2018 Vol.30(1)
Display Mode: |
2018, 30(1): 1-12.
doi: 10.21147/j.issn.1000-9604.2018.01.01
Abstract:
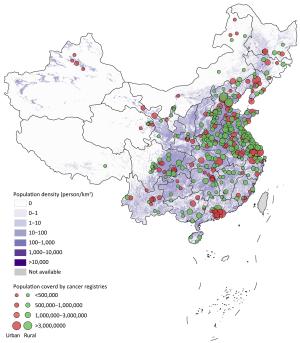
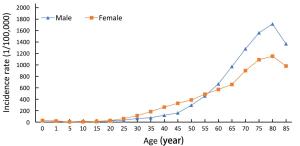
Background National Central Cancer Registry of China (NCCRC) updated nationwide cancer statistics using population-based cancer registry data in 2014 collected from all available cancer registries. Methods In 2017, 449 cancer registries submitted cancer registry data in 2014, among which 339 registries’ data met the criteria of quality control and were included in analysis. These cancer registries covered 288,243,347 population, accounting for about 21.07% of the national population in 2014. Numbers of nationwide new cancer cases and deaths were estimated using calculated incidence and mortality rates and corresponding national population stratified by area, sex, age group and cancer type. The world Segi’s population was applied for age-standardized rates. Results A total of 3,804,000 new cancer cases were diagnosed, the crude incidence rate was 278.07/100,000 (301.67/100,000 in males, 253.29/100,000 in females) and the age-standardized incidence rate by world standard population (ASIRW) was 186.53/100,000. Calculated age-standardized incidence rate was higher in urban areas than in rural areas (191.6/100,000 vs. 179.2/100,000). South China had the highest cancer incidence rate while Southwest China had the lowest incidence rate. Cancer incidence rate was higher in female for population between 20 to 54 years but was higher in male for population younger than 20 years or over 54 years. A total of 2,296,000 cancer deaths were reported, the crude mortality rate was 167.89/100,000 (207.24/100,000 in males, 126.54/100,000 in females) and the age-standardized mortality rate by world standard population (ASMRW) was 106.09/100,000. Calculated age-standardized mortality rate was higher in rural areas than in urban areas (110.3/100,000 vs. 102.5/100,000). East China had the highest cancer mortality rate while North China had the lowest mortality rate. The mortality rate in male was higher than that in female. Common cancer types and major causes of cancer death differed between age group and sex. Conclusions Heavy cancer burden and its disparities between area, sex and age group pose a major challenge to public health in China. Nationwide cancer registry plays a crucial role in cancer prevention and control.
2018, 30(1): 13-20.
doi: 10.21147/j.issn.1000-9604.2018.01.02
Abstract:
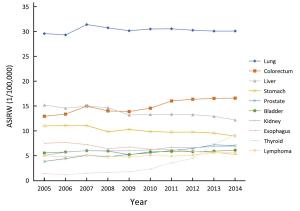
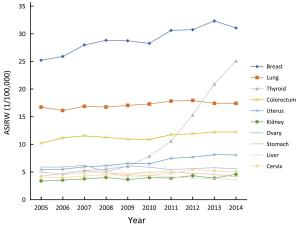
Objective To analyze cancer incidence data in Beijing in 2014 and temporal trends for selected common cancers during 2005 and 2014. Methods A total of 144 secondary and tertiary hospitals reported newly diagnosed cancer cases to Beijing Cancer Registry, which covers 13 million residents in Beijing. The cancer incidence rate was calculated in strata by cancer type, sex, age group and area. The population composition of China in 1982 and Segi’s population structure were used to calculate age-standardized rates. Extensive procedures were used to assure the quality of the data. Results The overall data quality indicators of the percentage of morphology verification (MV) (%), the percentage of death certificate-only (DCO) (%) and the mortality to incidence ratio (M/I) were 72.15%, 0.94% and 0.54 respectively. A total of 45,300 new cancer cases were diagnosed in Beijing in 2014. The incidence rate was 341.92/100,000 (343.50/100,000 in males, 340.33/100,000 in females), and the age-standardized incidence rates by Chinese standard population (ASIRC) and by world standard population (ASIRW) were 143.48/100,000 and 182.99/100,000, respectively. The cumulative incidence rate for cancer before 75 years was 20.61%. Cancers of lung, colorectum, liver, stomach and prostate were the top five common cancer types for males, while cancers of breast, lung, thyroid, colorectum and uterus were the top five common cancer types for females. The different patterns were also observed between rural and urban areas. Regarding temporal trends, the incidence of thyroid cancer has the fastest growth between 2005 and 2014. The incidence of liver cancer decreased, and stomach and esophageal cancer also decreased significantly for males in the last decade. Incidence rate for lung cancer was relatively stable during that period of time. Conclusions With more than 45,000 new cases in Beijing in 2014, cancer remains an important public health problem. Actions should be taken to diminish total cancer incidence in Beijing.
