2020 Vol.32(5)
Display Mode: |
2020, 32(5): 547-563.
doi: 10.21147/j.issn.1000-9604.2020.05.01
Abstract:
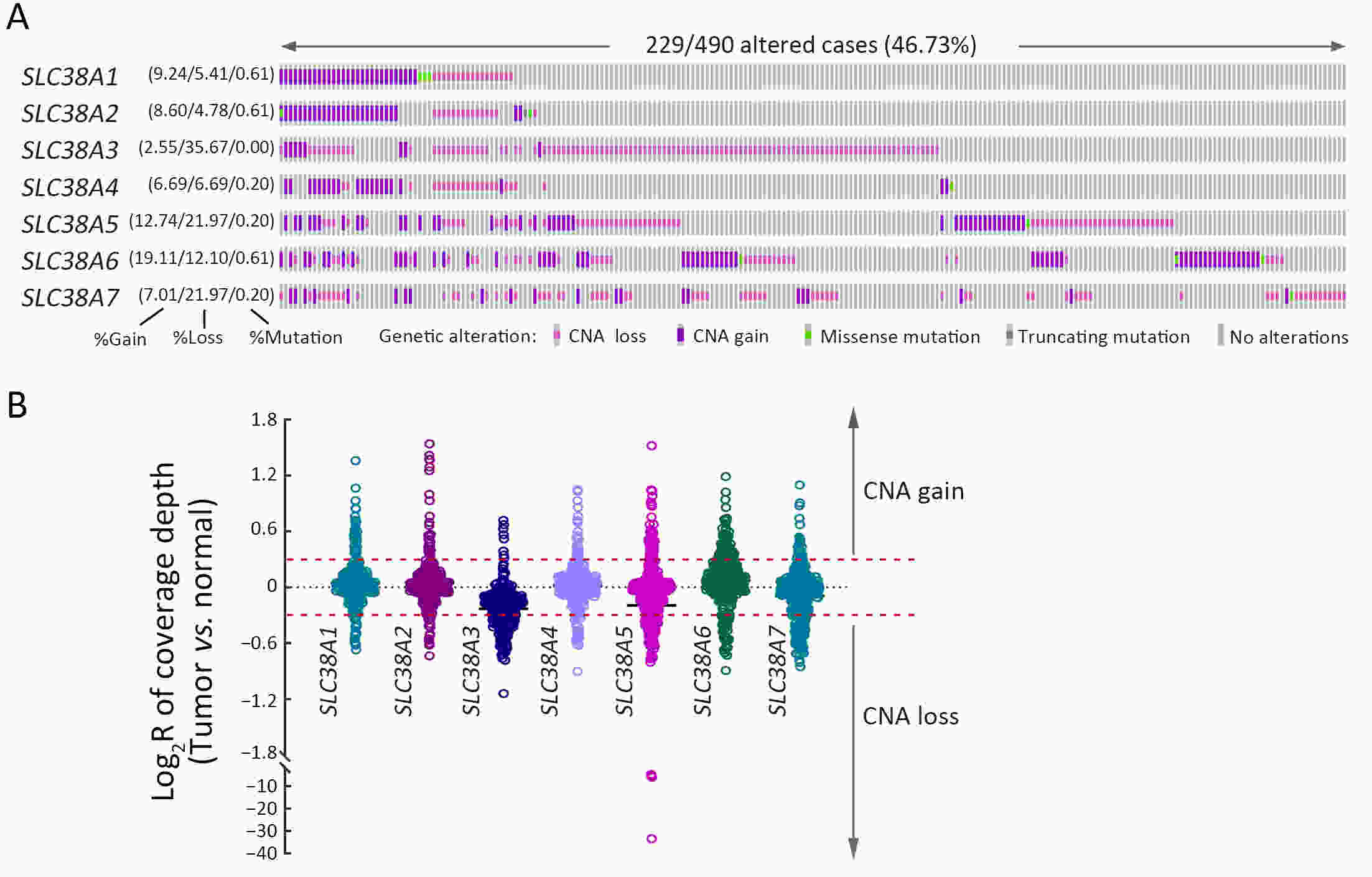
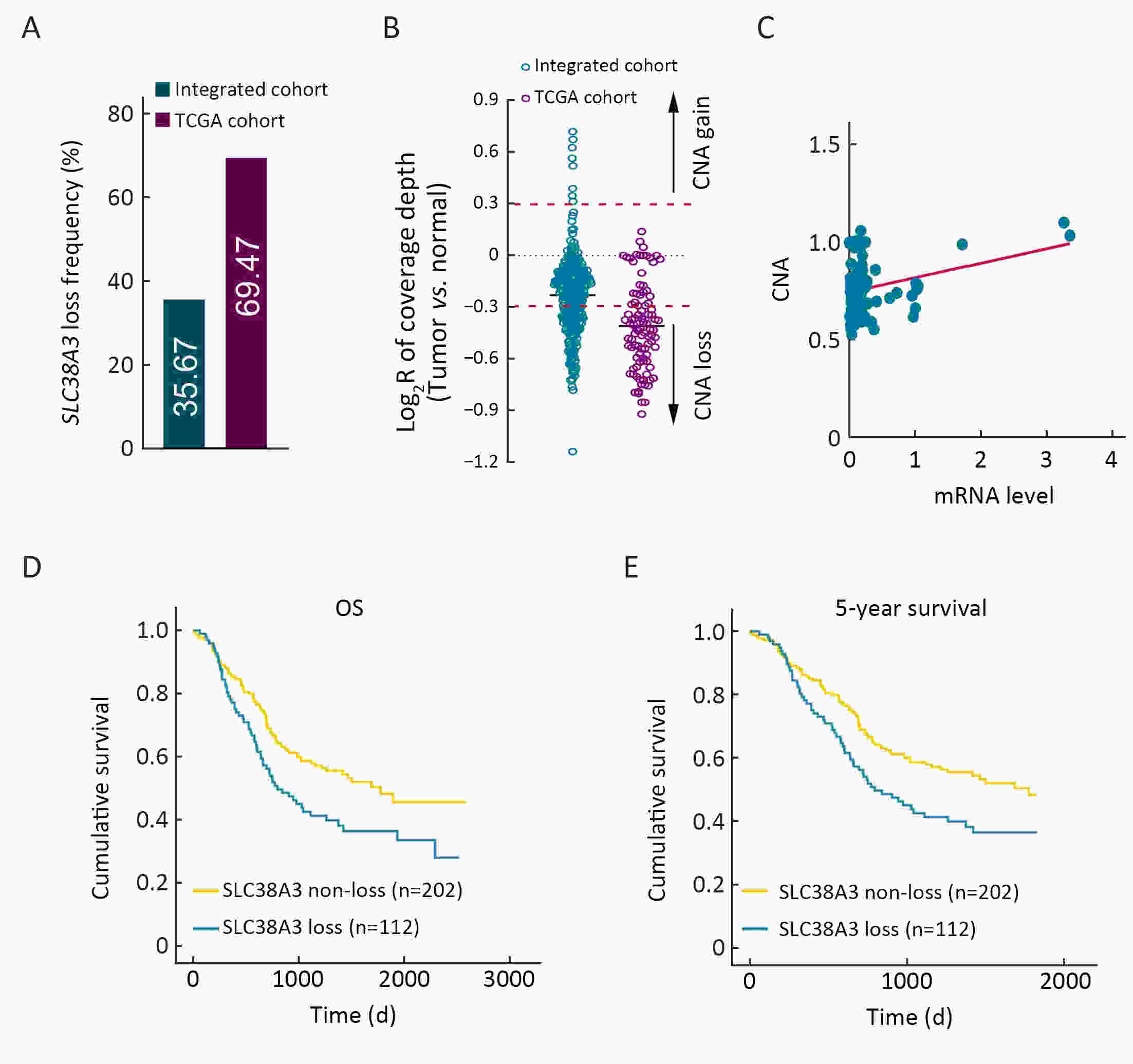
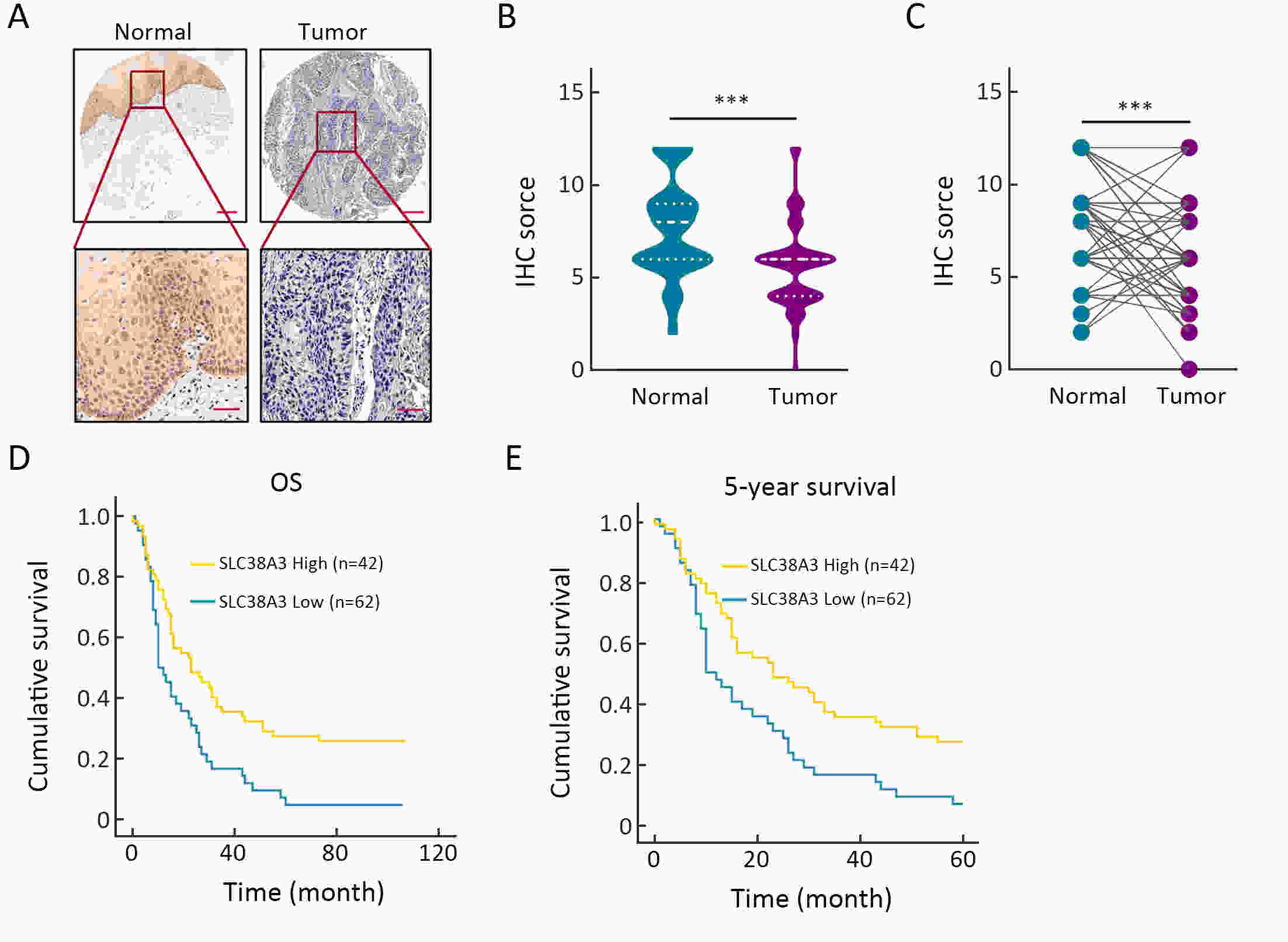
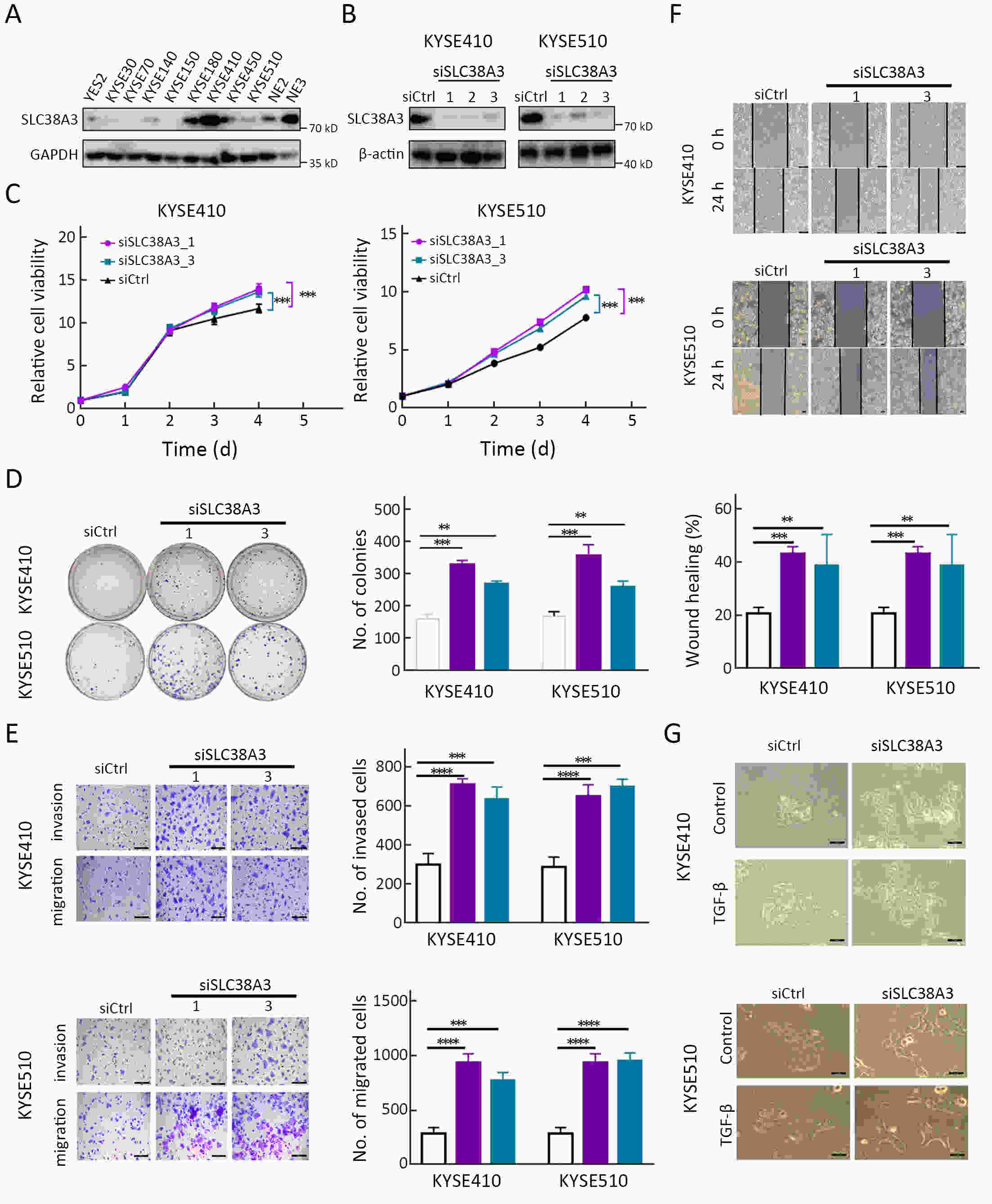
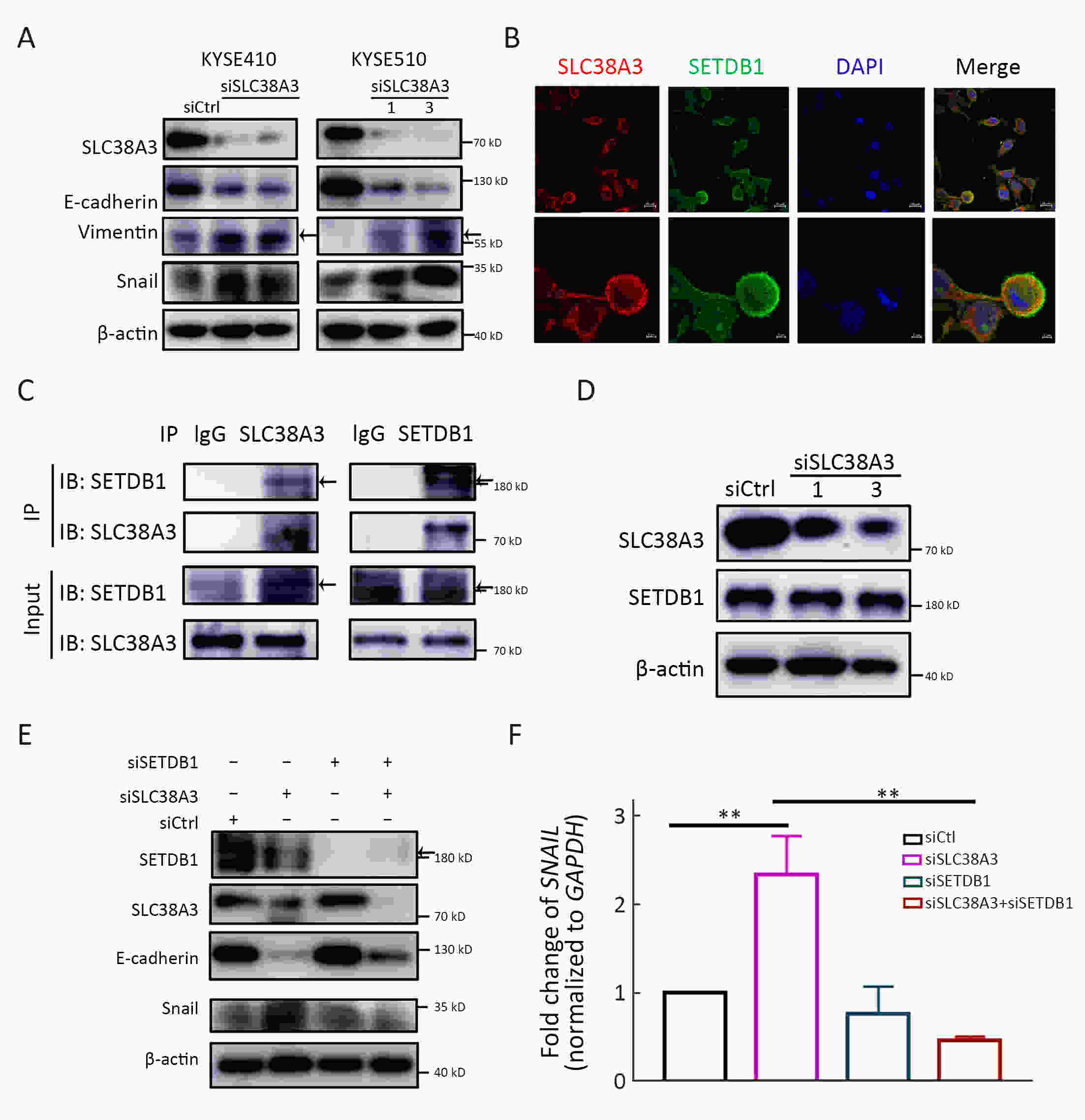
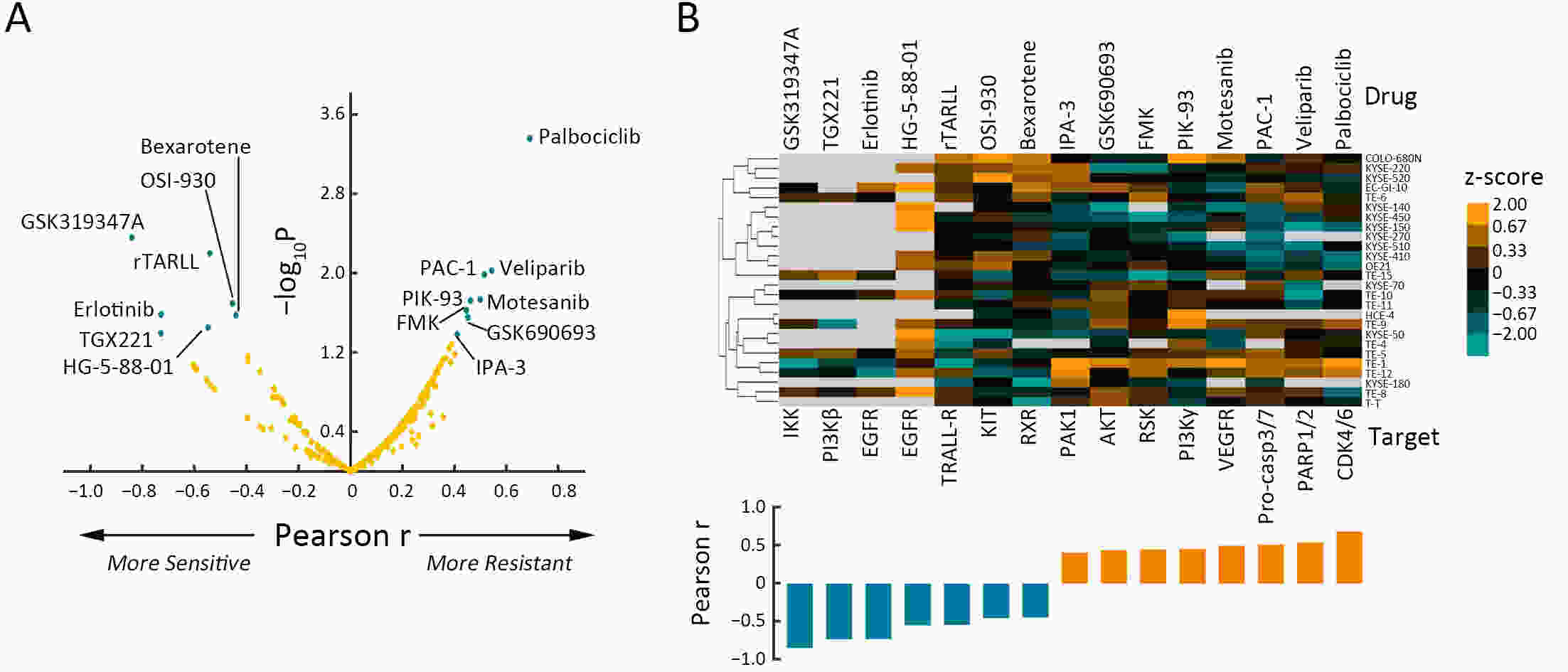
ObjectiveSolute carrier family 38 (SLC38s) transporters play important roles in amino acid transportation and signaling transduction. However, their genetic alterations and biological roles in tumors are still largely unclear. This study aimed to elucidate the genetic signatures of SLC38s transporters and their implications in esophageal squamous cell carcinoma (ESCC). MethodsAnalyses on somatic mutation and copy number alterations (CNAs) of SLC38A3 were performed as described. Immunohistochemistry (IHC) assay and Western blot assay were used to detect the protein expression level. MTS assay, colony formation assay, transwell assay and wound healing assay were used to explore the malignant phenotypes of ESCC cells. Immunofluorescence assay was used to verify the colocalization of two indicated proteins and immunopreciptation assay was performed to confirm the interaction of proteins. ResultsOur findings revealed that SLC38s family was significantly disrupted in ESCC, with high frequent CNAs and few somatic mutations. SLC38A3 was the most frequent loss gene among them and was linked to poor survival and lymph node metastasis. The expression of SLC38A3 was lower in tumor tissues compared to that in normal tissues, which was also significantly associated with worse clinical outcome. Further experiments revealed that depletion of SLC38A3 could