is very common in China and other countries except Japan and Korea, cannot be determined. Furthermore, the role of splenectomy for complete resection of No.10 and No.11 nodes has been controversial, and however, the final results from the randomized trial of JCOG0110 have yet to be completed. Gastric cancer with the No.14 and No.13 lymph node metastasis is defined as M1 stage in the current version of the Japanese classification. We propose that D2+No.14v and +No.13 lymphadenectomy may be an option in a potentially curative gastrectomy for tumors with apparent metastasis to the No.6 nodes or infiltrate to duodenum. The examined lymph node and extranodal metastasis are significantly associated with the survival of gastric cancer patients.
2016, 28(4): 404-412.
doi: 10.21147/j.issn.1000-9604.2016.04.03
Abstract:
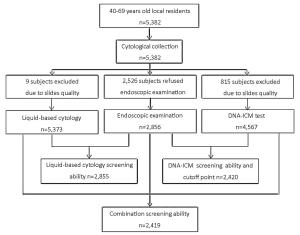
Objective To evaluate the feasibility of DNA image cytometry (DNA-ICM) as a primary screening method for esophageal squamous cell cancer (ESCC). Methods A total of 5,382 local residents aged 40–69 years from three high-risk areas in China (Linzhou in Henan province, Feicheng in Shandong province and Cixian in Hebei province) from 2008 to 2011 were recruited in this population-based screening study. And 2,526 subjects declined to receive endoscopic biopsy examination with Lugol’s iodine staining, while 9 and 815 subjects were excluded from liquid-based cytology and DNA-ICM test respectively due to slide quality. Finally, 2,856, 5,373 and 4,567 subjects were enrolled in the analysis for endoscopic biopsy examination, liquid-based cytology and DNA-ICM test, respectively. Sensitivity (SE), specificity (SP), negative predictive values (NPV) and positive predictive values (PPV) as well as their 95% confidence intervals (95% CI) for DNA-ICM, liquid-based cytology and the combination of the two methods were calculated. Receiver operating characteristic (ROC) curves were applied to determine the cutoff point of DNA-ICM for esophageal cancer. Results DNA-ICM results were significantly correlative with esophageal cancer and precancer lesions (χ2=18.016, P<0.001). The cutoff points were 5,802, 5,803 and 8,002 based on dissimilar pathological types of low grade intraepithelial neoplasia (LGIN), high grade intraepithelial neoplasia (HGIN), and ESCC, respectively, and 5,803 was chosen in this study considering the SE and SP. The SE, SP, PPV, NPV of DNA-ICM test (cutoff point 5,803) combined with liquid-based cytology [threshold atypical squamous cells of undetermined significance (ASCUS)] were separately 72.1% (95% CI: 70.3%-73.9%), 43.3% (95% CI: 41.3%-45.3%), 22.8% (95% CI: 21.1%-24.5%) and 87.0% (95% CI: 85.7%-88.3%) for LGIN, 85.7% (95% CI: 84.3%-87.1%), 41.3% (95% CI: 39.3%-43.3%), 4.6% (95% CI: 3.8%-5.4%) and 98.9% (95% CI: 98.5%-99.3%) for HGIN, and 96.0% (95% CI: 95.2%-96.8%), 40.8% (95% CI: 38.8%-42.8%), 1.7% (95% CI: 1.2%-2.2%) and 99.9% (95% CI: 99.8%-100.0%) for ESCC. Conclusions It is possible to use DNA-ICM test as a primary screening method before endoscopic screening for esophageal cancer.
2016, 28(4): 413-422.