2018, 30(1): 21-30.
doi: 10.21147/j.issn.1000-9604.2018.01.03
Abstract:
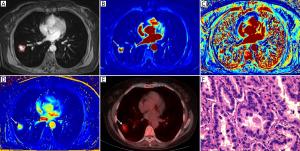
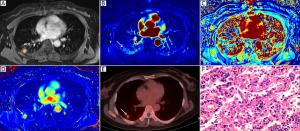
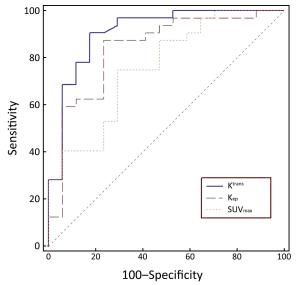
Objective To prospectively compare the discriminative capacity of dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI) with that of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in the differentiation of malignant and benign solitary pulmonary nodules (SPNs). Methods Forty-nine patients with SPNs were included in this prospective study. Thirty-two of the patients had malignant SPNs, while the other 17 had benign SPNs. All these patients underwent DCE-MRI and 18F-FDG PET/CT examinations. The quantitative MRI pharmacokinetic parameters, including the trans-endothelial transfer constant (Ktrans), redistribution rate constant (Kep), and fractional volume (Ve), were calculated using the Extended-Tofts Linear two-compartment model. The 18F-FDG PET/CT parameter, maximum standardized uptake value (SUVmax), was also measured. Spearman’s correlations were calculated between the MRI pharmacokinetic parameters and the SUVmax of each SPN. These parameters were statistically compared between the malignant and benign nodules. Receiver operating characteristic (ROC) analyses were used to compare the diagnostic capability between the DCE-MRI and 18F-FDG PET/CT indexes. Results Positive correlations were found between Ktrans and SUVmax, and between Kep and SUVmax (P<0.05). There were significant differences between the malignant and benign nodules in terms of the Ktrans, Kep and SUVmax values (P<0.05). The areas under the ROC curve (AUC) of Ktrans, Kep and SUVmax between the malignant and benign nodules were 0.909, 0.838 and 0.759, respectively. The sensitivity and specificity in differentiating malignant from benign SPNs were 90.6% and 82.4% for Ktrans; 87.5% and 76.5% for Kep; and 75.0% and 70.6% for SUVmax, respectively. The sensitivity and specificity of Ktrans and Kep were higher than those of SUVmax, but there was no significant difference between them (P>0.05). Conclusions DCE-MRI can be used to differentiate between benign and malignant SPNs and has the advantage of being radiation free.
2018, 30(1): 31-39.
doi: 10.21147/j.issn.1000-9604.2018.01.04
Abstract:
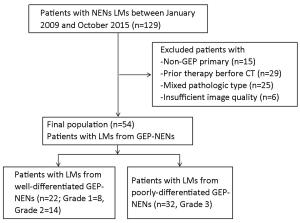
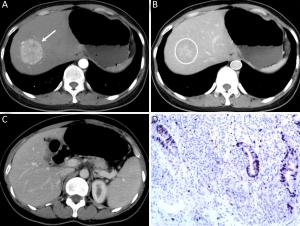
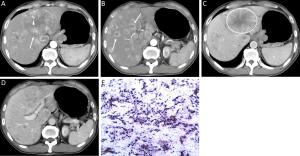
Objective To determine the capability of dynamic enhanced computed tomography (CT) to differentiate liver metastases (LMs) of well-differentiated from poorly-differentiated gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). Methods Patients with LMs of GEP-NENs who underwent dynamic enhanced CT examination in Peking University Cancer Hospital from January 2009 to October 2015 were included and data were retrospectively analyzed. We assessed the qualitative and quantitative CT features to identify the significant differentiating CT features of LMs of poorly-differentiated GEP-NENs from those of well-differentiated GEP-NENs using univariate analysis and a multivariate logistic regression model. Results The study included 22 patients with LMs of well-differentiated GEP-NENs and 32 patients with LMs of poorly-differentiated GEP-NENs. Univariate analysis revealed statistically significant differences between the LMs of well- and poorly-differentiated GEP-NENs in terms of feeding arteries (36.4% vs. 75.0%, χ2=8.061, P=0.005), intratumoral neovascularity (18.2% vs. 59.4%, χ2=9.047, P=0.003), lymphadenopathy (27.3% vs. 81.2%, χ2=15.733, P<0.001), tumor-to-aortic ratio in the hepatic arterial and portal venous phase (T-A/AP: 0.297±0.080 vs. 0.251±0.059, t=2.437, P=0.018; T-A/PVP: 0.639±0.138 vs. 0.529±0.117, t=3.163, P=0.003) and tumor-to-liver ratio in the hepatic arterial phase (T-L/AP: 1.108±0.267 vs. 0.907±0.240, t=2.882, P=0.006). The LMs of poorly-differentiated GEP-NENs showed more feeding arteries, more intratumoral neovascularity, more lymphadenopathy and a lower tumor-to-aortic ratio. Multivariate analysis suggested that intratumoral neovascularity [P=0.015, OR=0.108, 95% confidence interval (95% CI), 0.018–0.646], lymphadenopathy (P=0.001, OR=0.055, 95% CI, 0.009–0.323) and T-A/PVP (P=0.004, OR=5.3E–5, 95% CI, 0.000–0.044) were independent factors for differentiating LMs of poorly-differentiated from well-differentiated GEP-NENs. Conclusions Dynamic enhanced CT features (intratumoral neovascularity, lymphadenopathy and T-A/PVP) are useful in the pathological classification of LMs of GEP-NENs.