promote EMT in ESCC cell lines, and the interaction of SLC38A3 and SETDB1 might lead to the reduced transcription of Snail. Pharmacogenomic analyses demonstrated that fifteen inhibitors were showed significantly correlated with SLC38A3 expression. ConclusionsOur investigations have provided insights that SLC38A3 could act as a suppressor in EMT pathway and serve as a prognostic factor and predictor of differential drug sensitivities in ESCC.
2020, 32(5): 564-579.
doi: 10.21147/j.issn.1000-9604.2020.05.02
Abstract:
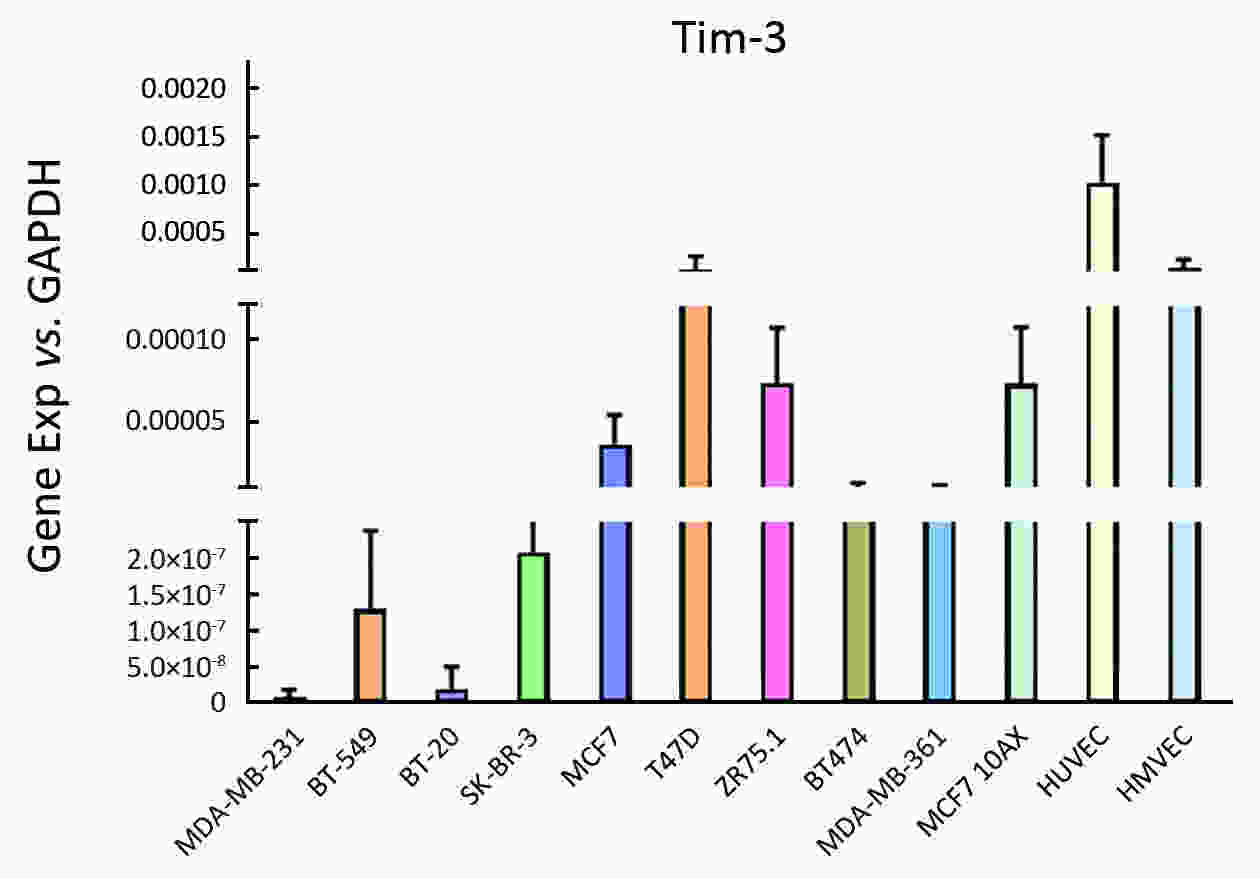
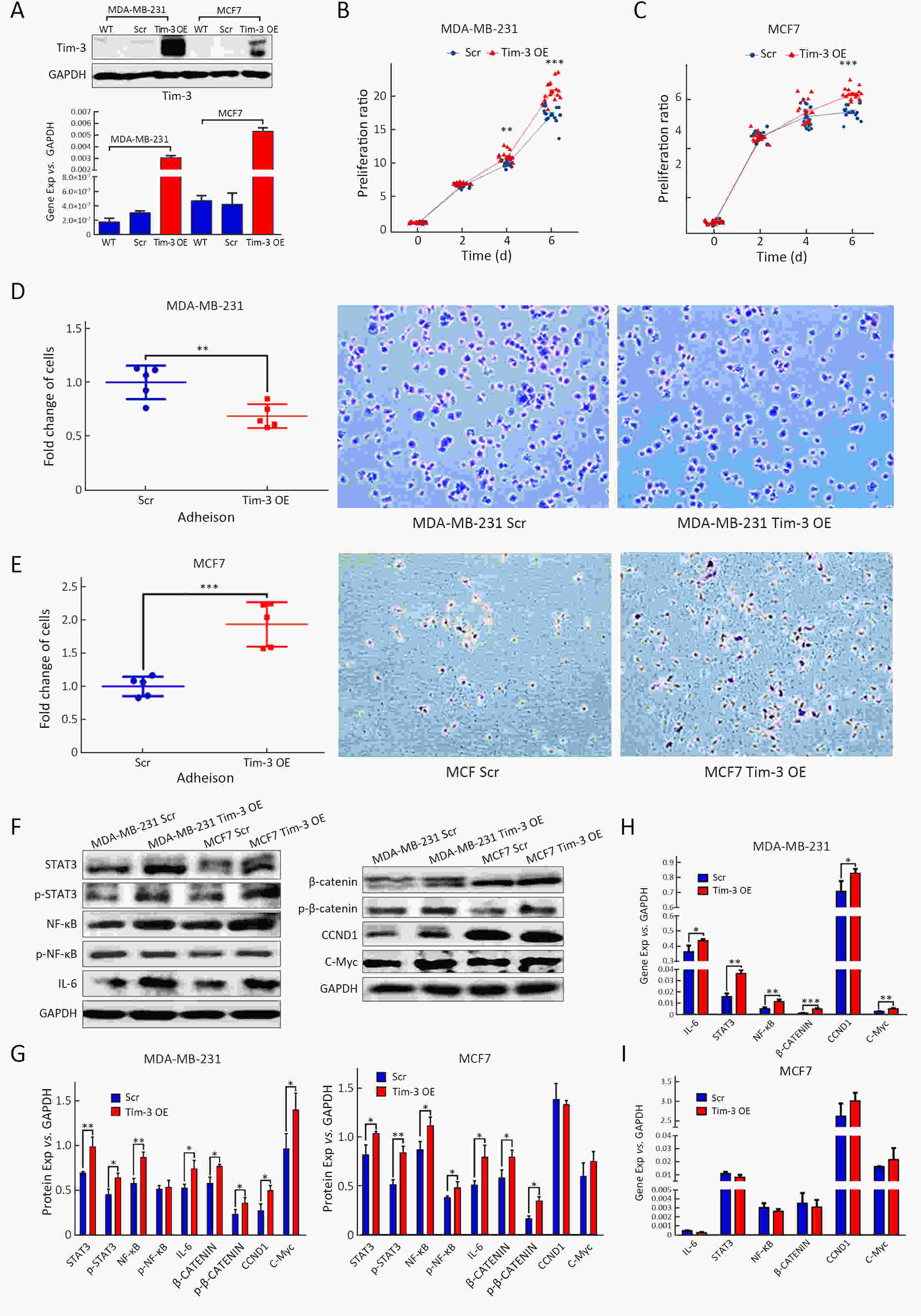
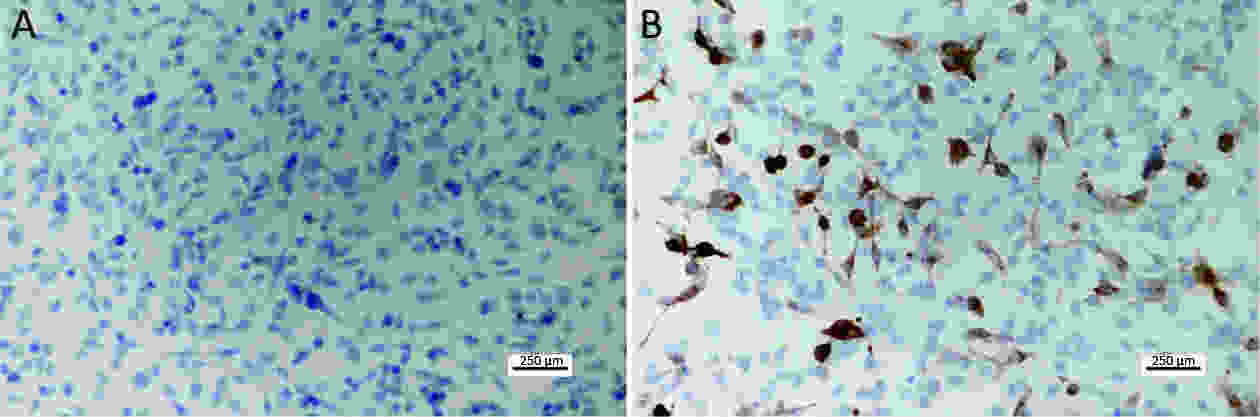
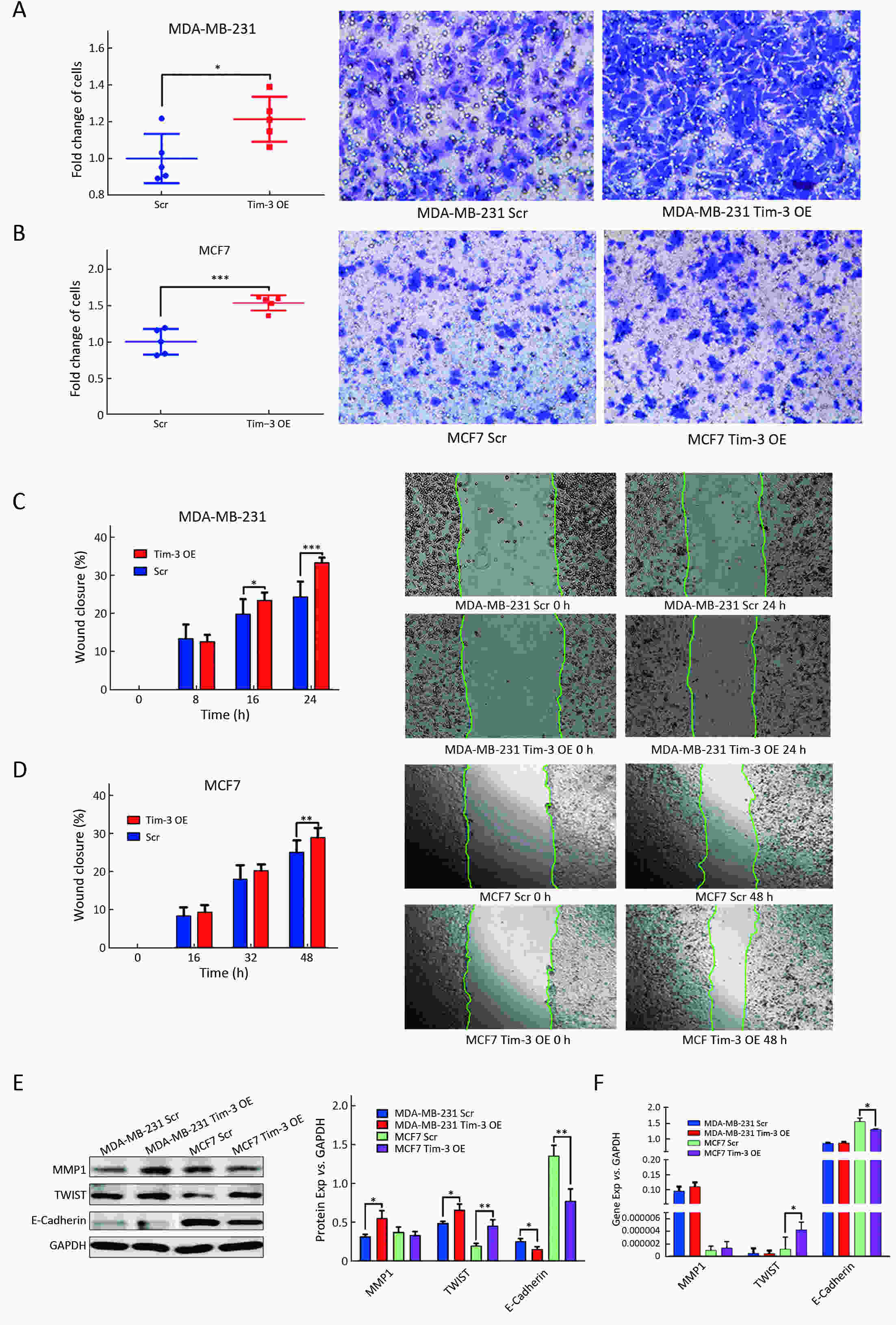
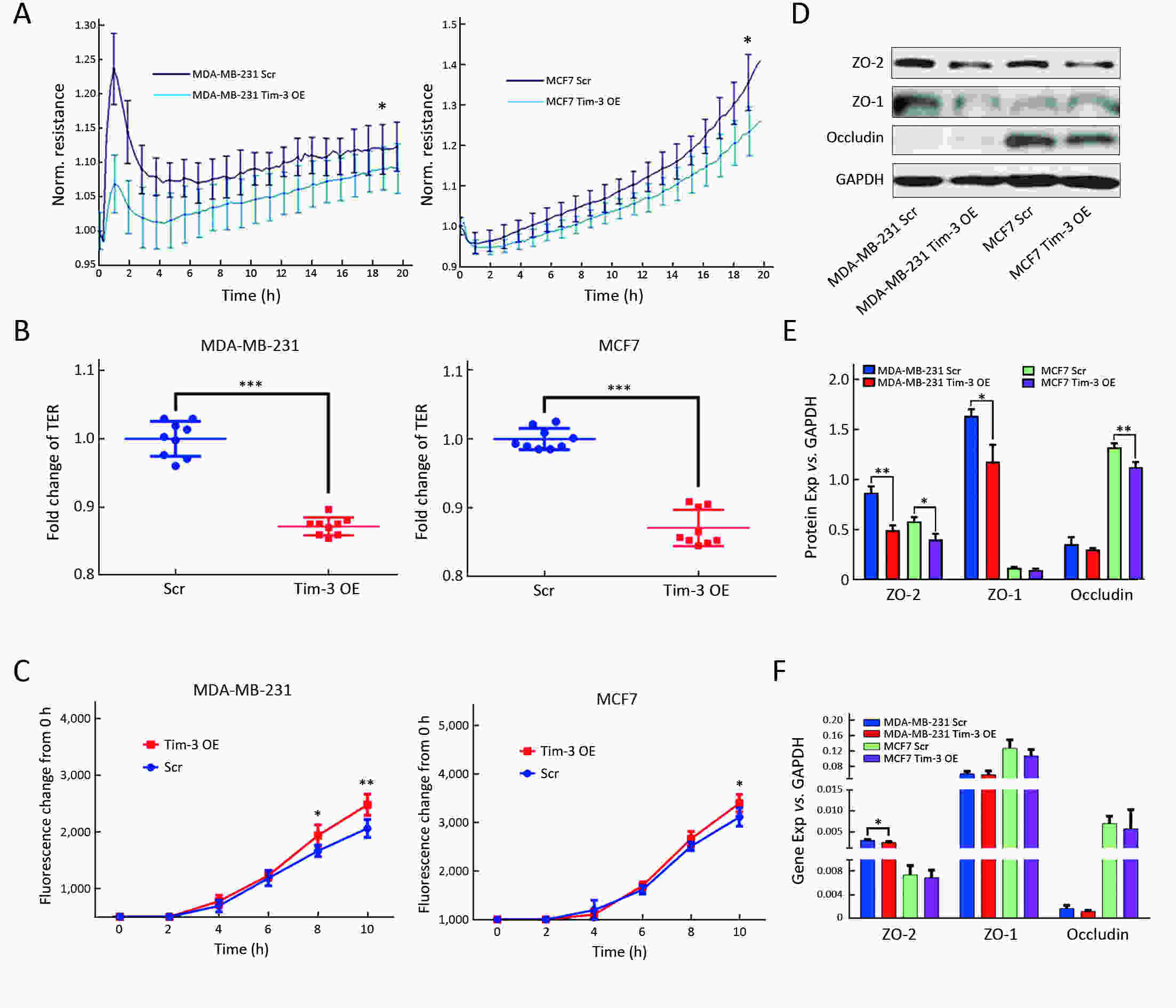
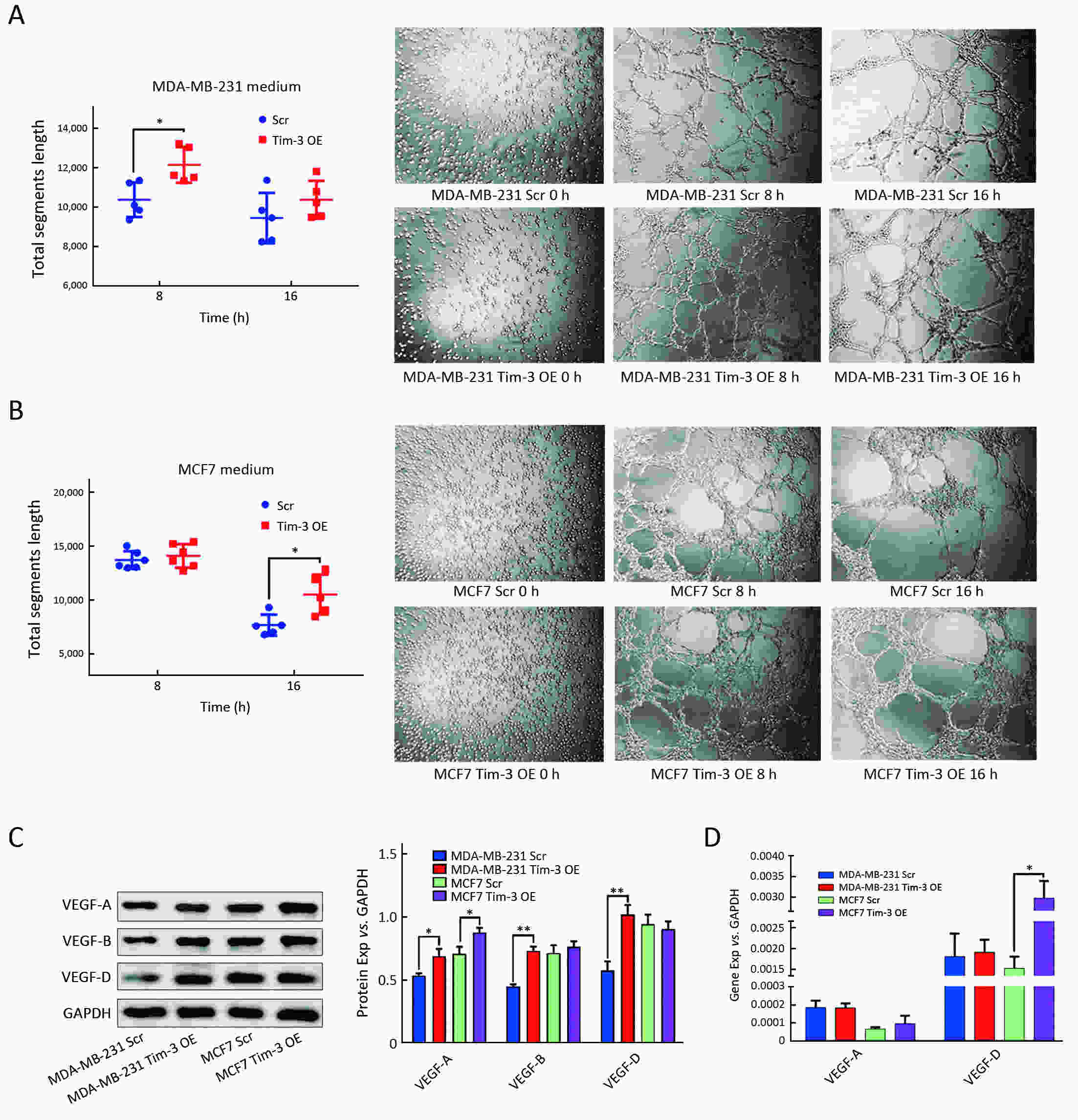
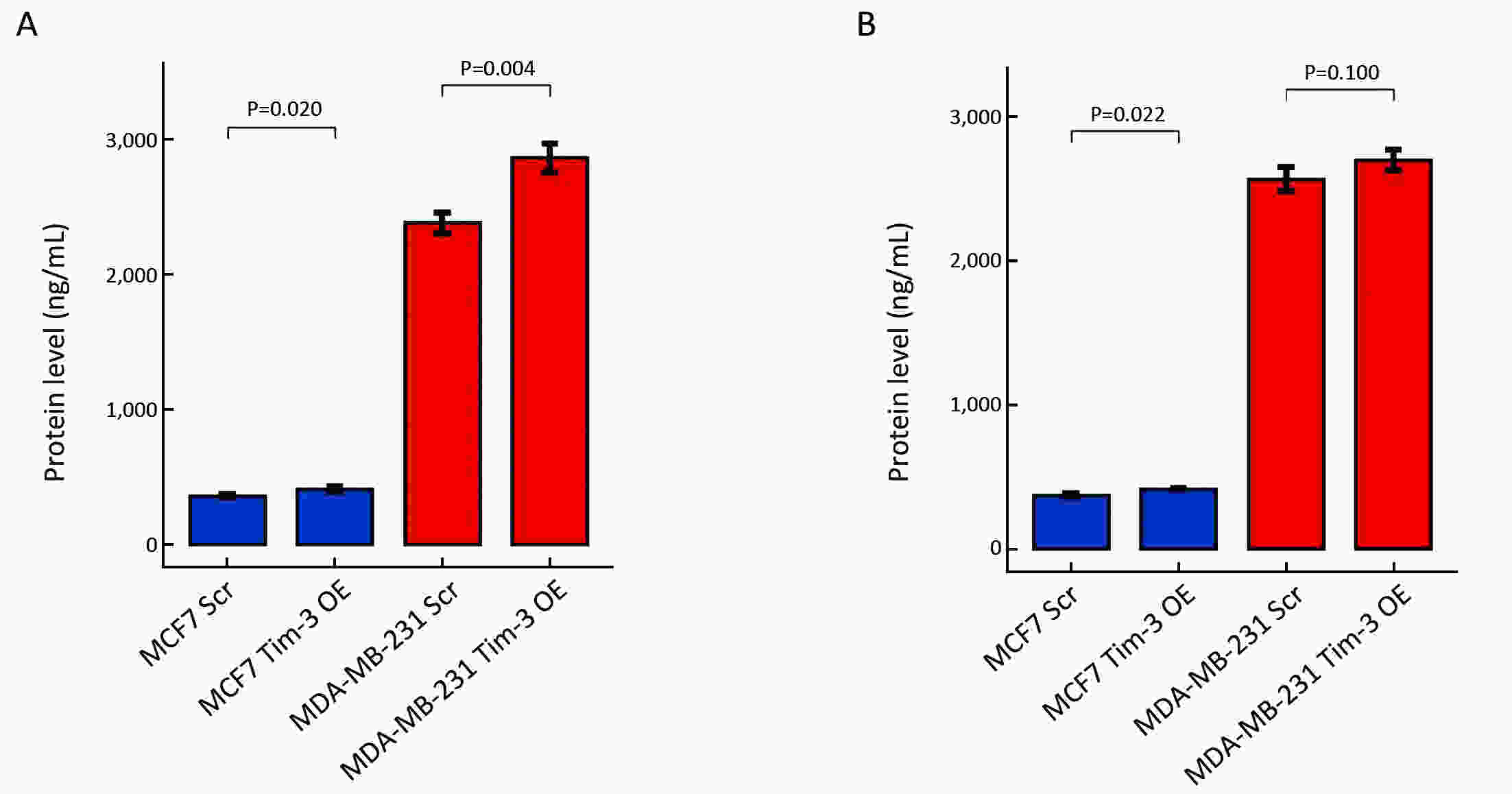
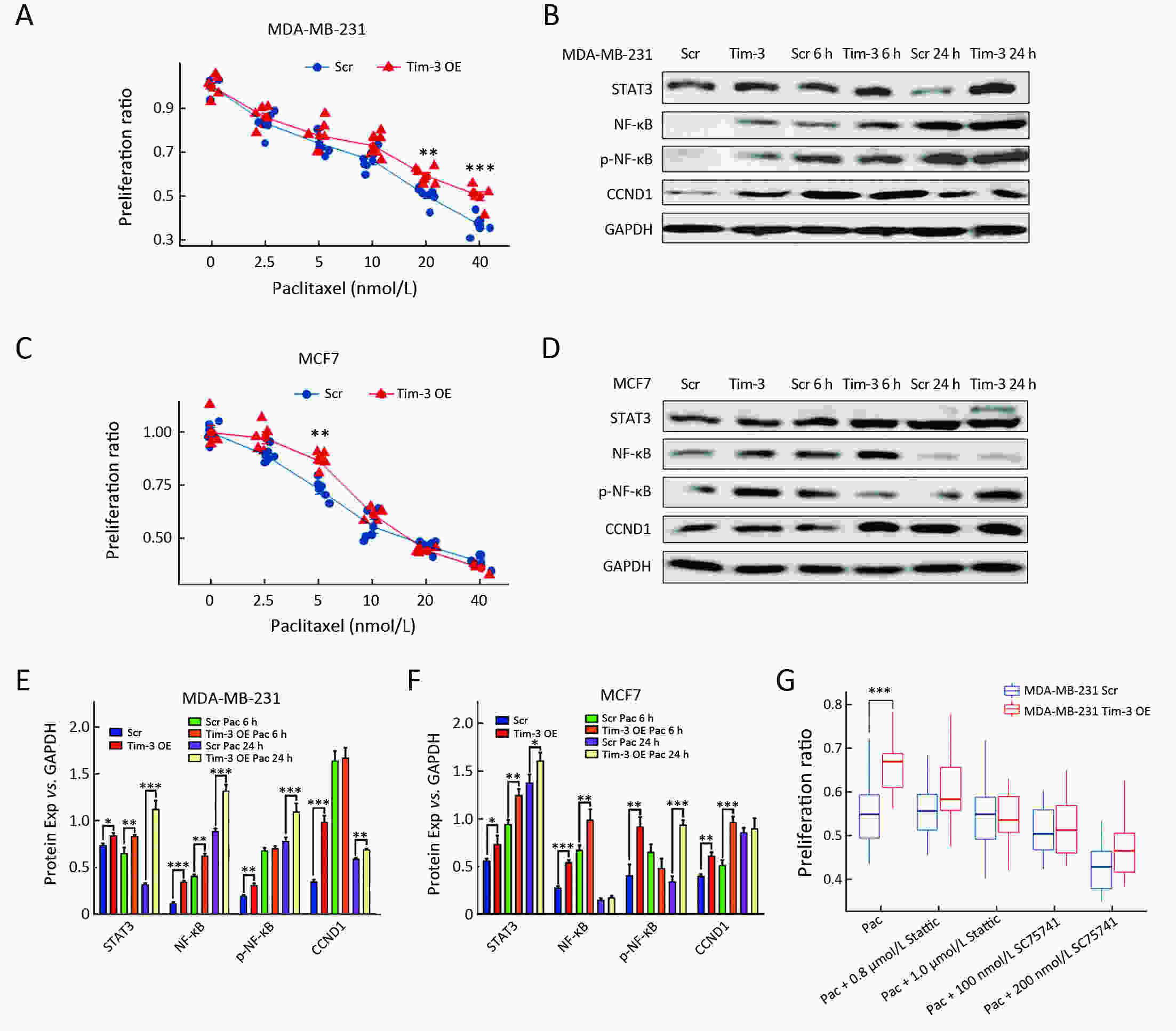
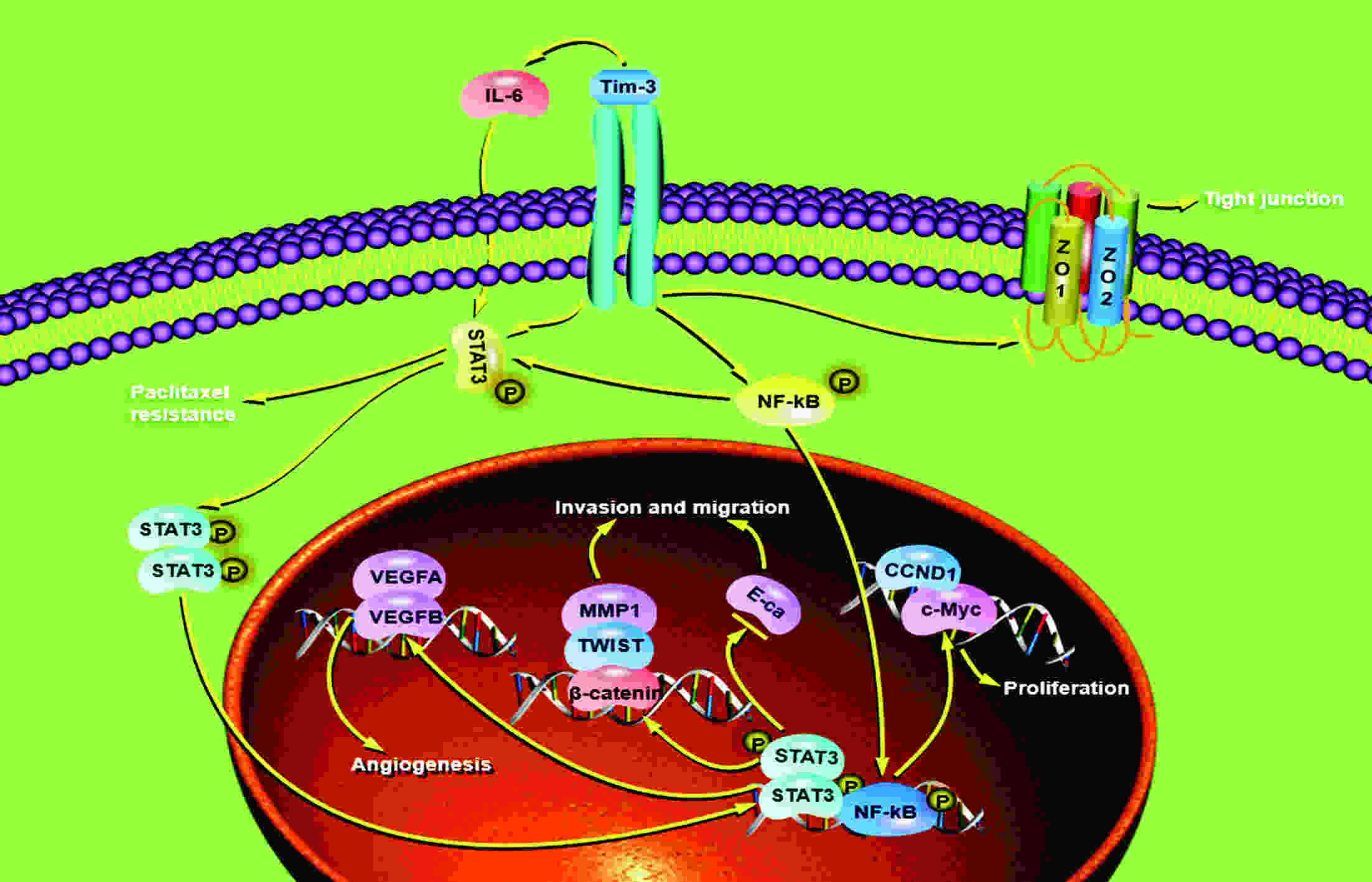
ObjectiveAlthough T-cell immunoglobulin and mucin-domain containing molecule-3 (Tim-3) has been recognized as a promising target for cancer immunotherapy, its exact role in breast cancer has not been fully elucidated. MethodsTim-3 gene expression in breast cancer and its prognostic significance were analyzed. Associated mechanisms were then explored in vitro by establishing Tim-3-overexpressing breast cancer cells. ResultsIn a pooled analysis of The Cancer Genome Atlas (TCGA) database, Tim-3 gene expression levels were significantly higher (P<0.001) in breast cancer tissue, compared with normal tissues. Tim-3 was a prognosis indicator in breast cancer patients [relapse-free survival (RFS), P=0.004; overall survival (OS), P=0.099]. Tim-3 overexpression in Tim-3low breast cancer cells promoted aggressiveness of