2017 Vol.29(2)
Display Mode: |
2017, 29(2): 93-99.
doi: 10.21147/j.issn.1000-9604.2017.02.01
Abstract:
Although a number of feasibility studies for sentinel node (SN) concepts in gastric cancer have been conducted since 2000, there remains a debate regarding detailed detection techniques and oncological safety. Two important multicenter phase II clinical trials were performed in Japan that used different methods and reached different conclusions; one confirmed acceptable results with a false-negative rate of 7%, and the other showed an unacceptably high false-negative rate of 46.4%. The Sentinel Node Oriented Tailored Approach (SENORITA) trial is a multicenter randomized controlled phase III trial being performed in Korea. Patient enrollment is now complete and the long-term results are currently awaited. Recently, an image-guided SN mapping technique using infrared ray/fluorescence was introduced. This method might be a promising technology because it allows the clear visualization of SNs. With regard to the primary tumor, the non-exposed endoscopic wall-inversion surgery technique and non-exposure endolaparoscopic full-thickness resection with simple suturing technique have been reported. These methods prevent abdominal infection and tumor seeding and can be good alternatives to conventional laparoscopic gastric wedge resection. For indications, SN navigation surgery can be extended to patients who underwent non-curative endoscopic resection. Although a few studies have been performed on these patients, sentinel concepts may be beneficial to patients as they omit the need for additional gastrectomy. SN navigation surgery can lead to actual organ-preserving surgery and plays a key role in improving the quality of life of patients with early gastric cancer in the future.
Although a number of feasibility studies for sentinel node (SN) concepts in gastric cancer have been conducted since 2000, there remains a debate regarding detailed detection techniques and oncological safety. Two important multicenter phase II clinical trials were performed in Japan that used different methods and reached different conclusions; one confirmed acceptable results with a false-negative rate of 7%, and the other showed an unacceptably high false-negative rate of 46.4%. The Sentinel Node Oriented Tailored Approach (SENORITA) trial is a multicenter randomized controlled phase III trial being performed in Korea. Patient enrollment is now complete and the long-term results are currently awaited. Recently, an image-guided SN mapping technique using infrared ray/fluorescence was introduced. This method might be a promising technology because it allows the clear visualization of SNs. With regard to the primary tumor, the non-exposed endoscopic wall-inversion surgery technique and non-exposure endolaparoscopic full-thickness resection with simple suturing technique have been reported. These methods prevent abdominal infection and tumor seeding and can be good alternatives to conventional laparoscopic gastric wedge resection. For indications, SN navigation surgery can be extended to patients who underwent non-curative endoscopic resection. Although a few studies have been performed on these patients, sentinel concepts may be beneficial to patients as they omit the need for additional gastrectomy. SN navigation surgery can lead to actual organ-preserving surgery and plays a key role in improving the quality of life of patients with early gastric cancer in the future.
2017, 29(2): 100-108.
doi: 10.21147/j.issn.1000-9604.2017.02.02
Abstract:
Objective Though D2 lymphadenectomy has been increasingly regarded as standard surgical procedure for advanced gastric cancer (GC), the modified D2 (D1 + 7, 8a and 9) lymphadenectomy may be more suitable than D2 dissection for T2 stage GC. The purpose of this study is to elucidate whether the surgical outcome of modified D2 lymphadenectomy was comparable to that of standard D2 dissection in T2 stage GC patients. Methods A retrospective cohort study with 77 cases and 77 controls matched for baseline characteristics was conducted. Patients were categorized into two groups according to the extent of lymphadenectomy: the modified D2 group (mD2) and the standard D2 group (D2). Surgical outcome and recurrence date were compared between the two groups. Results The 5-year overall survival (OS) rate was 71.4% for patients accepted mD2 lymphadenectomy and 70.1% for those accepted standard D2, respectively, and the difference was not statistically significant. Multivariate survival analysis revealed that curability, tumor size, TNM stage and postoperative complications were independently prognostic factors for T2 stage GC patients. Patients in the mD2 group tended to have less intraoperative blood loss (P=0.001) and shorter operation time (P<0.001) than those in the D2 group. While there were no significant differences in recurrence rate and types, especially lymph node recurrence, between the two groups. Conclusions The surgical outcome of mD2 lymphadenectomy was equal to that of standard D2, and the use of mD2 instead of standard D2 can be a better option for T2 stage GC.