2018, 30(1): 40-50.
doi: 10.21147/j.issn.1000-9604.2018.01.05
Abstract:
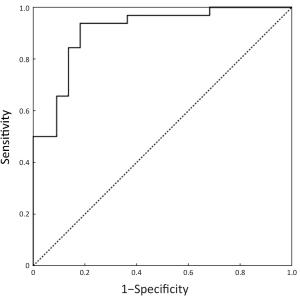
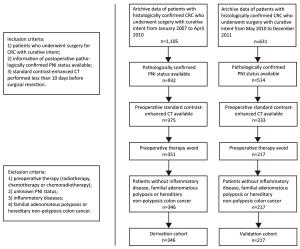
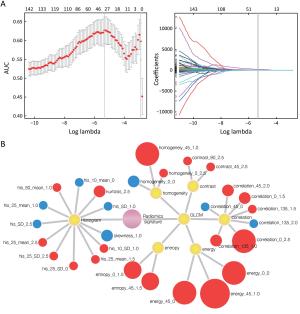
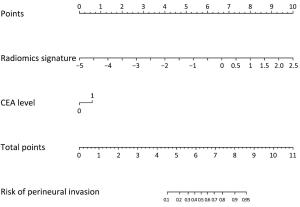
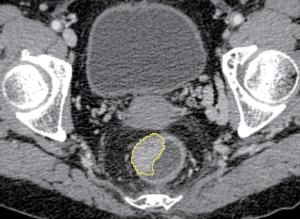
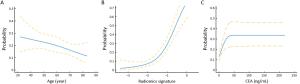
Objective To develop and validate a radiomics prediction model for individualized prediction of perineural invasion (PNI) in colorectal cancer (CRC). Methods After computed tomography (CT) radiomics features extraction, a radiomics signature was constructed in derivation cohort (346 CRC patients). A prediction model was developed to integrate the radiomics signature and clinical candidate predictors [age, sex, tumor location, and carcinoembryonic antigen (CEA) level]. Apparent prediction performance was assessed. After internal validation, independent temporal validation (separate from the cohort used to build the model) was then conducted in 217 CRC patients. The final model was converted to an easy-to-use nomogram. Results The developed radiomics nomogram that integrated the radiomics signature and CEA level showed good calibration and discrimination performance [Harrell’s concordance index (c-index): 0.817; 95% confidence interval (95% CI): 0.811–0.823]. Application of the nomogram in validation cohort gave a comparable calibration and discrimination (c-index: 0.803; 95% CI: 0.794–0.812). Conclusions Integrating the radiomics signature and CEA level into a radiomics prediction model enables easy and effective risk assessment of PNI in CRC. This stratification of patients according to their PNI status may provide a basis for individualized auxiliary treatment.
2018, 30(1): 51-60.
doi: 10.21147/j.issn.1000-9604.2018.01.06
Abstract:
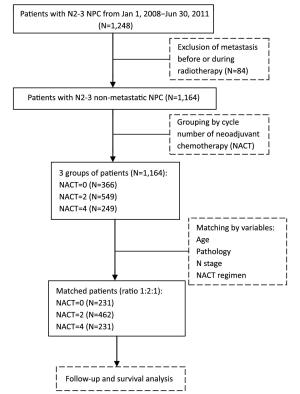
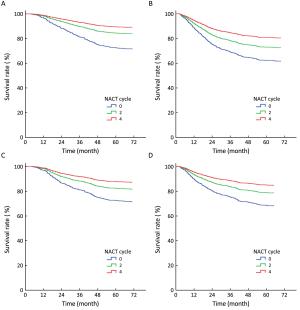
Objective Stage N2-3 nasopharyngeal carcinoma (NPC) shows a high risk of distant metastasis, which will finally cause death. This study aimed to evaluate the impact of neoadjuvant chemotherapy (NACT) of various cycles before radical radiotherapy on distant metastasis and survival of patients with stage N2-3 diseases. Methods In this study, a total of 1,164 consecutive patients with non-metastatic N2-3 NPC were recruited and prospectively observed. Then 231 patients who received NACT of 4 cycles (NACT=4 group) were matched 1:2:1 to 462 patients treated with NACT of 2 cycles (NACT=2 group) and 231 patients treated without NACT (NACT=0 group), according to age, histological subtype, N stage and NACT regimen. Five candidate variables (sex, T stage, concurrent chemotherapy, intensity-modulated radiation therapy and cycle number of NACT) were analyzed for their association with patients' survival. Results After matching, the overall survival (OS), disease-free survival (DFS), local-recurrence-free survival (RFS) and distant-metastasis-free survival (MFS) of the NACT=4 group (89.2%, 81.0%, 83.3% and 84.8%, respectively) were better than those of the NACT=2 group (83.3%, 72.5%, 81.2% and 77.9%, respectively) and the NACT=0 group (74.0%, 63.2%, 74.0% and 68.8%, respectively). In multivariate analysis, the cycle number of NACT maintained statistical significance on the OS, DFS, RFS and MFS (all P<0.05). Conclusions For N2-3 NPC, cycle number of NACT appeared to be an independent factor associated with an improvement of survival.