breast cancer cells, as evidenced by enhanced proliferation, migration, invasion, tight junction deterioration and tumor-associated tubal formation. Tim-3 also enhanced cellular resistance to paclitaxel. Furthermore, Tim-3 exerted its function by activating the NF-κB/STAT3 signalling pathway and by regulating gene expression [cyclin D1 (CCND1), C-Myc, matrix metalloproteinase-1(MMP1), TWIST, vascular endothelial growth factor (VEGF) upregulation, concomitant with E-cadherin downregulation). Lastly, Tim-3 downregulated tight junction-associated molecules zona occludens (ZO)-2, ZO-1 and occludin, which may further facilitate tumor progression. ConclusionsTim-3 plays an oncogenic role in breast cancer and may represent a potential target for antitumor therapy.
2020, 32(5): 580-595.
doi: 10.21147/j.issn.1000-9604.2020.05.03
Abstract:
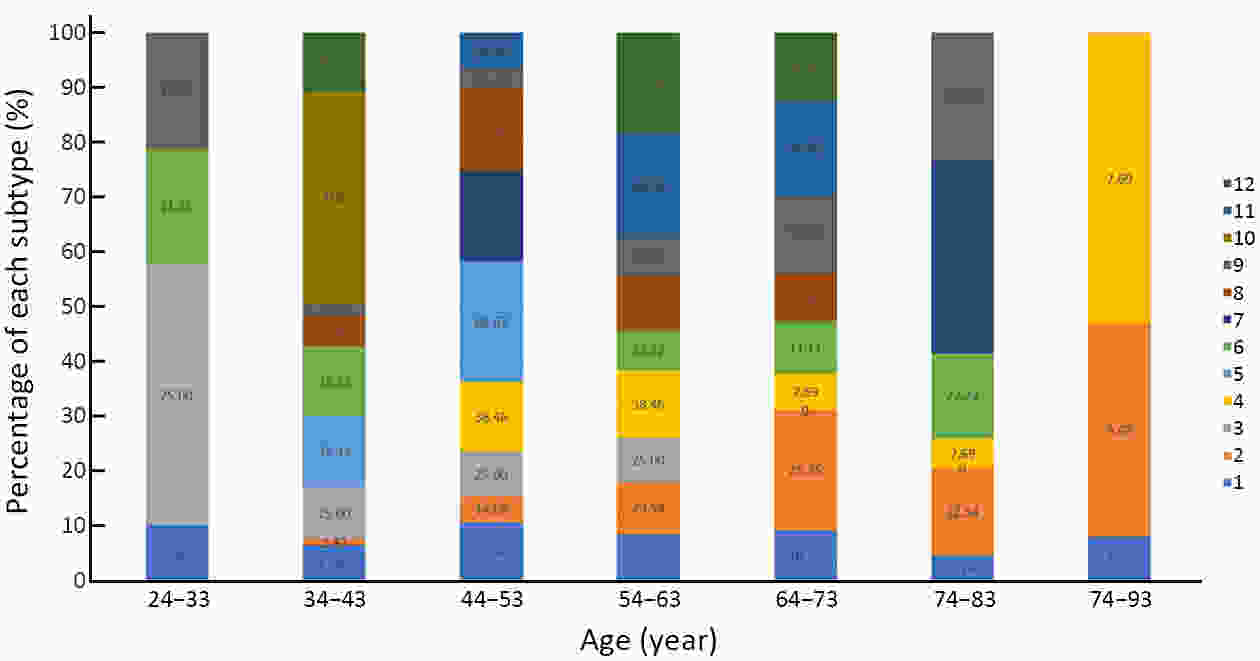
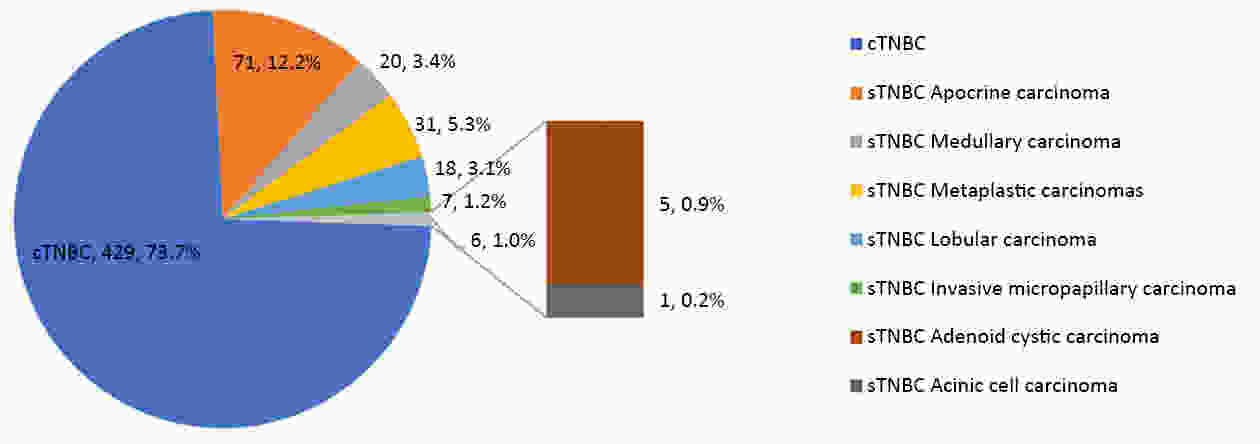
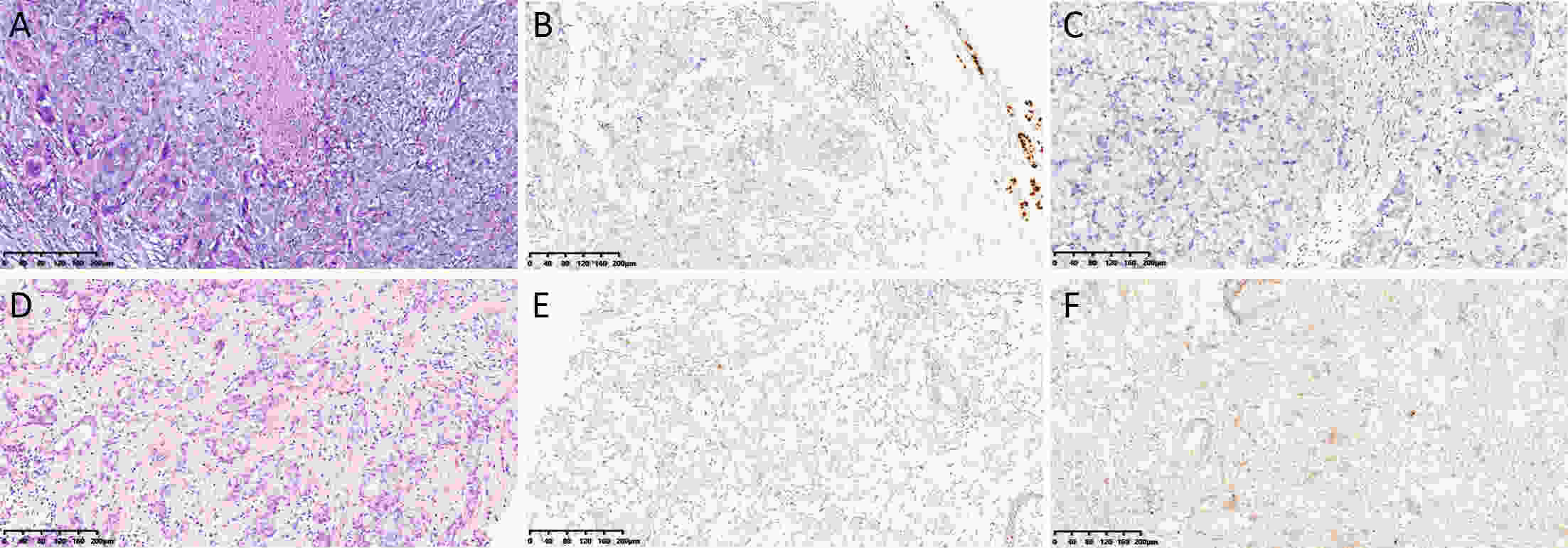
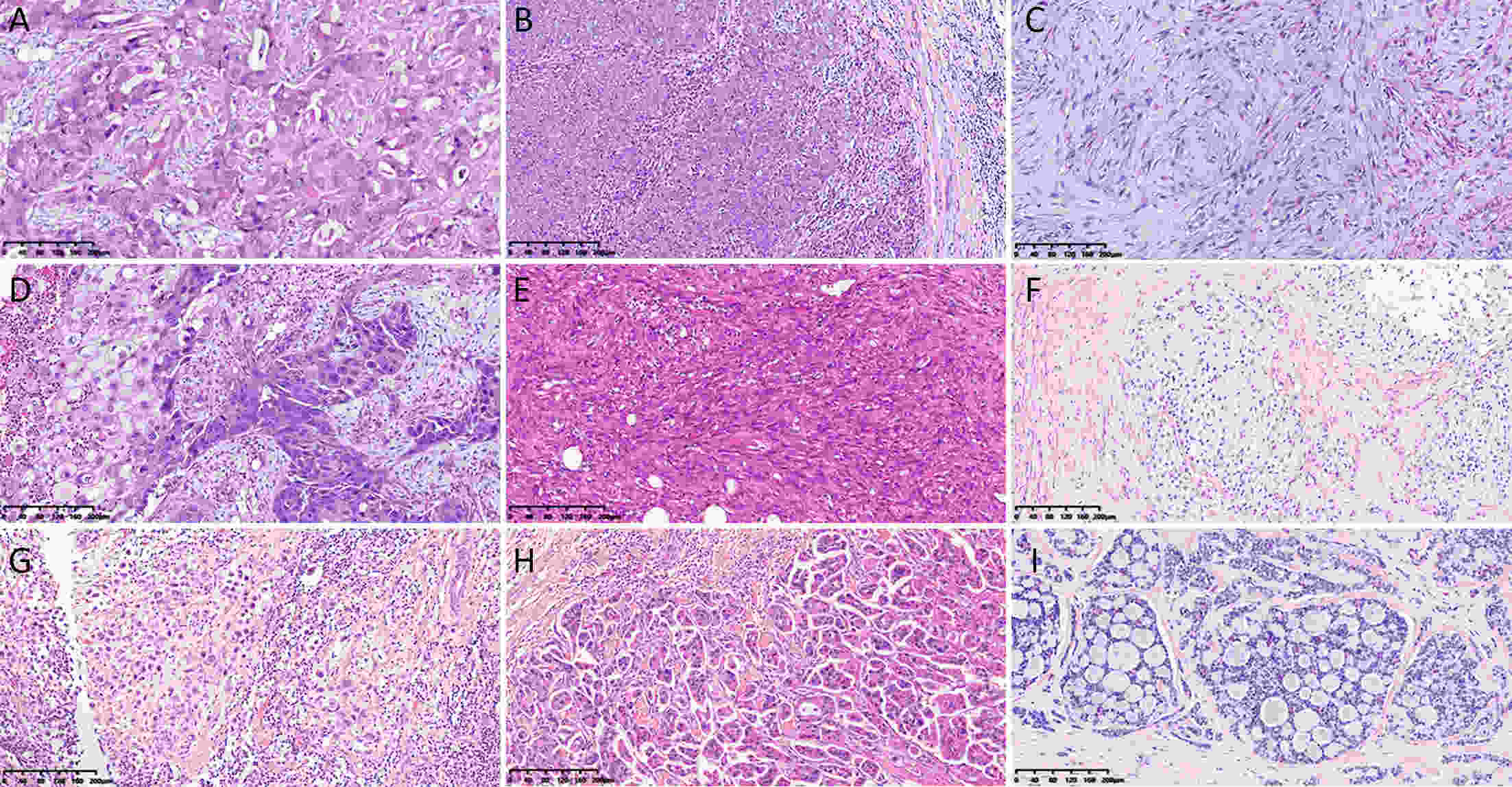
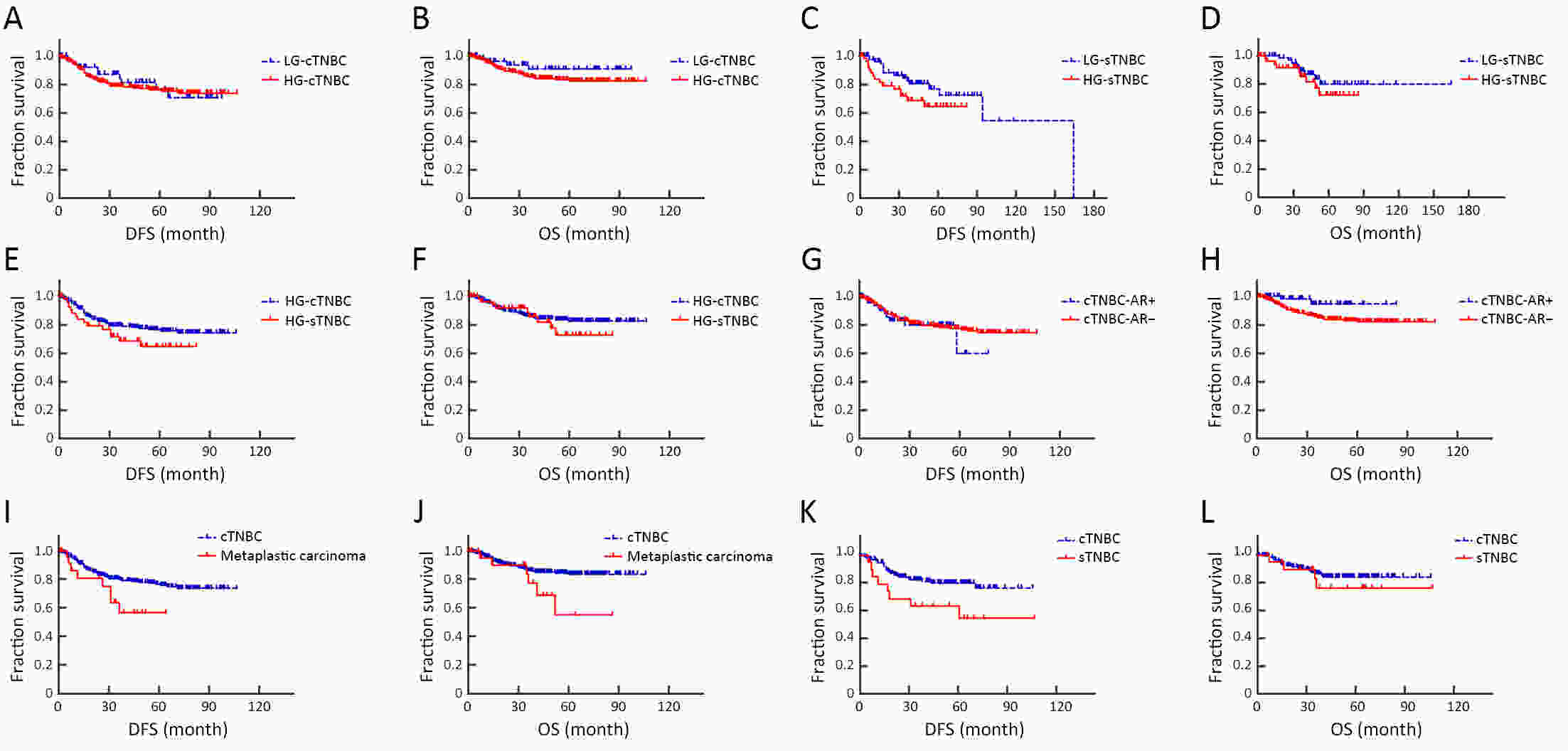
ObjectiveTo investigate histo-pathological distribution and clinico-pathological significance in a large Chinese triple-negative breast cancer (TNBC) patients serials based on the latest understanding of its clinico-pathological diversity, and to provide more information to clinicians to improve precision of individualized treatment of TNBC. MethodsA retrospective analysis was performed on patients with TNBC at Breast Disease Center, Peking University First Hospital between January 2010 and December 2019. Histo- and clinico-pathological characteristics were analyzed by Chi-square test and Student’s t-test, and prognoses were calculated using Kaplan-Meier method