2017, 29(2): 109-117.
doi: 10.21147/j.issn.1000-9604.2017.02.03
Abstract:
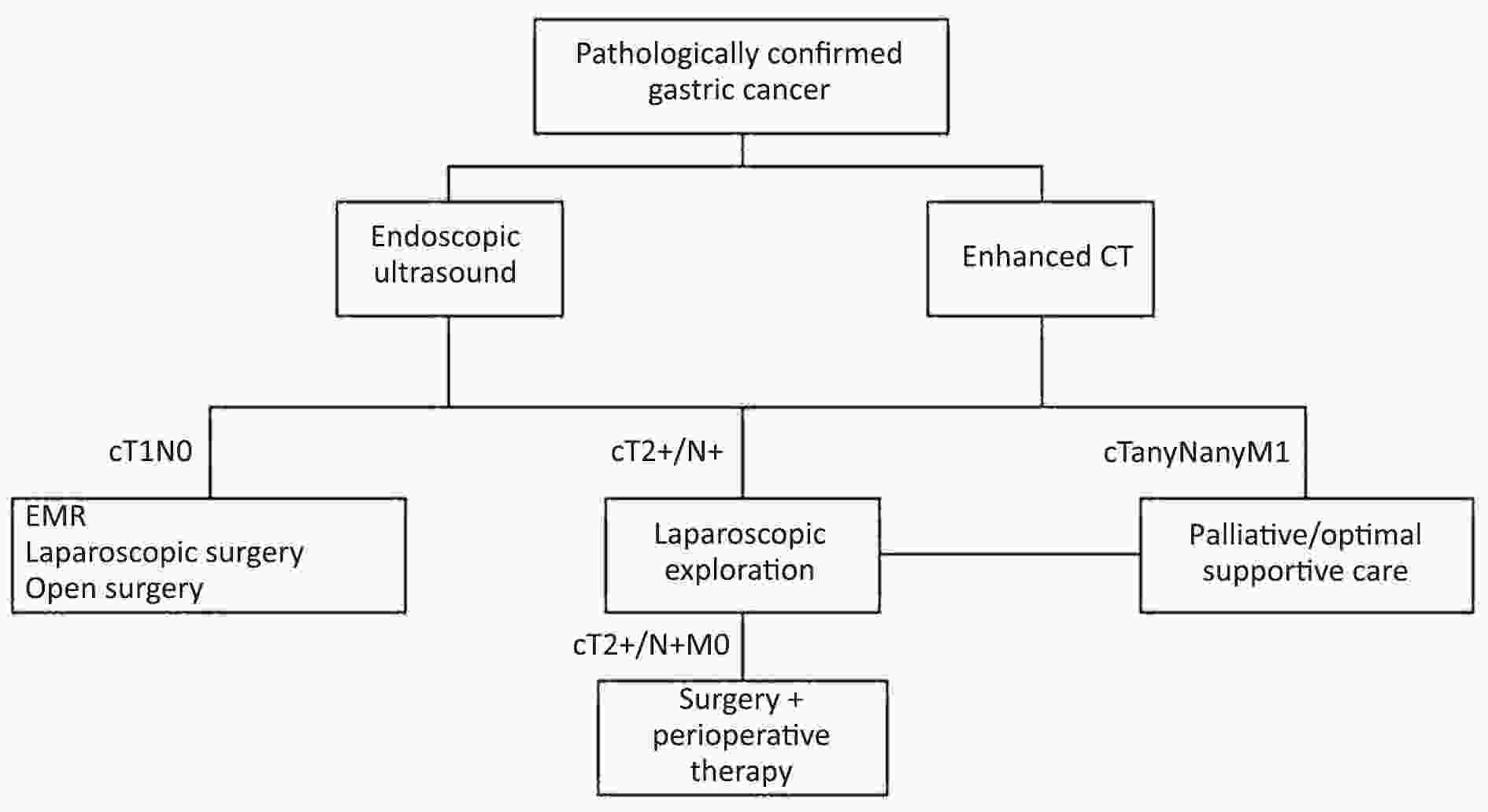
ObjectiveDiagnostic laparoscopy is recommended for the pretherapeutic staging of gastric cancer to detect any unexpected or unconfirmed intra-abdominal metastasis. The aim of this study was to evaluate the role and indications of diagnostic laparoscopy in the detection of intra-abdominal metastasis. MethodsStandard diagnostic laparoscopy with peritoneal cytology examination was performed prospectively on patients who were clinically diagnosed with primary local advanced gastric cancer (cT≥2M0). We calculated the rate of intra-abdominal metastases identified by diagnostic laparoscopy, and examined the relationship between peritoneal dissemination (P) and cytology results (CY). Split-sample method was applied to find clinical risk factors for intra-abdominal metastasis. Multivariate logistic regression analysis and receiver-operator characteristic (ROC) analysis were performed in training set to find out risk factors of intra-abdominal metastasis, and then validate it in testing set. ResultsOut of 249 cM0 patients, 51 (20.5%) patients with intra-abdominal metastasis were identified by diagnostic laparoscopy, including 20 (8.0%) P1CY1, 17 (6.8%) P0CY1 and 14 (5.6%) P1CY0 patients. In the training set, multivariate logistic regression analysis and ROC analysis showed that the depth of tumor invasion on computer tomography (CT) scan ≥21 mm and tumor-occupied ≥2 portions of stomach are predictive factors of metastasis. In the testing set, when diagnostic laparoscopy was performed on patients who had one or two of these risk factors, the sensitivity and positive predictive value for detecting intra-abdominal metastasis were 90.0% and 32.1%, respectively. ConclusionsAccording to our results, depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis.