2018, 30(1): 61-71.
doi: 10.21147/j.issn.1000-9604.2018.01.07
Abstract:
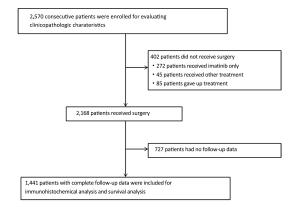
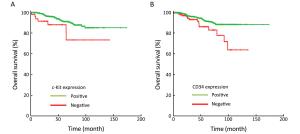
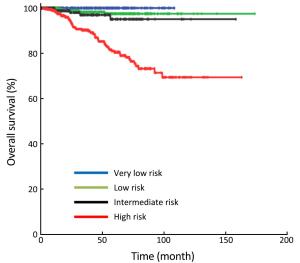
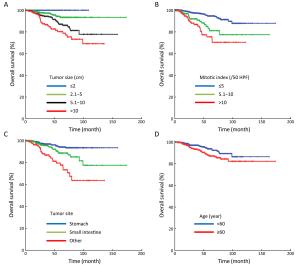
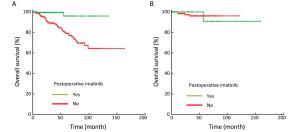
Objective We aimed to evaluate the clinicopathologic characteristics, immunohistochemical expression and prognostic factors of patients with primary gastrointestinal stromal tumors (GISTs). Methods Data from 2,570 consecutive GIST patients from four medical centers in China (January 2001–December 2015) were reviewed. Survival curves were constructed by the Kaplan-Meier method, and Cox regression models were used to identify independent prognostic factors. Results Of the included patients, 1,375 (53.5%) were male, and the patient age range was 18 to 95 (median, 58) years. The tumors were mostly found in the stomach (64.5%), small intestine (25.1%) and colorectal region (5.1%). At the time of diagnosis, the median tumor size was 4.0 (range: 0.1–55.0) cm, and the median mitotic index per 50 high power fields (HPFs) was 3 (range: 0–254). Of the 2,168 resected patients, 2,009 (92.7%) received curative resection. According to the modified National Institutes of Health (NIH) classification, 21.9%, 28.9%, 14.1% and 35.1% were very low-, low-, intermediate- and high-risk tumors, respectively. The rate of positivity was 96.4% for c-Kit, 87.1% for CD34, 96.9% for delay of germination 1 (DOG-1), 8.0% for S-100, 31.0% for smooth muscle actin (SMA) and 5.1% for desmin. However, the prognostic value of each was limited. Multivariate analysis showed that age, tumor size, mitotic index, tumor site, occurrence of curative resection and postoperative imatinib were independent prognostic factors. Furthermore, we found that high-risk patients benefited significantly from postoperative imatinib (P<0.001), whereas intermediate-risk patients did not (P=0.954). Conclusions Age, tumor size, mitotic index, tumor site, occurrence of curative resection and postoperative imatinib were independent prognostic factors in patients with GISTs. Moreover, determining whether intermediate-risk patients can benefit from adjuvant imatinib would be of considerable interest in future studies.
2018, 30(1): 72-83.
doi: 10.21147/j.issn.1000-9604.2018.01.08
Abstract:
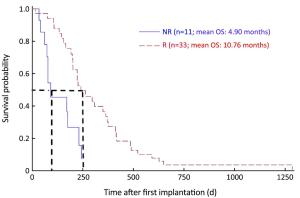
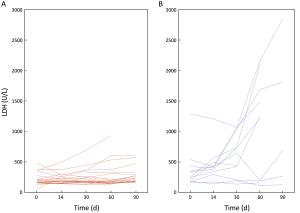
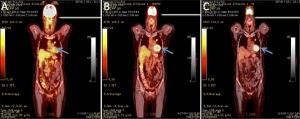
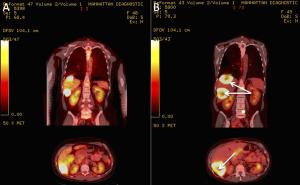
Objective The complexity, heterogeneity and capacity of malignant neoplastic cells and tumors for rapid change and evolution suggest that living-cell-based biological-systems approaches to cancer treatment are merited. Testing this hypothesis, the tumor marker, metabolic activity, and overall survival (OS) responses, to the use of one such system, implantable macrobeads [RENCA macrobeads (RMBs)], in phase I and IIa clinical trials in advanced, treatment-resistant metastatic colorectal cancer (mCRC) are described here. Methods Forty-eight mCRC patients (30 females; 18 males), who had failed all available, approved treatments, underwent RMB implantation (8 RMB/kg body weight) up to 4 times in phase I and phase IIa open-label trials. Physicals, labs [tumor and inflammation markers, lactate dehydrogenase (LDH)] and positron emission tomography-computed tomography (PET-CT) imaging to measure number/volume and metabolic activity of the tumors were performed pre- and 3-month-post-implantation to evaluate safety and initial efficacy (as defined by biological responses). PET-CT maximum standard uptake value (SUVmax) (baseline and d 90; SUVmax ≥2.5), LDH, and carcinoembryonic antigen (CEA) and/or cancer antigen 19-9 (CA 19-9) response (baseline, d 30 and/or d 60) were assessed and compared to OS. Results Responses after implantation were characterized by an at least 20% decrease in CEA and/or CA 19-9 in 75% of patients. Fluorodeoxyglucose (FDG)-positive lesions (phase I, 39; 2a, 82) were detected in 37/48 evaluable patients, with 35% stable volume and stable or decreased SUV (10) plus four with necrosis; 10, increased tumor volume, SUV. LDH levels remained stable and low in Responders (R) (d 0–60, 290.4–333.9), but increased steadily in Non-responders (NR) (d 0–60, 382.8–1,278.5) (d 60, P=0.050). Responders to RMBs, indicated by the changes in the above markers, correlated with OS (R mean OS=10.76 months; NR mean OS=4.9 months; P=0.0006). Conclusions The correlations of the tumor marker, tumor volume and SUV changes on PET-CT, and LDH levels themselves, and with OS, support the concept of a biological response to RMB implantation and the validity of the biological-systems approach to mCRC. A phase III clinical trial is planned.