and a Cox proportionate hazards model. Bonferroni correction was used to correct for multiple comparison. ResultsConventional type of TNBC (cTNBC) were identified in 73.7% of 582 TNBC, while special type of TNBC (sTNBC) were 26.3%, including 71 apocrine carcinoma, 20 medullary carcinoma, 31 metaplastic carcinoma, 18 invasive lobular carcinoma, 7 invasive micropapillary carcinoma, 5 adenoid cystic carcinoma and 1 acinic cell carcinoma. Compared to sTNBC, cTNBC was associated with high histologic grade (P<0.001) and lower androgen receptor (AR) expression (P<0.001). TNM stage of low-grade cTNBC was significantly lower than that of high-grade cTNBC (P=0.002). Although no significant difference, there was a trend that the rate of 5-year disease-free survival (DFS) and 5-year overall survival (OS) were longer in high-grade cTNBC than in high-grade sTNBC (P=0.091 and 0.518), and were longer in low-grade sTNBC than in high-grade sTNBC (P=0.051 and 0.350). Metaplastic carcinomas showed larger tumor size (P=0.008) and higher proliferative Ki67 index (P=0.004) than cTNBCs. ConclusionsResults from our cohort imply that sub-categorization or subtyping and histological grading could be meaningful in pathological evaluation of TNBC, and need to be clarified in more large collections of TNBC.
2020, 32(5): 596-604.
doi: 10.21147/j.issn.1000-9604.2020.05.04
Abstract:
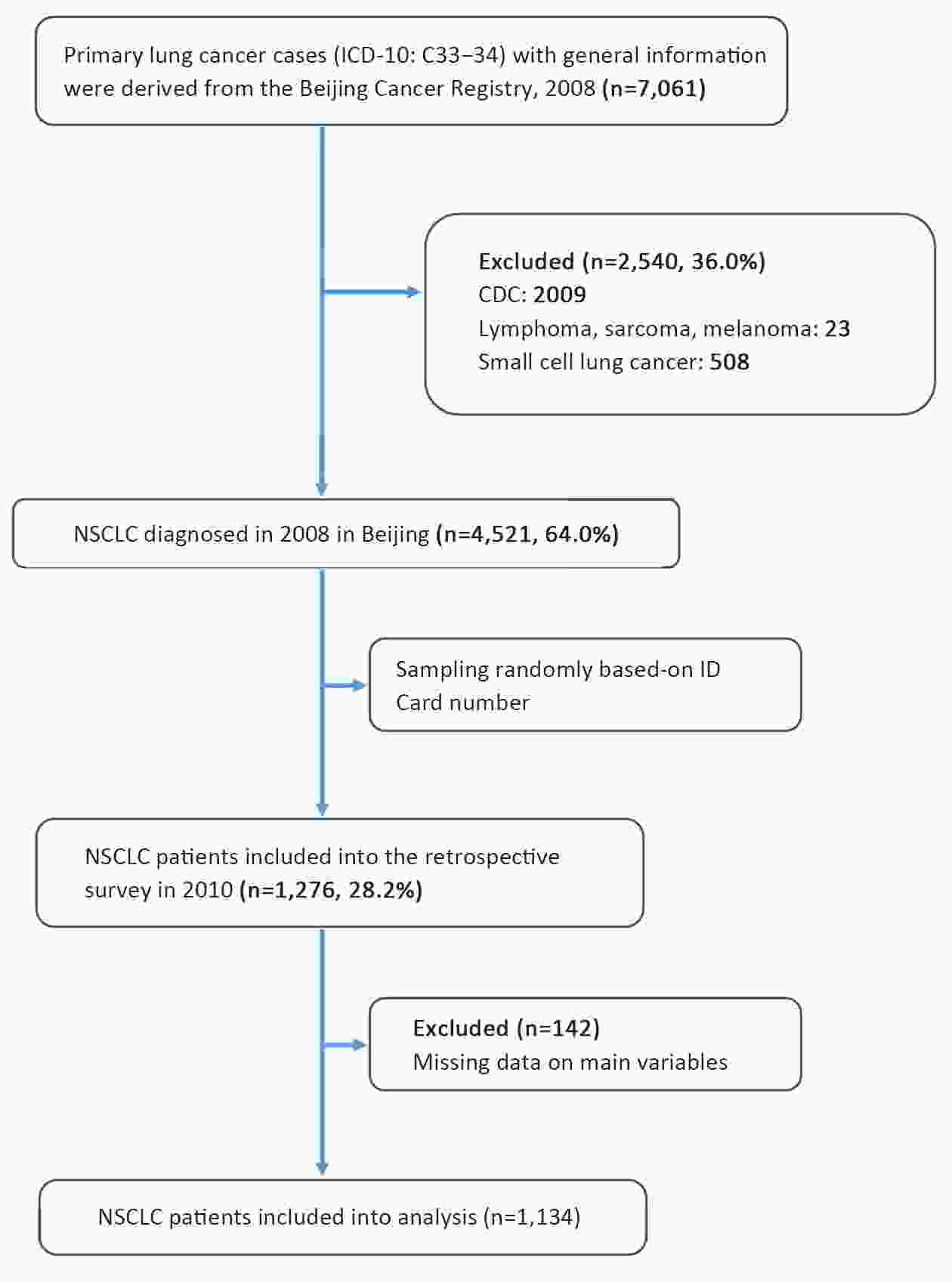
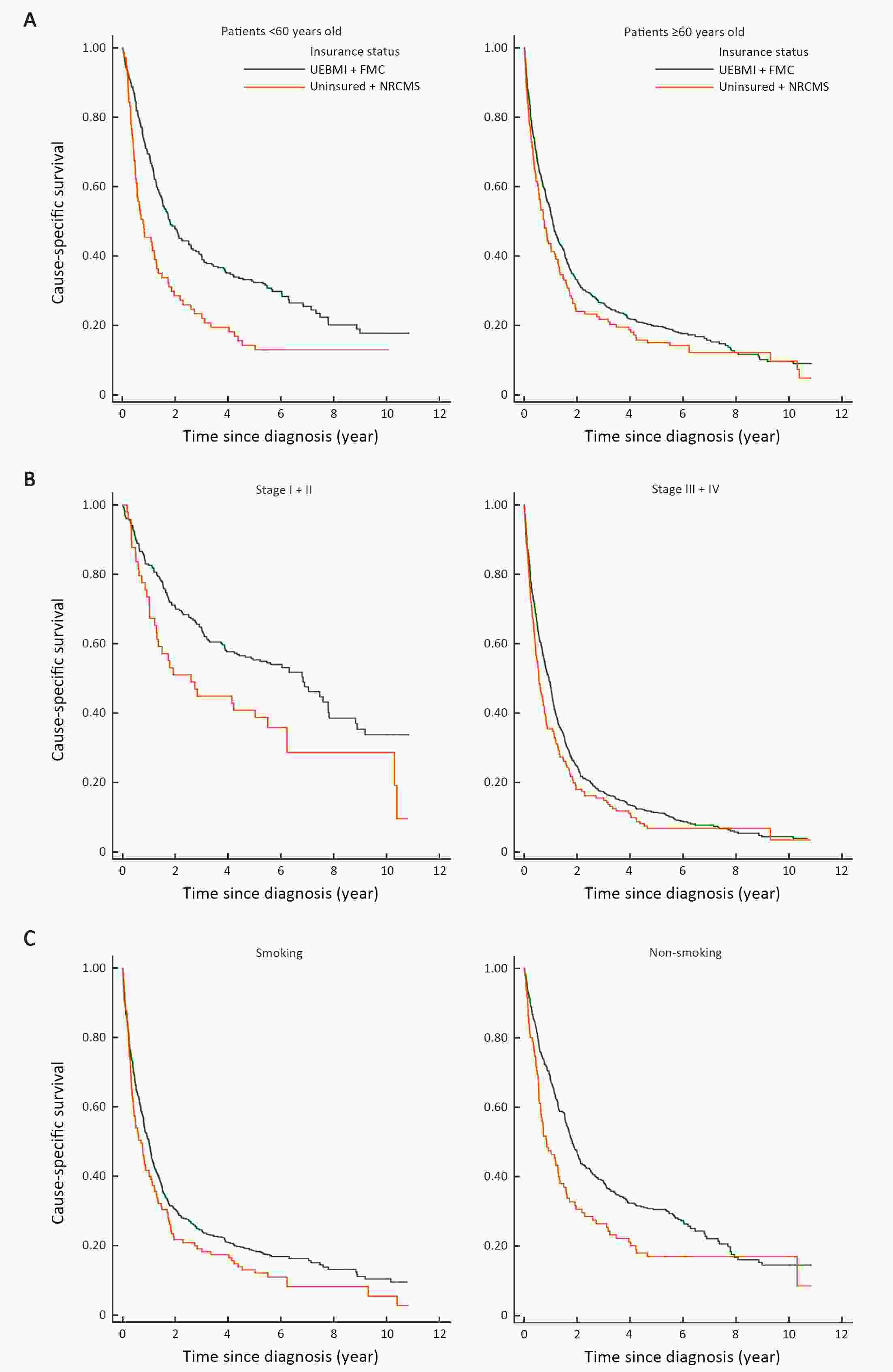