2017, 29(2): 118-126.
doi: 10.21147/j.issn.1000-9604.2017.02.04
Abstract:
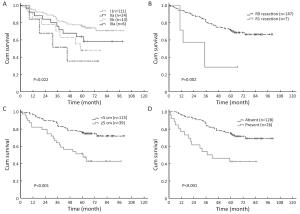
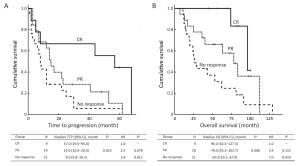
Objective To investigate the role of apparent diffusion coefficient (ADC) from diffusion-weighted magnetic resonance imaging (DW-MRI) when applied to the 7th TNM classification in the staging and prognosis of gastric cancer (GC). Methods Between October 2009 and May 2014, a total of 89 patients with non-metastatic, biopsy proven GC underwent 1.5T DW-MRI, and then treated with radical surgery. Tumor ADC was measured retrospectively and compared with final histology following the 7th TNM staging (local invasion, nodal involvement and according to the different groups — stage I, II and III). Kaplan-Meier curves were also generated. The follow-up period is updated to May 2016. Results Median follow-up period was 33 months and 45/89 (51%) deaths from GC were observed. ADC was significantly different both for local invasion and nodal involvement (P<0.001). Considering final histology as the reference standard, a preoperative ADC cut-off of 1.80×10–3 mm2/s could distinguish between stages I and II and an ADC value of ≤1.36×10–3 mm2/s was associated with stage III (P<0.001). Kaplan-Meier curves demonstrated that the survival rates for the three prognostic groups were significantly different according to final histology and ADC cut-offs (P<0.001). Conclusions ADC is different according to local invasion, nodal involvement and the 7th TNM stage groups for GC, representing a potential, additional prognostic biomarker. The addition of DW-MRI could aid in the staging and risk stratification of GC.
2017, 29(2): 127-136.
doi: 10.21147/j.issn.1000-9604.2017.02.05
Abstract:
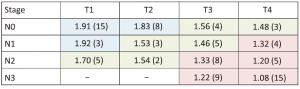
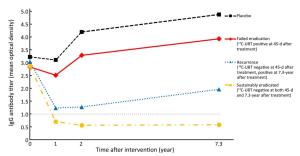
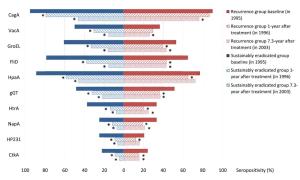
Objective To identify serum biomarkers that may predict the short or long term outcomes of anti-Helicobacter pylori (H. pylori) treatment, a follow-up study was performed based on an intervention trial in Linqu County, China. Methods A total of 529 subjects were selected randomly from 1,803 participants to evaluate total anti-H. pylori immunoglobulin G (IgG) and 10 specific antibody levels before and after treatment at 1-, 2- and 7.3-year. The outcomes of anti-H. pylori treatment were also parallelly assessed by 13C-urea breath test at 45-d after treatment and 7.3-year at the end of follow-up. Results We found the medians of anti-H. pylori IgG titers were consistently below cut-off value through 7.3 years in eradicated group, however, the medians declined in recurrence group to 1.2 at 1-year after treatment and slightly increased to 2.0 at 7.3-year. While the medians were significantly higher (>3.0 at 2- and 7.3-year) among subjects who failed the eradication or received placebo. For specific antibody responses, baseline seropositivities of FliD and HpaA were reversely associated with eradication failure [for FliD, odds ratio (OR)=0.44, 95% confidence interval (95% CI): 0.27–0.73; for HpaA, OR=0.32, 95% CI: 0.17–0.60]. The subjects with multiple positive specific antibodies at baseline were more likely to be successfully eradicated in a linear fashion (Ptrend=0.006). Conclusions Our study suggested that total anti-H. pylori IgG level may serve as a potential monitor of long-term impact on anti-H. pylori treatment, and priority for H. pylori treatment may be endowed to the subjects with multiple seropositive antibodies at baseline, especially for FliD and HapA.