doi: 10.21147/j.issn.1000-9604.2016.04.04
Abstract:
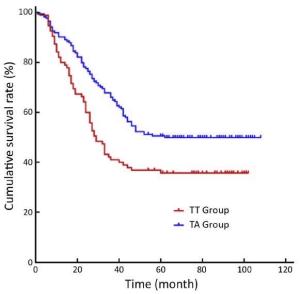
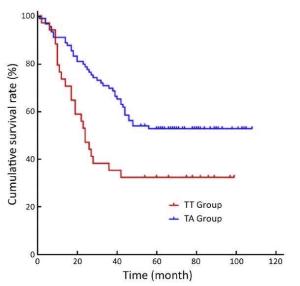
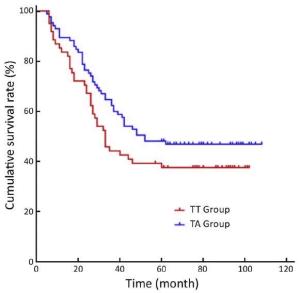
Objective To compare the survival outcomes of transabdominal (TA) and transthoracic (TT) surgical approaches in patients with Siewert-II/III esophagogastric junction adenocarcinoma. Methods This retrospective study was conducted in patients with Siewert-II/III esophagogastric junction adenocarcinoma who underwent either TT or TA operations in the West China Hospital between January 2006 and December 2009. Results A total of 308 patients (109 in the TT and 199 in the TA groups) were included in this study with a follow-up rate of 87.3%. The median (P25, P75) number of harvested perigastric lymph nodes was 8 (5, 10) in the TT group and 23 (16, 34) in the TA group (P<0.001), and the number of positive perigastric lymph nodes was 2 (0, 5) in the TT group and 3 (1, 8) in the TA group (P<0.004). The 5-year overall survival (OS) rate was 36% in the TT group and 51% in the TA group (P=0.005). Subgroup analysis by Siewert classification showed that 5-year OS rates for patients with Siewert II tumors were 38% and 48% in TT and TA groups, respectively (P=0.134), whereas the 5-year OS rate for patients with Siewert III tumors was significantly lower in the TT group than that in the TA group (33% vs. 53%; P=0.010). Multivariate analysis indicated that N2 and N3 stages, R1/R2 resection and a TT surgical approach were prognostic factors for poor OS. Conclusions Improved perigastric lymph node dissection may be the main reason for better survival outcomes observed with a TA gastrectomy approach than with TT gastrectomy for Siewert III tumor patients.
2016, 28(4): 423-428.
doi: 10.21147/j.issn.1000-9604.2016.04.05
Abstract:
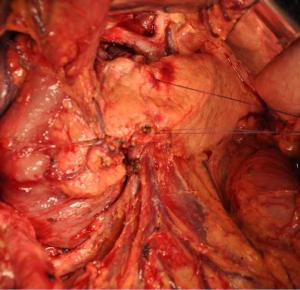
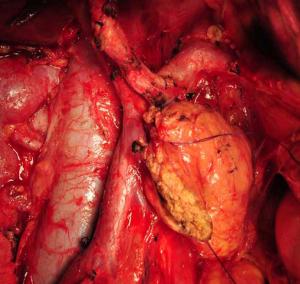
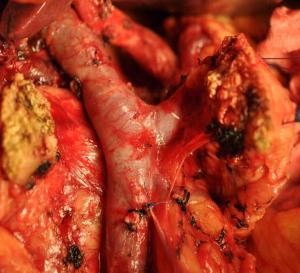
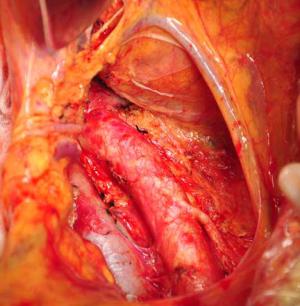
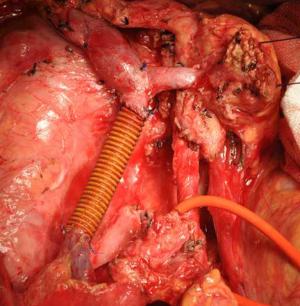
Objective To evaluate the feasibility and safety of total mesopancreas excision (TMpE) in the treatment of pancreatic head cancer. Methods The clinical and pathological data of 120 patients with pancreatic head cancer who had undergone TMpE in our center from May 2010 to January 2014 were retrospectively analyzed. Results The mean operative time was (275.0±50.2) min and the average intra-operative blood loss was (390.0±160.5) mL. Post-operative complications were reported in 45 patients, while no peri-operative death was noted. The specimen margins were measured in three dimensions, and 86 patients (71.6%) achieved R0 resection. Conclusions TMpE is safe and feasible for pancreatic head cancer and is particularly helpful to increase the R0 resection rate.