2018, 30(1): 84-92.
doi: 10.21147/j.issn.1000-9604.2018.01.09
Abstract:
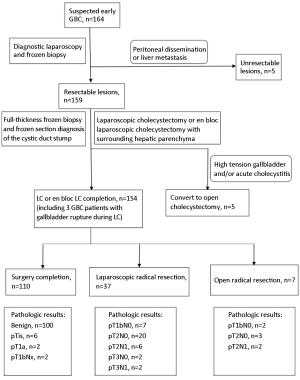
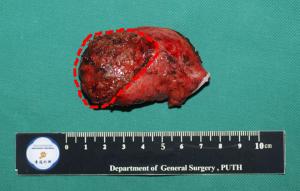
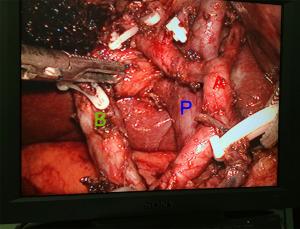
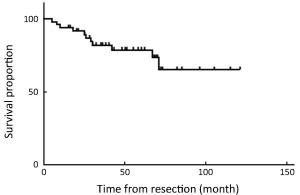
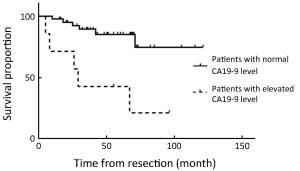
Objective Although laparoscopic treatment of gallbladder cancer (GBC) has been explored in the last decade, long-term results are still rare. This study evaluates long-term results of intended laparoscopic treatment for suspected GBC confined to the gallbladder wall, based on our experience over 10 years. Methods Between August 2006 and December 2015, 164 patients with suspected GBC confined to the wall were enrolled in the protocol for laparoscopic surgery. The process for GBC treatment was analyzed to evaluate the feasibility of computed tomography (CT) and/or magnetic resonance imaging (MRI) combined with frozen-section examination in identifying GBC confined to the wall. Of 159 patients who underwent the intended laparoscopic radical treatment, 47 with pathologically proven GBC were investigated to determine the safety and oncologic outcomes of a laparoscopic approach to GBC. Results Among the 164 patients, 5 patients avoided further radical surgery because of unresectable disease and 12 were converted to open surgery; in the remaining 147 patients, totally laparoscopic treatment was successfully accomplished. Extended cholecystectomy was performed in 37 patients and simple cholecystectomy in 10. The T stages based on final pathology were Tis (n=6), T1a (n=2), T1b (n=9), T2 (n=26), and T3 (n=4). Recurrence was detected in 11 patients over a median follow-up of 51 months. The disease-specific 5-year survival rate of these 47 patients was 68.8%, and rose to 85% for patients with a normal cancer antigen 19-9 (CA19-9) level. Conclusions The favorable long-term outcomes demonstrate the feasibility of combined CT/MRI and frozen-section examination in the selection of patients with GBC confined to the gallbladder wall, confirm the oncologic safety of laparoscopic treatment in selected GBC patients, and favor measurement of preoperative CA19-9 in the selection of GBCs suitable for laparoscopic treatment.
2018, 30(1): 93-103.