2017, 29(2): 149-155.
doi: 10.21147/j.issn.1000-9604.2017.02.08
Abstract:
Objective No standard postoperative adjuvant chemotherapy has ever been established in node-positive esophageal squamous cell carcinoma (ESCC). This is a study to explore the effect of postoperative paclitaxel (PTX) and cisplatin (DDP) in lymph node-positive, completely resected thoracic ESCC patients. Methods We conducted a prospective phase II trial. Patients had pathologically node-positive thoracic ESCC with negative margins. Outcomes of disease-free survival (DFS) and overall survival (OS) were compared with a matched historical control cohort. The postoperative chemotherapy regimen consisted of 4 to 6 cycles of PTX 150 mg/m2 administered intravenously on d 1 followed by DDP 50 mg/m2 on d 2 every 14 d. Results Forty-three patients were accrued from December 2007 to May 2012 at Cancer Hospital of Chinese Academy of Medical Sciences for adjuvant chemotherapy. The historical control group consisted of 80 patients who received complete resection but no adjuvant chemotherapy during the same period of time. Of the 43 patients with adjuvant chemotherapy, 37 (86.0%) patients completed 4 to 6 cycles of chemotherapy. The 3-year DFS rates were 56.3% in the adjuvant group and 34.6% in the control group (P=0.006). The 3-year OS rates were 55.0% in the adjuvant group and 37.5% in the control group (P=0.013). Multivariate analysis revealed that postoperative chemotherapy was the significant predictor for improved OS (P=0.005). Conclusions Biweekly adjuvant PTX and DDP might improve 3-year DFS and OS in lymph node-positive, curatively resected thoracic ESCC patients. These conclusions warrant further study in randomized phase III clinical trials.
2017, 29(2): 156-165.
doi: 10.21147/j.issn.1000-9604.2017.02.09
Abstract:
Objective This study aims to evaluate the natural history of patients with chronic lymphocytic leukemia (CLL) and a 17p deletion (17p-) and identify the predictive factors within this subgroup. Methods The sample of patients with CLL were analyzed by fluorescence in situ hybridization for deletions in chromosome bands 11q22, 13q14 and 17p13; trisomy of bands 12q13; and translocation involving band 14q32. The data from 456 patients with or without a 17p- were retrospectively collected and analyzed. Results The overall response rate (ORR) in patients with a 17p- was 56.9%, and patients with a high percentage of 17p- (defined as more than 25% of cells harbouring a 17p-) had a lower ORR. The median overall survival (OS) in patients with a 17p- was 78.0 months, which was significantly shorter than the OS in patients without this genetic abnormality (median 162.0 months, P<0.001). Within the subgroup with a 17p-, the progression-free survival was significantly shorter in patients at Binet stage B-C and patients with elevated lactate dehydrogenase (LDH), B symptoms, unmutated IGHV and a high percentage of 17p-. Conclusions These results indicated that patients with a 17p- CLL have a variable prognosis that might be predicted using simple clinical and laboratory characteristics.