2016, 28(4): 429-434.
doi: 10.21147/j.issn.1000-9604.2016.04.06
Abstract:
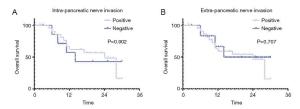
Objective To explore the clinical value of the radical nerve dissection (RND) for the carcinoma of head of pancreas (CHP). Methods The clinical and pathological data of 30 CHP patients who underwent RND in our hospital were retrospectively analyzed, with an attempt to explore the safety and short-term efficacy of this procedure. Results Among these 30 patients, the operative time was (351±61) min, the intra-operative blood loss was 350 (range, 300-600) mL, and the grades B and C pancreatic fistula was 23.33%. During the follow-up (range, 2-30 months; median: 17 months), the 1-year survival rate was 63.33% and the 1-year disease-free survival rate was 56.67%. Among the 23 patients (76.66%) with positive extra-pancreatic perineural invasion (PNI), the 1-year casefatality rate was 34.78%, which was not significantly different from that (28.57%) of patients with negative PNI (P=0.760). Conclusions Our results suggested potential advantages of RND in the fields of surgery-associated risk and prognosis compared with the Whipple operation in the treatment of CHP. Due to the low sample size of this study, further well-designed research of large sample size is needed.
2016, 28(4): 435-452.
doi: 10.21147/j.issn.1000-9604.2016.04.07
Abstract:
Objective The inhibition of the neovascularization in tumors is a potential therapeutic target of cancer. Vascular endothelial growth inhibitor (VEGI) is a member of the TNF superfamily which has the ability to suppress the formation of new vessels in tumors. In order to study the association between VEGI gene polymorphisms and breast cancer risk, a case-control study was conducted in Chinese Han women in Northeast China. Methods Our study involved 708 female breast cancer patients and 685 healthy volunteers. Four SNPs of VEGI gene were analyzed through the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The association between VEGI gene polymorphisms and breast cancer risk was analyzed in our study. The relation between VEGI gene variants and clinical features of breast cancer including lymph node (LN) metastasis, estrogen receptor (ER), progestrogen receptor (PR), tumor protein 53 (p53), human epidermal growth factor receptor 2 (Her-2) and triple negative (ER-/PR-/Her-2-) status was analyzed as well. Results We found that the CT genotype and T allele of rs6478106 were more frequent in patients than in controls. There was also a statistical difference in the distribution of Crs6478106Grs4263839 haplotype between patients and controls. In addition, SNP rs6478106 and rs4979462 were related with the Her-2 status. Conclusions Our results suggest that VEGI gene variants may be related to the breast cancer risk and the clinical features of breast cancer in Chinese Han women in Northeast China.
2016, 28(4): 444-451.
doi: 10.21147/j.issn.1000-9604.2016.04.08
Abstract:
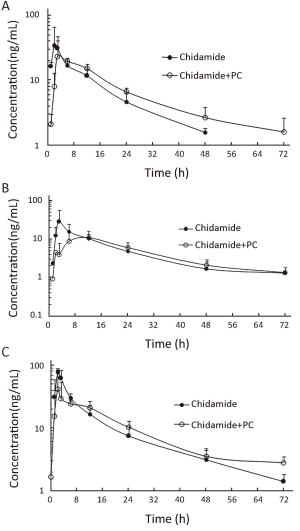
Objective This phase I study was to evaluate safety, maximum tolerated dose, pharmacokinetics and preliminary antitumor activity of chidamide, a novel subtype-selective histone deacetylase (HDAC) inhibitor, in combination with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer (NSCLC). Methods Ten patients received oral chidamide 20, 25, or 30 mg twice per week continuously with paclitaxel (175 mg/m2) and carboplatin [area under the curve (AUC) 5 mg/mL/min] administered in a 3-week cycle. Patients with response and stable disease after four cycles maintained chidamide monotherapy until disease progression or unacceptable toxicity. Blood samples were collected for pharmacokinetic analysis after the first single oral of chidamide and first combination treatment in cycle 1 from all patients. Results Two dose-limiting toxicities were recorded in the 30 mg cohort, including thrombocytopenia and prolonged neutropenia in the first cycle. Grade 3/4 neutropenia in any cycle was observed in all patients, but was not associated with significant complications. Other grade 3/4 hematologic toxicities included thrombocytopenia and leucopenia. No significant changes were observed in pharmacokinetic parameters for both chidamide and paclitaxel. One patient in the 20 mg cohort had confirmed partial response (PR). Two out of 5 patients with brain metastases had intracranial complete remission after 4-cycle treatment. Conclusions Chidamide combined with paclitaxel and carboplatin was generally tolerated without unanticipated toxicities or clinically relevant pharmacokinetic interactions. The recommended dose for chidamide in this combination was established at 20 mg, and a phase II trial is ongoing with this regimen in patients with advanced NSCLC.