doi: 10.21147/j.issn.1000-9604.2018.01.10
Abstract:
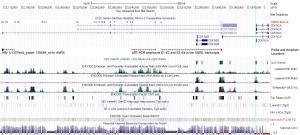
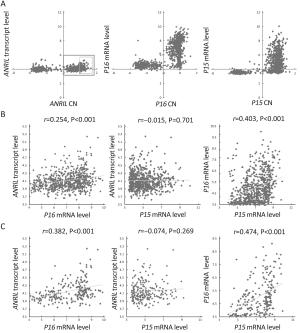
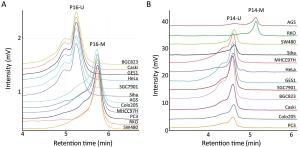
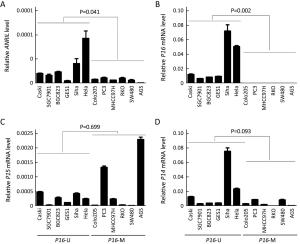
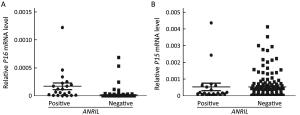
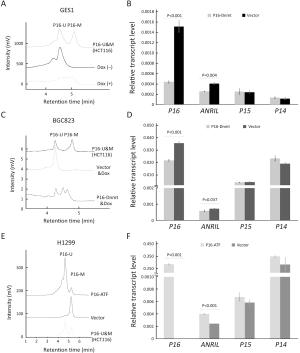
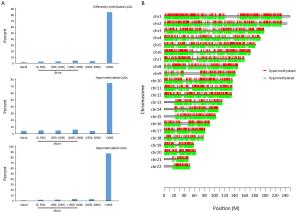
ObjectiveTo investigate the relationship between the transcription of ANRIL, P15, P14 and P16 at the same locus and the regulation mechanism of ANRIL. MethodsPublicly available database of Cancer Cell Line Encyclopedia (CCLE) was used in bioinformatic analyses. Methylation of CpG islands was detected by denaturing high performance liquid chromatography (DHPLC). Gene transcript levels were determined using quantitative real-time polymerase chain reaction (qRT-PCR) assays. An engineered P16-specific transcription factor and DNA methyltransferase were used to induce P16-specific DNA demethylation and methylation. ResultsThe expression level of ANRIL was positively and significantly correlated with that of P16 but not with that of P15 in the CCLE database. This was confirmed in human cell lines and patient colon tissue samples. In addition, ANRIL was significantly upregulated in colon cancer tissues. Transcription of ANRIL and P16 was observed only in cell lines in which the P16 alleles were unmethylated and not in cell lines with fully methylated P16 alleles. Notably, P16-specific methylation significantly decreased transcription of P16 and ANRIL in BGC823 and GES1 cells. In contrast, P16-specific demethylation re-activated transcription of ANRIL and P16 in H1299 cells (P<0.001). Alteration ofANRIL expression was not induced by P16 expression changes. ConclusionsANRIL and P16 are coordinately transcribed in human cells and regulated by the methylation status of the P16 CpG islands around the transcription start site.
2018, 30(1): 104-111.
doi: 10.21147/j.issn.1000-9604.2018.01.11
Abstract:
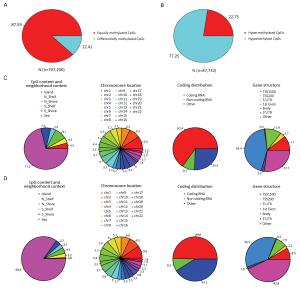
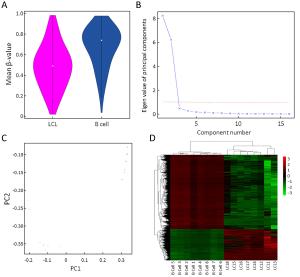
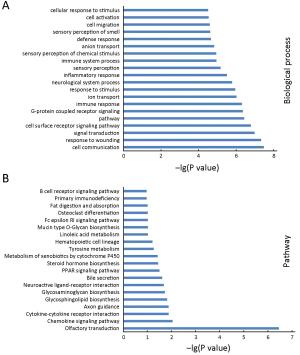
Objective This study aimed to comprehensively assess Epstein-Barr virus (EBV)-induced methylation alterations of B cell across whole genome. Methods We compared DNA methylation patterns of primary B cells and corresponding lymphoblastoid cell lines (LCLs) from eight participants. The genome-wide DNA methylation profiles were compared at over 850,000 genome-wide methylation sites. Results DNA methylation analysis revealed 87,732 differentially methylated CpG sites, representing approximately 12.41% of all sites in LCLs compared to primary B cells. The hypermethylated and hypomethylated CpG sites were about 22.75% or 77.25%, respectively. Only 0.8% of hypomethylated sites and 4.5% of hypermethylated sites were located in CpG islands, whereas 8.0% of hypomethylated sites and 16.3% of hypermethylated sites were located in shore (N_shore and S_shore). Using principal component analysis of the DNA methylation profiles, primary B cells and LCLs could be accurately predicted. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of differently methylated genes revealed that most of the top GO biological processes were related to cell activation and immune response, and some top enrichment pathways were related with activation and malignant transformation of human B cells. Conclusions Our study demonstrated genome-wide DNA methylation variations between primary B cells and corresponding LCLs, which might yield new insight on the methylation mechanism of EBV-induced immortalization.