ObjectiveTo evaluate the effects of health insurance status on long-term cancer-specific survival of non-small cell lung cancer (NSCLC) in Beijing, China, using a population-based cancer registry data. MethodsInformation on NSCLC patients diagnosed in 2008 was derived from the Beijing Cancer Registry. The medical records of 1,134 cases were sampled and re-surveyed to obtain information on potential risk factors. Poorly-insured status was defined as Uninsured and New Rural Cooperative Medical Insurance Scheme (NRCMS), while well-insured included Urban Employees Basic Medical Insurance (UEBMI) and Free Medical Care (FMC). To estimate survival outcomes, individuals were followed-up until December 31, 2018. Cancer-specific survival probabilities at 5 and 10 years after diagnosis were estimated using the Kaplan-Meier method. Log-rank test was used to compare long-term survival with different characteristics. Multivariable Cox proportional hazard regression model was used to examine the relative effect of insurance status on cancer-specific mortality. ResultsWell-insured NSCLC patients have longer cancer-specific survival than poorly-insured individuals [hazard ratio (HR)=0.81; 95% confidence interval (95% CI): 0.67−0.97), even after adjusting for age, gender, cancer stage, smoking status, family history and residential area. Older age and rural residence were associated with a higher risk of cancer-specific mortality (HR=1.03; 95% CI: 1.02−1.03 and HR=1.25; 95% CI: 1.07−1.46, respectively). Smoking individuals had a 41% higher long-term cancer-specific mortality risk than non-smoking ones (HR=1.41; 95% CI: 1.20−1.66). ConclusionsNSCLC patients with good insurance status had better survival rates than those with poor insurance. An association was significant even after 10 years. Large population-based studies are needed to validate that high reimbursement insurance status can lead to the improvement of long-term cancer prognosis in China.