2017, 29(2): 137-143.
doi: 10.21147/j.issn.1000-9604.2017.02.06
Abstract:
Type 1 gastric neuroendocrine tumors (gNETs) are usually small lesions, restricted to mucosal and sub-mucosal layers of corpus and fundus, with low aggressive behavior, for the majority of cases. Nevertheless, some cases present aggressive behavior. The increasing incidence of gNETs brings together a new relevant problem: how to identify potentially aggressive type 1 gNETs. The challenging problem seems to be finding out signs or features able to predict potentially aggressive cases, allowing a tailored approach, since the involved societies dedicated to provide guidelines for management of these neoplasms apparently failed in producing staging systems able to accurately predict prognosis of these tumors. Additionally, it is also important to try to find out explanations for increasing incidence, as well as to identify potential targets aiming to reach better control of this neoplasia. Here, we discuss potential pathways implicated in aggressive behavior, as well as new strategies to improve clinical management of these tumors.
Type 1 gastric neuroendocrine tumors (gNETs) are usually small lesions, restricted to mucosal and sub-mucosal layers of corpus and fundus, with low aggressive behavior, for the majority of cases. Nevertheless, some cases present aggressive behavior. The increasing incidence of gNETs brings together a new relevant problem: how to identify potentially aggressive type 1 gNETs. The challenging problem seems to be finding out signs or features able to predict potentially aggressive cases, allowing a tailored approach, since the involved societies dedicated to provide guidelines for management of these neoplasms apparently failed in producing staging systems able to accurately predict prognosis of these tumors. Additionally, it is also important to try to find out explanations for increasing incidence, as well as to identify potential targets aiming to reach better control of this neoplasia. Here, we discuss potential pathways implicated in aggressive behavior, as well as new strategies to improve clinical management of these tumors.
2017, 29(2): 144-148.
doi: 10.21147/j.issn.1000-9604.2017.02.07
Abstract:
Background The ACTS-GC study had shown postoperative adjuvant therapy with S-1 improved survival of patients with locally advanced gastric cancer. Addition of oxaliplatin to S-1 is considered to be acceptable as one of the treatment options for gastric cancer patients after radical gastrectomy with D2 lymph node excision. Methods We have commenced a randomized phase III trial in December 2016 to evaluate S-1 plus oxaliplatin compared with S-1 alone in the adjuvant setting for locally advanced gastric cancer. A total of 564 patients will be accrued from 13 Chinese institutions in two years. The primary endpoint is 3-year relapse-free survival. The secondary endpoints are 5-year overall survival, proportion of patients who complete the postoperative chemotherapy and incidence of adverse events. Ethic and dissemination The trial has been approved by the institutional review board of each participating institution and it was activated on December, 2016. The enrollment will be finished in December, 2018. Patient’s follow-up will be ended until December, 2023. Trial registration ClinicalTrials.gov, identifier: NCT02867839. Registered on August 4, 2016.
2017, 29(2): 166-167.
doi: 10.21147/j.issn.1000-9604.2017.02.10
Abstract:
2017, 29(2): 168-170.
doi: 10.21147/j.issn.1000-9604.2017.02.11
Abstract:
Multidisciplinary team (MDT) model is a diagnostic and treatment model characterized by interdisciplinarity, integration, centralism, individualization, and precision and is becoming more common in the management of complex malignancies. MDT emphasizes team spirit and a personalized treatment strategy according to the actual condition of each patient. A cooperative and effective multidisciplinary team is an important guarantee for delivering high-quality services to patients. Under the guidance of a medical humanistic concept, MDT provides reasonable, effective, convenient, and a full range of excellent quality medical service to patients. The MDT maximizes patient benefits, and it is the developmental direction for large-scale general hospitals. At the same time, the MDT is also an important measure to strengthen the core competitiveness of hospitals. Here, we introduce the clinical application of the model in tumor therapy as well as the current state and development in our hospital.
Multidisciplinary team (MDT) model is a diagnostic and treatment model characterized by interdisciplinarity, integration, centralism, individualization, and precision and is becoming more common in the management of complex malignancies. MDT emphasizes team spirit and a personalized treatment strategy according to the actual condition of each patient. A cooperative and effective multidisciplinary team is an important guarantee for delivering high-quality services to patients. Under the guidance of a medical humanistic concept, MDT provides reasonable, effective, convenient, and a full range of excellent quality medical service to patients. The MDT maximizes patient benefits, and it is the developmental direction for large-scale general hospitals. At the same time, the MDT is also an important measure to strengthen the core competitiveness of hospitals. Here, we introduce the clinical application of the model in tumor therapy as well as the current state and development in our hospital.

 Abstract
Abstract FullText HTML
FullText HTML PDF 351KB
PDF 351KB