2018, 30(1): 112-121.
doi: 10.21147/j.issn.1000-9604.2018.01.12
Abstract:
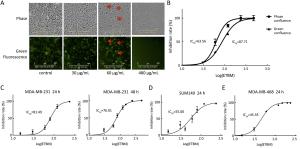
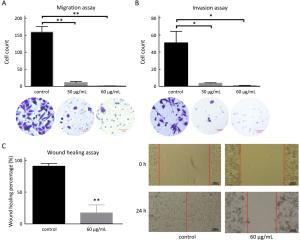
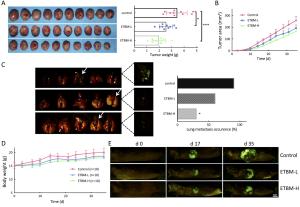
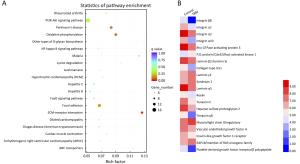
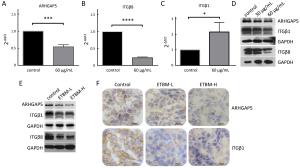
Objective Triple-negative breast cancer (TNBC) is highly invasive and metastatic, which is in urgent need of transformative therapeutics. Tubeimu (TBM), the rhizome of Bolbostemma paniculatum (Maxim.) Franquet, is one of the Chinese medicinal herbs used for breast diseases since the ancient times. The present study evaluated the efficacy, especially the anti-metastatic effects of the dichloromethane extract of Tubeimu (ETBM) on TNBC orthotopic mouse models and cell lines. Methods We applied real-time imaging on florescent orthotopic TNBC mice model and tested cell migration and invasion abilities with MDA-MB-231 cell line. Digital gene expression sequencing was performed and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis applied to explore the pathways influenced by ETBM. Moreover, quantitative real-time polymerase chain reactions (qRT-PCR) and Western blot were delivered to confirm the gene expression changes. Results ETBM exhibited noticeable control on tumor metastasis and growth of TNBC tumors with no obvious toxicity. In compliance with this, it also showed inhibition of cell migration and invasion in vitro. Its impact on the changed biological behavior in TNBC may be a result of decreased expression of integrin β1 (ITGβ1), integrin β8 (ITGβ8) and Rho GTPase activating protein 5 (ARHGAP5), which disabled the focal adhesion pathway and caused change in cell morphology. Conclusions This study reveals that ETBM has anti-metastatic effects on MDA-MB-231-GFP tumor and may lead to a new therapeutic agent for the integrative treatment of highly invasive TNBC.
2018, 30(1): 122-130.
doi: 10.21147/j.issn.1000-9604.2018.01.13
Abstract:
Gastric cancer is one of the leading causes of cancer-related deaths worldwide. Among which, about 1%–3% of gastric cancer patients were characterized by inherited gastric cancer predisposition syndromes, knowing as hereditary diffuse gastric cancer (HDGC). Studies reported that CDH1 germline mutations are the main cause of HDGC. With the help of rapid development of genetic testing technologies and data analysis tools, more and more researchers focus on seeking candidate susceptibility genes for hereditary cancer syndromes. In addition, National Comprehensive Cancer Network (NCCN) guidelines recommend that the patients of HDGC carrying CDH1 mutations should undergo prophylactic gastrectomy or routine endoscopic surveillances. Therefore, genetic counseling plays a key role in helping individuals with pathogenic mutations make appropriate risk management plans. Moreover, experienced and professional genetic counselors as well as a systematic multidisciplinary team (MDT) are also required to facilitate the development of genetic counseling and benefit pathogenic mutation carriers who are in need of regular and standardized risk management solutions. In this review, we provided an overview about the germline mutations of several genes identified in HDGC, suggesting that these genes may potentially act as susceptibility genes for this malignant cancer syndrome. Furthermore, we introduced information for prevention, diagnosis and risk management of HDGC. Investigations on key factors that may have effect on risk management decision-making and genetic data collection of more cancer syndrome family pedigrees are required for the development of HDGC therapeutic strategies.
Gastric cancer is one of the leading causes of cancer-related deaths worldwide. Among which, about 1%–3% of gastric cancer patients were characterized by inherited gastric cancer predisposition syndromes, knowing as hereditary diffuse gastric cancer (HDGC). Studies reported that CDH1 germline mutations are the main cause of HDGC. With the help of rapid development of genetic testing technologies and data analysis tools, more and more researchers focus on seeking candidate susceptibility genes for hereditary cancer syndromes. In addition, National Comprehensive Cancer Network (NCCN) guidelines recommend that the patients of HDGC carrying CDH1 mutations should undergo prophylactic gastrectomy or routine endoscopic surveillances. Therefore, genetic counseling plays a key role in helping individuals with pathogenic mutations make appropriate risk management plans. Moreover, experienced and professional genetic counselors as well as a systematic multidisciplinary team (MDT) are also required to facilitate the development of genetic counseling and benefit pathogenic mutation carriers who are in need of regular and standardized risk management solutions. In this review, we provided an overview about the germline mutations of several genes identified in HDGC, suggesting that these genes may potentially act as susceptibility genes for this malignant cancer syndrome. Furthermore, we introduced information for prevention, diagnosis and risk management of HDGC. Investigations on key factors that may have effect on risk management decision-making and genetic data collection of more cancer syndrome family pedigrees are required for the development of HDGC therapeutic strategies.