2020, 32(5): 605-613.
doi: 10.21147/j.issn.1000-9604.2020.05.05
Abstract:
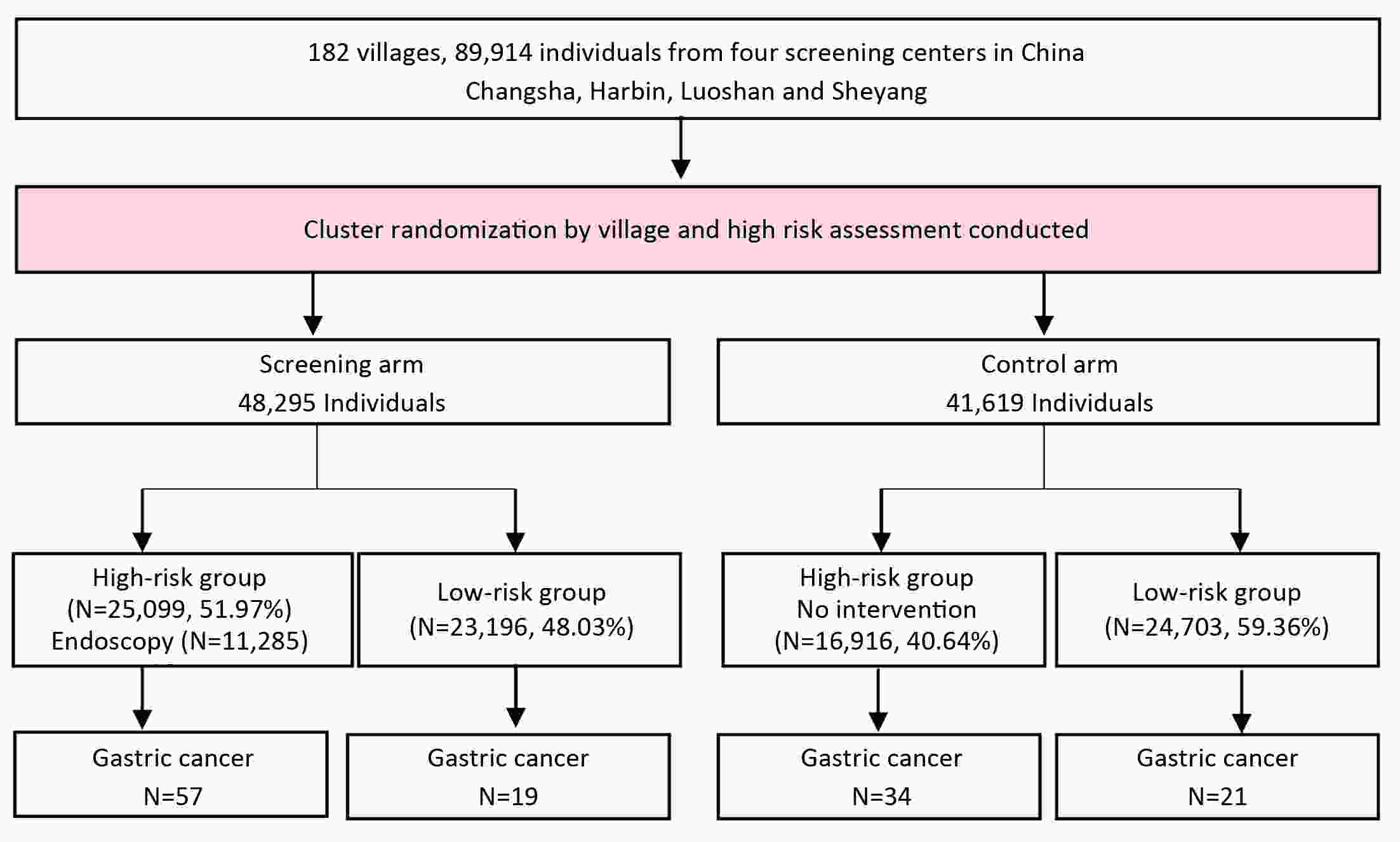
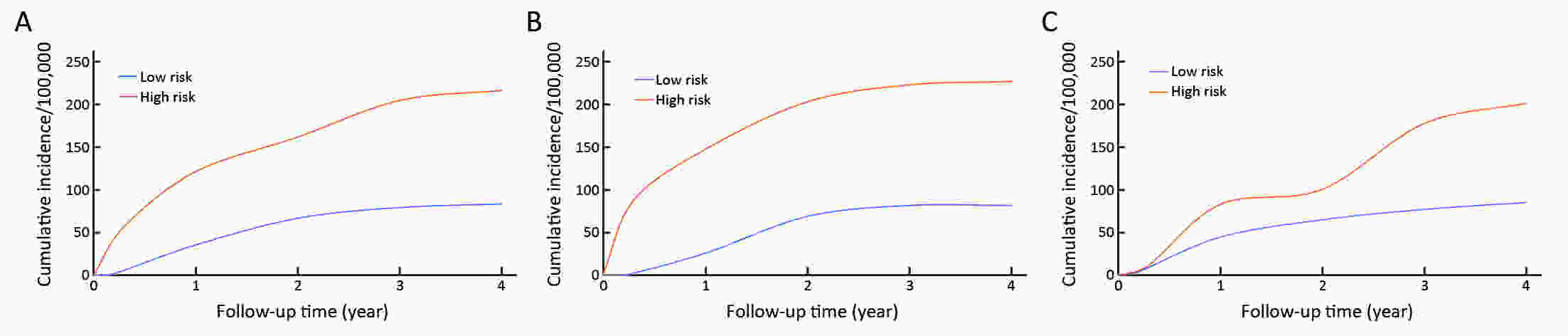
ObjectiveThis study aimed at evaluating the efficacy of the questionnaire-based prediction model in an independent prospective cohort. MethodsA cluster-randomized controlled trial was conducted in Changsha, Harbin, Luoshan, and Sheyang in eastern China in 2015−2017. A total of 182 villages/communities were regarded as clusters, and allocated to screening arm or control arm randomly. Face-to-face interview through a questionnaire interview, including of relevant risk factors of gastric cancer, was administered for each subject. Participants were further classified into high-risk or low-risk groups based on their exposure to risk factors. All participants were followed up until December 31, 2019. Cumulative incidence rates from gastric cancer between high-risk and low-risk groups were calculated and compared using the log-rank test. Cox proportional hazard regression models were applied to estimate hazard ratio (HR) and 95% confidence interval (95% CI). ResultsTotally, 89,914 residents were recruited with a mean follow-up of 3.47 years. And 42,015 (46.73%) individuals were classified into high-risk group and 47,899 (53.27%) subjects were categorized into low-risk group. Gastric cancer was diagnosed in 131 participants, of which 91 were in high-risk group. Compared with the low-risk participants, high-risk individuals were more likely to develop gastric cancer (adjusted HR=2.15, 95% CI, 1.23−3.76). The sensitivity of the questionnaire-based model was estimated at 61.82% (95% CI, 47.71−74.28) in a general population. ConclusionsOur questionnaire-based model is effective at identifying high-risk individuals for gastric cancer.
2020, 32(5): 614-620.
doi: 10.21147/j.issn.1000-9604.2020.05.06
Abstract:
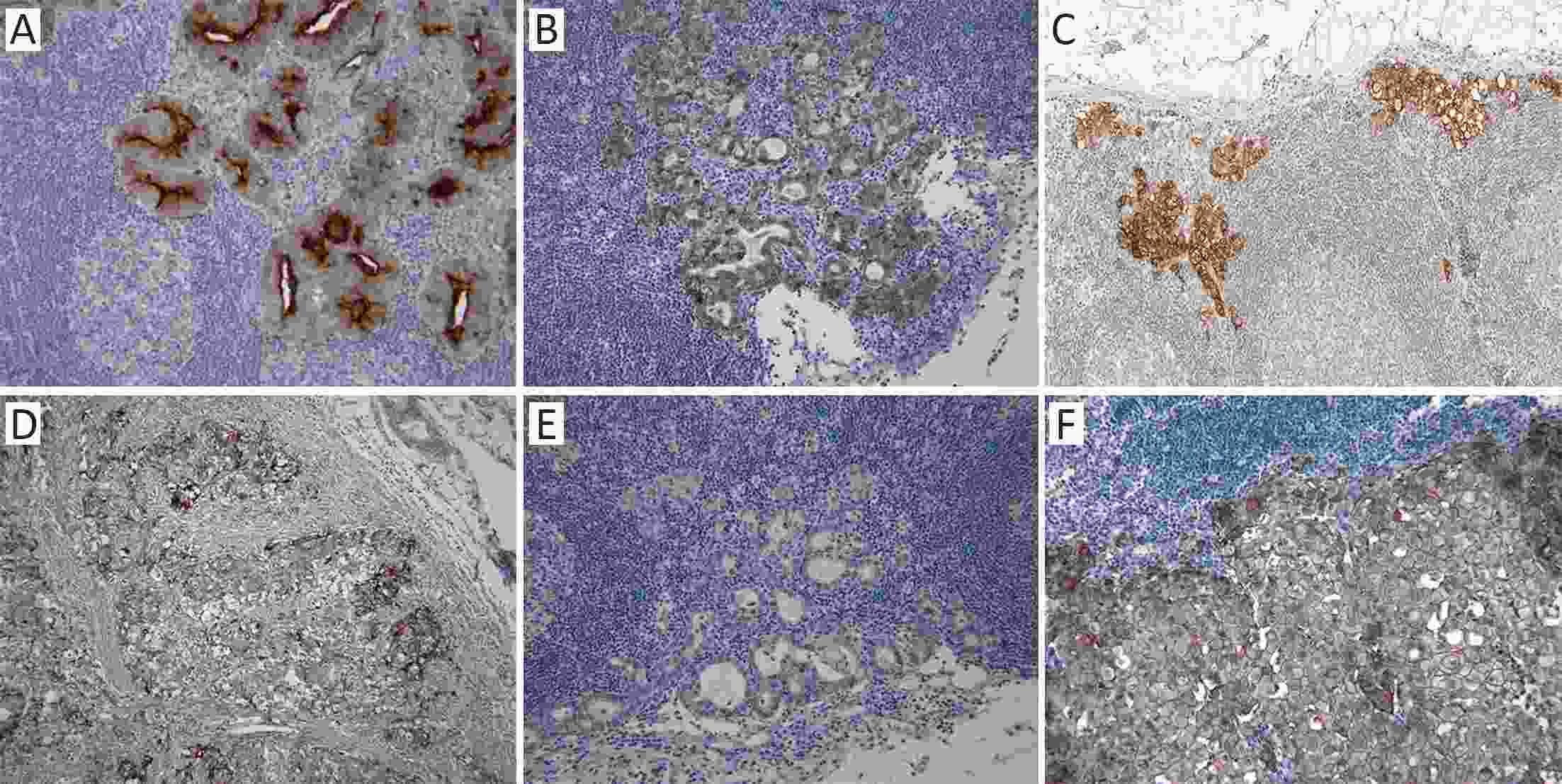
ObjectiveThere has been a demand for a tumor-specific marker for metastatic lymph nodes in sentinel navigation surgery for gastric cancer. The aim of this study is to analyze protein expression in both primary tumors and metastatic lymph nodes in early gastric cancer patients. MethodsWe collected primary tumors and metastatic lymph nodes from 71 patients who underwent curative gastrectomy and pathologically diagnosed with T1N1 or T1N2 (8th Union for International Cancer Control 8th edition/American Joint Committee on Cancer staging system) gastric cancer. Immunohistochemistry was used to determine the expression of six cell membrane proteins, including carcinoembryonic antigen (CEA), E-cadherin, epithelial cell adhesion molecule (EpCAM), P-cadherin, CD44v6, and c-erbB2 in the patient samples. ResultsThe expression of CEA, E-cadherin, EpCAM, P-cadherin, CD44v6 and c-erbB2 in the evaluable primary tumor samples was 75.4%, 97.1%, 100%, 89.9%, 11.1% and 7.2%, respectively. Among cases wherein both the primary tumor and metastatic lymph nodes were evaluable, double positivity (expression in both primary tumor and metastatic lymph nodes) was observed for CEA, E-cadherin, EpCAM, P-cadherin, CD44v6 and c-erbB2 in 53.2%, 97.9%, 98.1%, 76.6%, 0 and 6.8% of the cases, respectively. The proportion of metastatic lymph nodes positive for CEA, E-cadherin, EpCAM, P-cadherin, CD44v6 and c-erbB2 was 71.4%, 100%, 98.1%, 83.7%, 0, and 75%, respectively in primary tumors positive for the same markers. ConclusionsE-cadherin and EpCAM had an overlap of 100% and 98.1% between the primary tumor and metastatic lymph nodes, respectively. Thus, E-cadherin and EpCAM are potential molecular markers to detect metastatic lymph nodes in patients with early gastric cancer.