2016, 28(4): 452-460.
doi: 10.21147/j.issn.1000-9604.2016.04.09
Abstract:
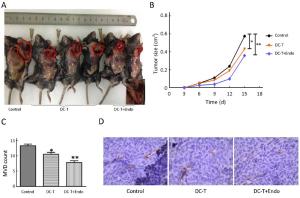
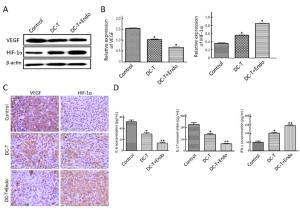
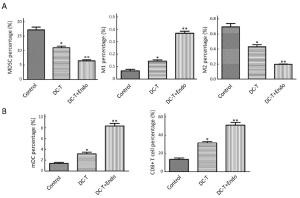
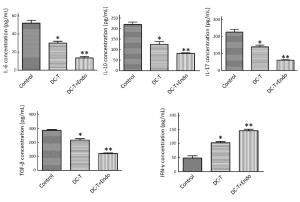
Objective To investigate the antitumor effect of endostatin combined with tumor antigen-pulsed dendritic cell (DC)-T cell therapy on lung cancer. Methods Transplanted Lewis lung cancer (LLC) models of C57BL/6 mice were established by subcutaneous injection of LLC cells in left extremity axillary. Tumor antigen-pulsed DC-T cells from spleen cells and bone of mice were cultured in vitro. Tumor-bearing mice were randomly divided into three groups, including DC-T+endostatin group, DC-T group, and phosphate-buffered saline (PBS) control group. Microvessel density (MVD) of tumor tissue in tumor-bearing mice was determined by immunohistochemistry (IHC). The expressions of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) were determined by Western blotting and IHC staining. The proportions of CD8+ T cells, mature dendritic cells (mDC), tumor-associated macrophages [TAM (M1/M2)], and myeloid-derived suppressor cells (MDSC) in suspended cells of tumor tissue were determined by flow cytometry. The expressions of interleukin (IL)-6, IL-10, IL-17, transforming growth factor-β (TGF-β) and interferon-γ (IFN-γ) in suspended cells of tumor tissue were detected by enzyme-linked immune sorbent assay (ELISA). Results DC-T cells combined with endostatin remarkably suppressed tumor growth. MVD of mice in DC-T+endostatin group was significantly lower than that of the control group and DC-T monotherapy group. The expressions of VEGF, IL-6 and IL-17 in tumors were markedly decreased, but IFN-γ and HIF-1α increased after treating with DC-T cells combined with endostatin, compared to control group and DC-T group. In the DC-T+endostatin group, the proportions of MDSC and TAM (M2 type) were significantly decreased, mDC and TAM (M1 type) were up-regulated, and CD8+ T cells were recruited to infiltrate tumors, in contrast to PBS control and DC-T monotherapy. DC-T cells combined with endostatin potently reduced the expressions of IL-6, IL-10, TGF-β and IL-17 in tumor tissue, and enhanced the expression of IFN-γ. Conclusions The study indicated the synergic antitumor effects between endostatin and tumor antigen-pulsed DC-T cells, which may be a prospective therapy strategy to achieve potent antitumor effects on lung cancer.
2016, 28(4): 461-462.
doi: 10.21147/j.issn.1000-9604.2016.04.10
Abstract:
2016, 28(4): 463-466.
doi: 10.21147/j.issn.1000-9604.2016.04.11
Abstract:

 Abstract
Abstract FullText HTML
FullText HTML PDF 1898KB
PDF 1898KB