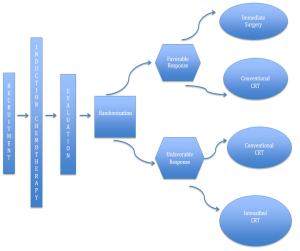
2018, 30(1): 131-146.
doi: 10.21147/j.issn.1000-9604.2018.01.14
Abstract:
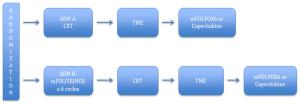
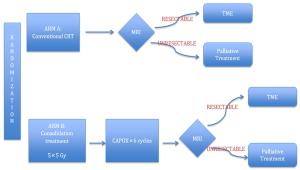
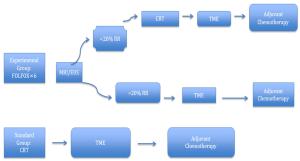
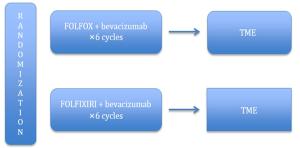
Advancements in rectal cancer treatment have resulted in improvement only in locoregional control and have failed to address distant relapse, which is the predominant mode of treatment failure in rectal cancer. As the efficacy of conventional chemoradiotherapy (CRT) followed by total mesorectal excision (TME) reaches a plateau, the need for alternative strategies in locally advanced rectal cancer (LARC) has grown in relevance. Several novel strategies have been conceptualized to address this issue, including: 1) neoadjuvant induction and consolidation chemotherapy before CRT; 2) neoadjuvant chemotherapy alone to avoid the sequelae of radiation; and 3) nonoperative management for patients who achieved pathological or clinical complete response after CRT. This article explores the issues, recent advances and paradigm shifts in the management of LARC and emphasizes the need for a personalized treatment plan for each patient based on tumor stage, location, gene expression and quality of life.
Advancements in rectal cancer treatment have resulted in improvement only in locoregional control and have failed to address distant relapse, which is the predominant mode of treatment failure in rectal cancer. As the efficacy of conventional chemoradiotherapy (CRT) followed by total mesorectal excision (TME) reaches a plateau, the need for alternative strategies in locally advanced rectal cancer (LARC) has grown in relevance. Several novel strategies have been conceptualized to address this issue, including: 1) neoadjuvant induction and consolidation chemotherapy before CRT; 2) neoadjuvant chemotherapy alone to avoid the sequelae of radiation; and 3) nonoperative management for patients who achieved pathological or clinical complete response after CRT. This article explores the issues, recent advances and paradigm shifts in the management of LARC and emphasizes the need for a personalized treatment plan for each patient based on tumor stage, location, gene expression and quality of life.
2018, 30(1): 147-156.
doi: 10.21147/j.issn.1000-9604.2018.01.15
Abstract:
Microvessels promote proliferation of tumor cells by delivering oxygen and nutrients, but rapid growth of tumors results in unmet demands for oxygen and nutrients, thereby creating a hypoxia microenvironment. Under hypoxic conditions, vascular endothelial cells (ECs) initiate the formation of immature and abnormal microvasculature. This results in leakage and tortuosity that facilitates tumor cell invasion, metastasis and resistance to cytotoxic treatment. Radiotherapy (RT) is a vital tumor treatment modality. Currently, more than 60% of patients with malignant tumors receive RT at certain points during their treatment. Hypoxia induced by abnormal microvessels can hamper the cytotoxic effect of ionizing radiation, particularly, stereotactic body radiotherapy (SBRT). Anti-angiogenesis (AA) agents are known to reduce and renormalize microvessels in tumors, and hence alleviate hypoxia. The combination of AA agents with SBRT may have a synergistic role in inhibiting the growth of tumors. On the contrary, large doses of irradiation may affect tumor microvessels itself. In this review, we aim to clarify the relationship between SBRT and microvessel formation in tumors. In addition, we provide a retrospective analysis of the combination therapy involving SBRT and AA agents in preclinical and clinical practice to define its role in anti-tumor treatment.
Microvessels promote proliferation of tumor cells by delivering oxygen and nutrients, but rapid growth of tumors results in unmet demands for oxygen and nutrients, thereby creating a hypoxia microenvironment. Under hypoxic conditions, vascular endothelial cells (ECs) initiate the formation of immature and abnormal microvasculature. This results in leakage and tortuosity that facilitates tumor cell invasion, metastasis and resistance to cytotoxic treatment. Radiotherapy (RT) is a vital tumor treatment modality. Currently, more than 60% of patients with malignant tumors receive RT at certain points during their treatment. Hypoxia induced by abnormal microvessels can hamper the cytotoxic effect of ionizing radiation, particularly, stereotactic body radiotherapy (SBRT). Anti-angiogenesis (AA) agents are known to reduce and renormalize microvessels in tumors, and hence alleviate hypoxia. The combination of AA agents with SBRT may have a synergistic role in inhibiting the growth of tumors. On the contrary, large doses of irradiation may affect tumor microvessels itself. In this review, we aim to clarify the relationship between SBRT and microvessel formation in tumors. In addition, we provide a retrospective analysis of the combination therapy involving SBRT and AA agents in preclinical and clinical practice to define its role in anti-tumor treatment.

 Abstract
Abstract FullText HTML
FullText HTML PDF 1049KB
PDF 1049KB