2020, 32(5): 621-630.
doi: 10.21147/j.issn.1000-9604.2020.05.07
Abstract:
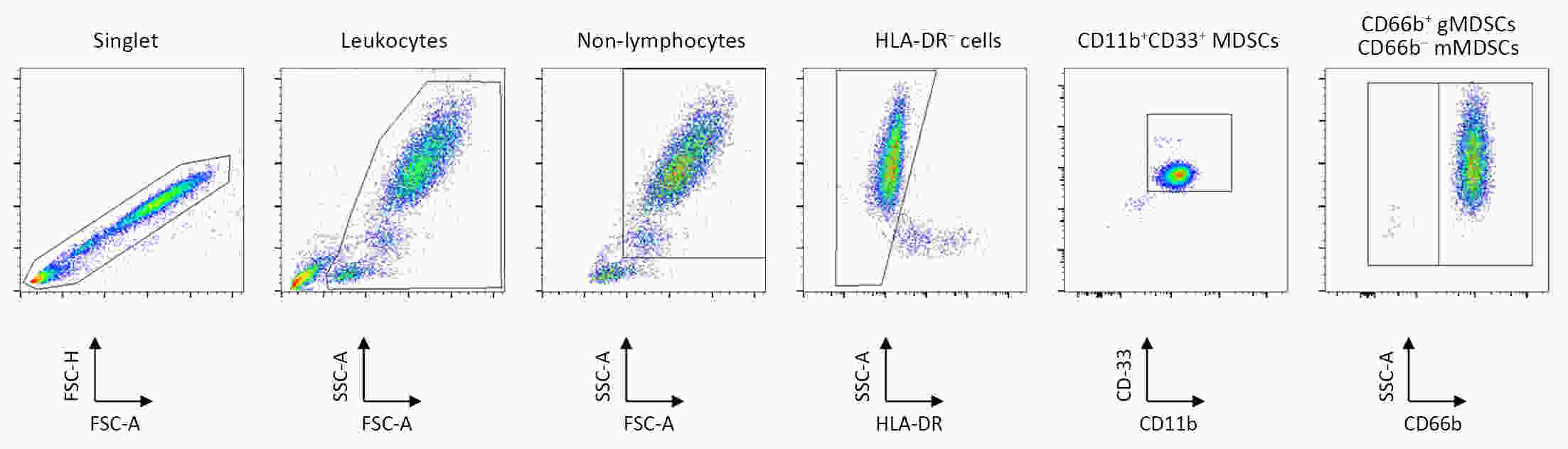
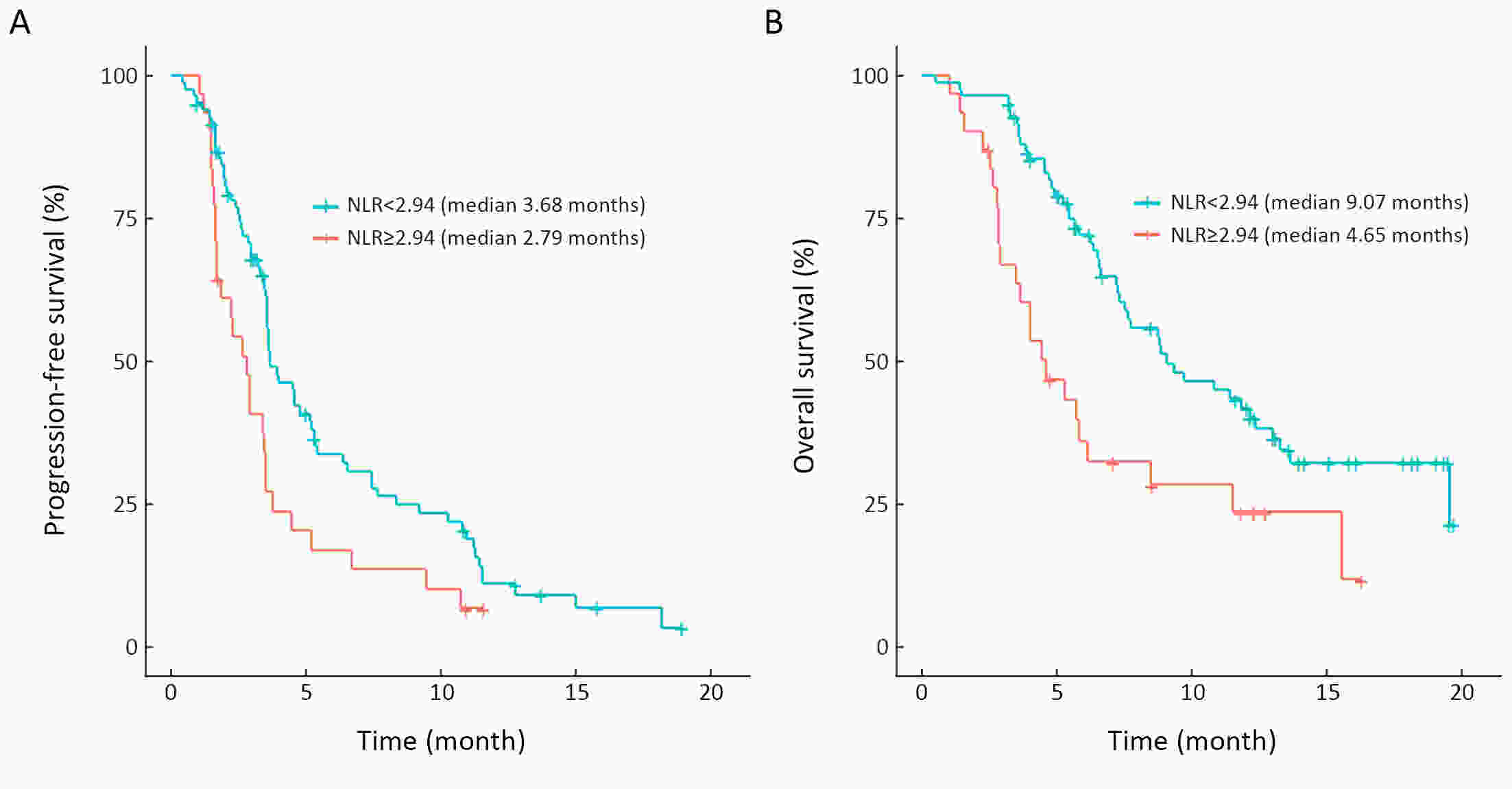
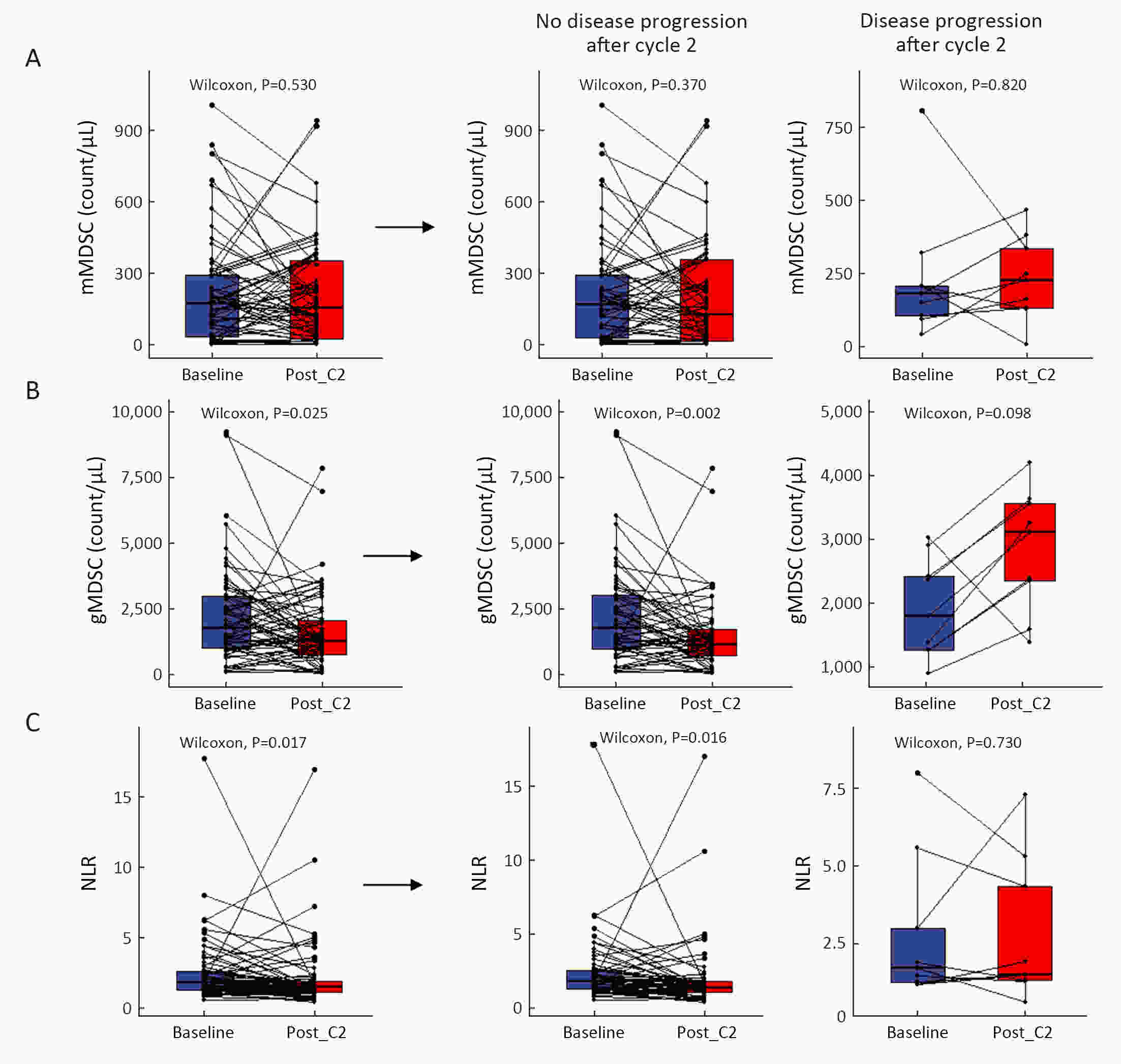
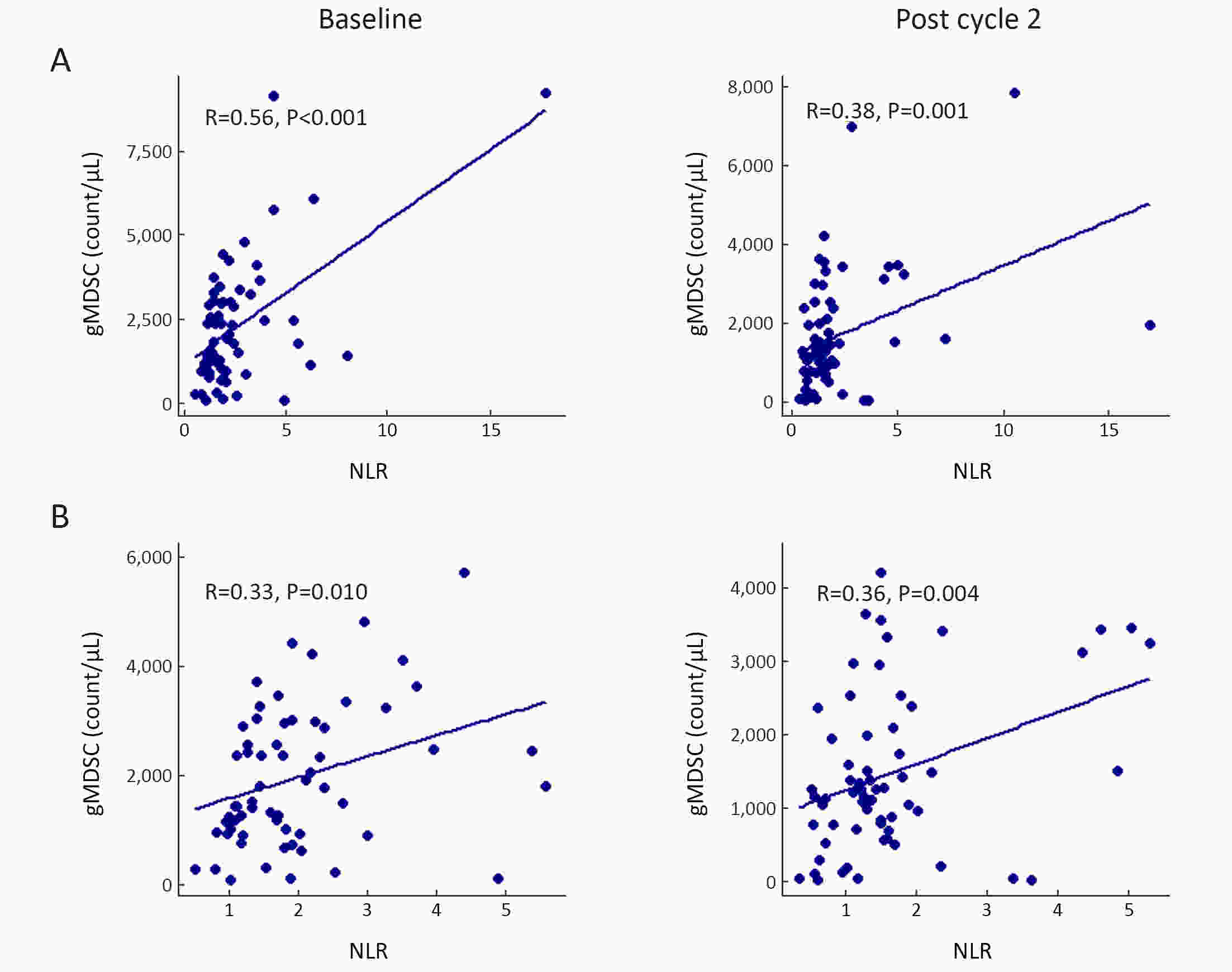
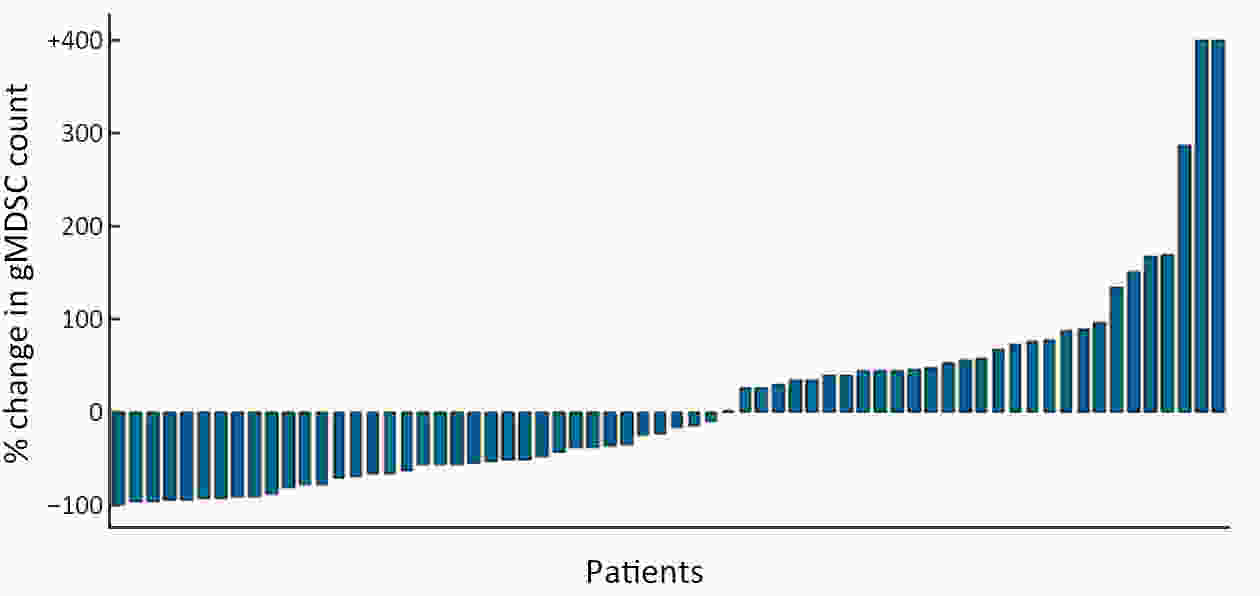
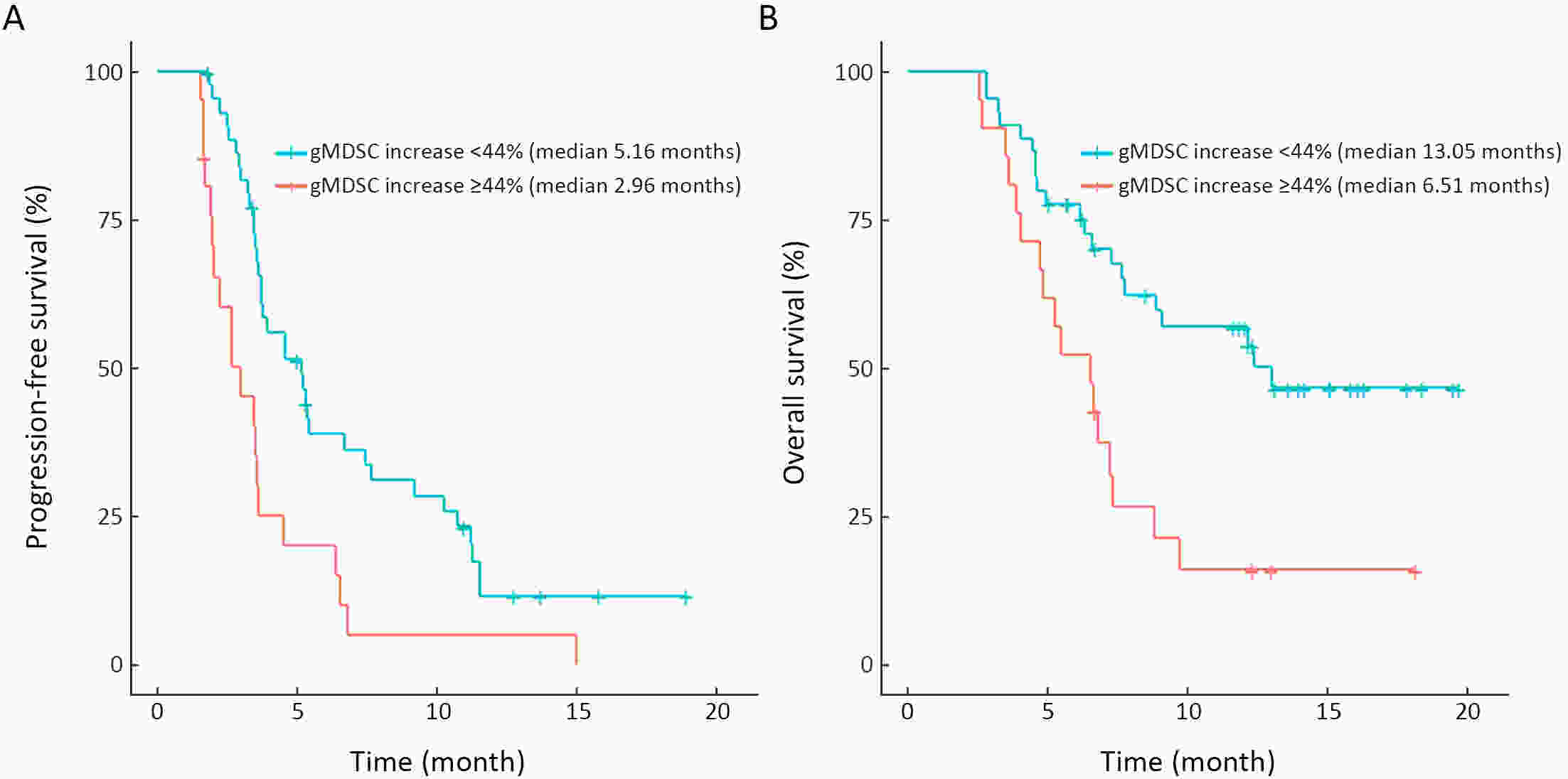
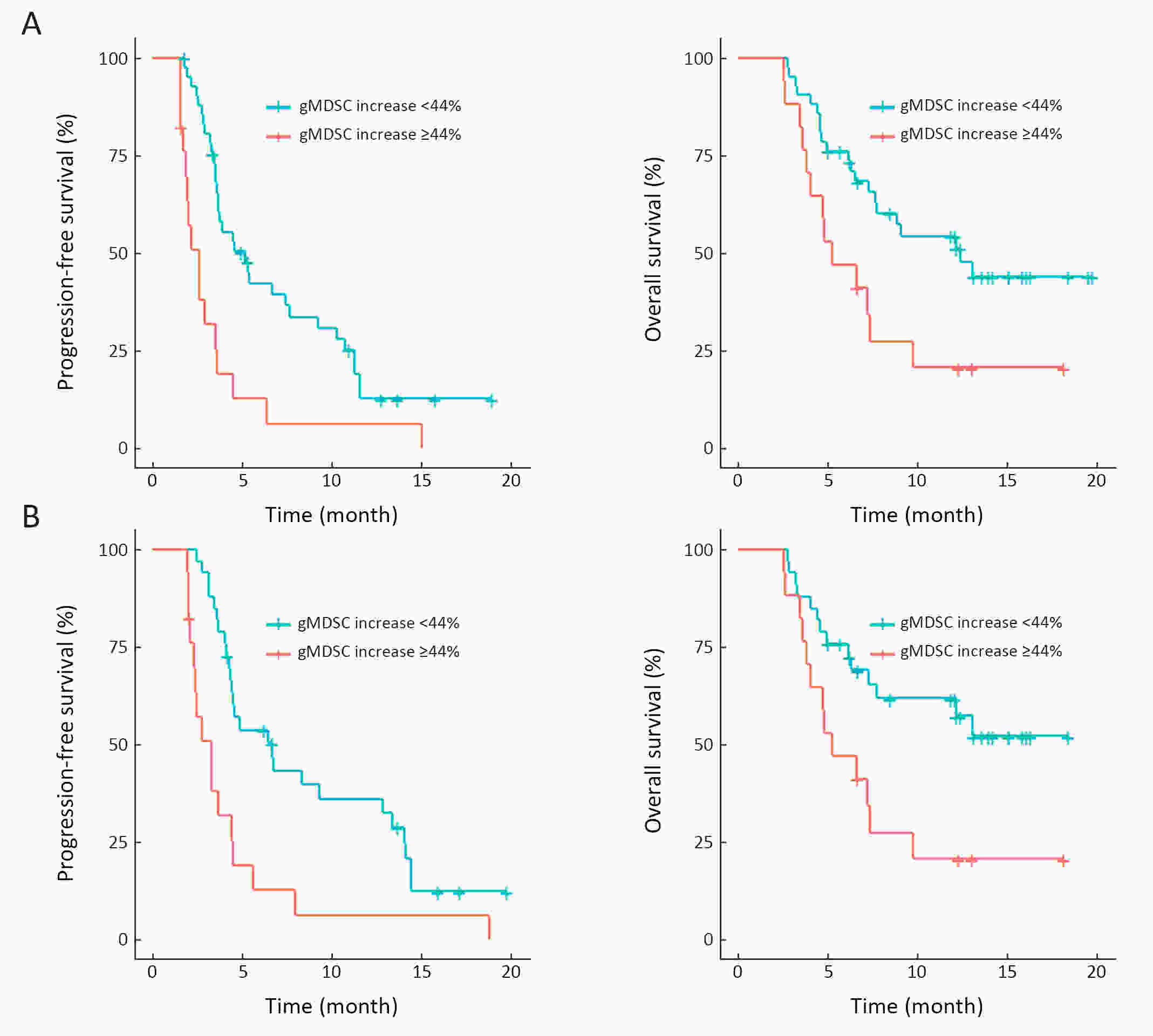
ObjectiveWe aimed to investigate the prognostic value of neutrophil-to-lymphocyte ratio (NLR) and myeloid-derived suppressor cells (MDSCs) in gastric cancer patients treated with second-line ramucirumab plus paclitaxel. MethodsA total of 116 patients with advanced or metastatic gastric cancer who receive ramucirumab plus paclitaxel were prospectively enrolled. Fresh blood samples were collected before and after treatment, and flow cytometry was performed to assess the proportions of monocytic (mMDSCs) and granulocytic MDSCs (gMDSCs). ResultsMedian age was 58 years and 71 (61.2%) patients were male. A baseline NLR≥2.94 was associated with significantly poorer progression-free survival (PFS) and overall survival (OS) vs. an NLR<2.94 (P=0.011 and P=0.002, respectively). In multivariate analysis, an NLR≥2.94 was independently associated with poorer PFS [hazard ratio (HR)=1.58; 95% confidence interval (95% CI): 1.01−2.49, P=0.046] and OS (HR=1.77; 95% CI: 1.04−3.04, P=0.036). While mMDSC counts did not significantly change following two cycles of therapy (P=0.530), gMDSC counts decreased significantly after two treatment cycles (P=0.025) but tended to increase in patients with progressive disease after two treatment cycles (P=0.098). A progressive increase in gMDSC counts (≥44%) was associated with a significantly shorter PFS and OS vs. a gMDSC count increase <44% (P=0.001 and P=0.003, respectively). ConclusionsThe baseline NLR may help guide clinical decisions during ramucirumab plus paclitaxel therapy for gastric cancer. Our gMDSC kinetics data warrant further clinical validation and mechanistic investigation.
2020, 32(5): 631-644.
doi: 10.21147/j.issn.1000-9604.2020.05.08
Abstract:
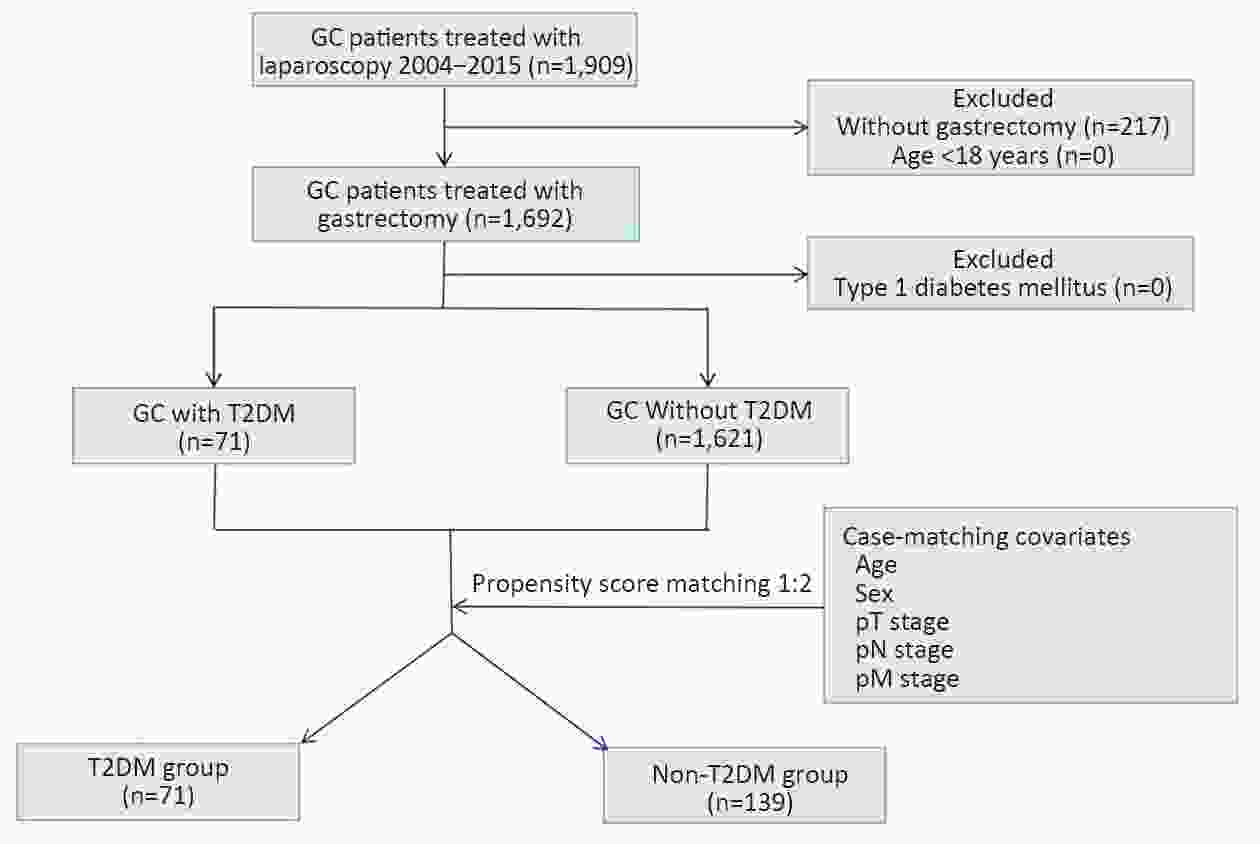
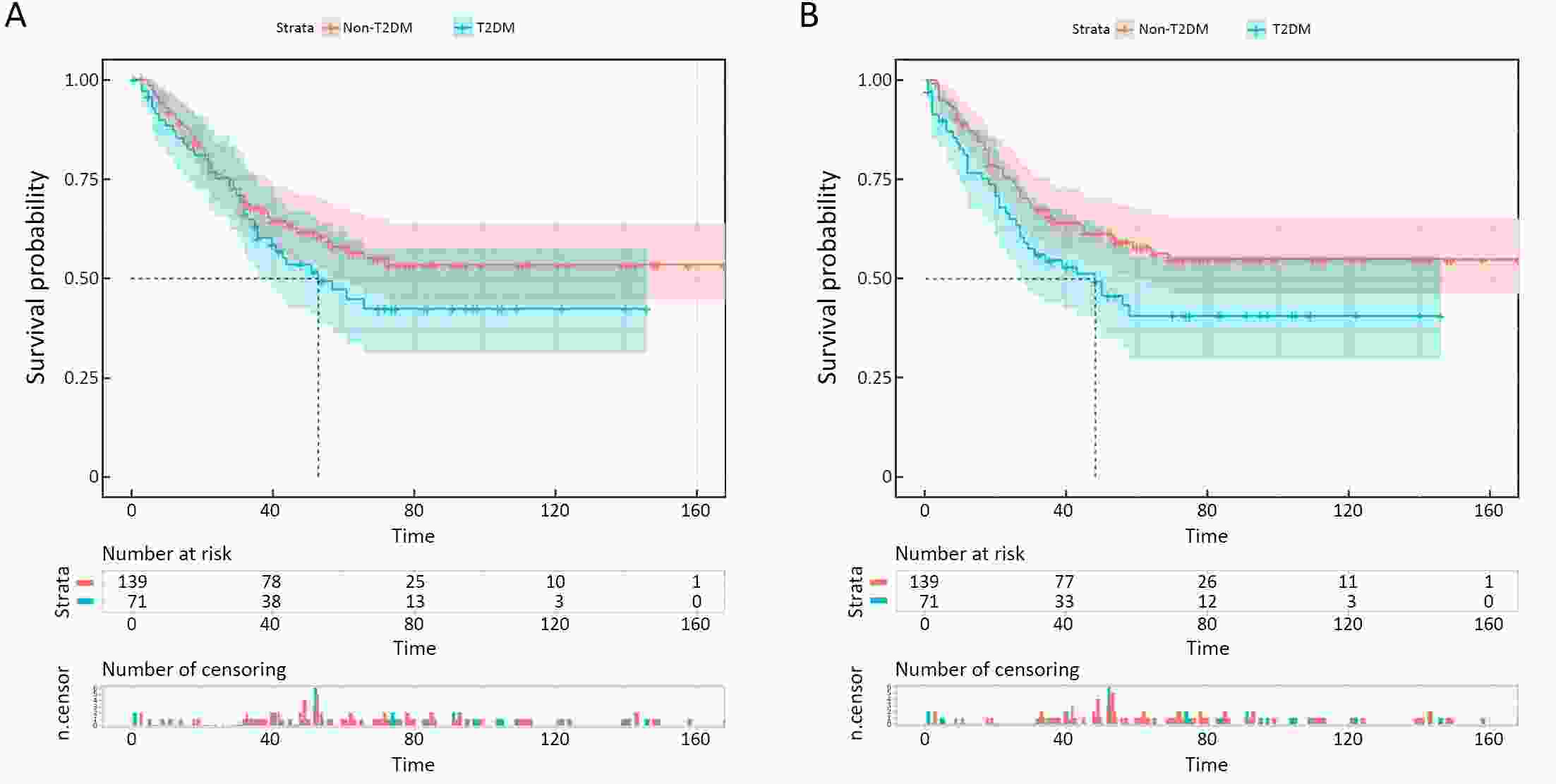
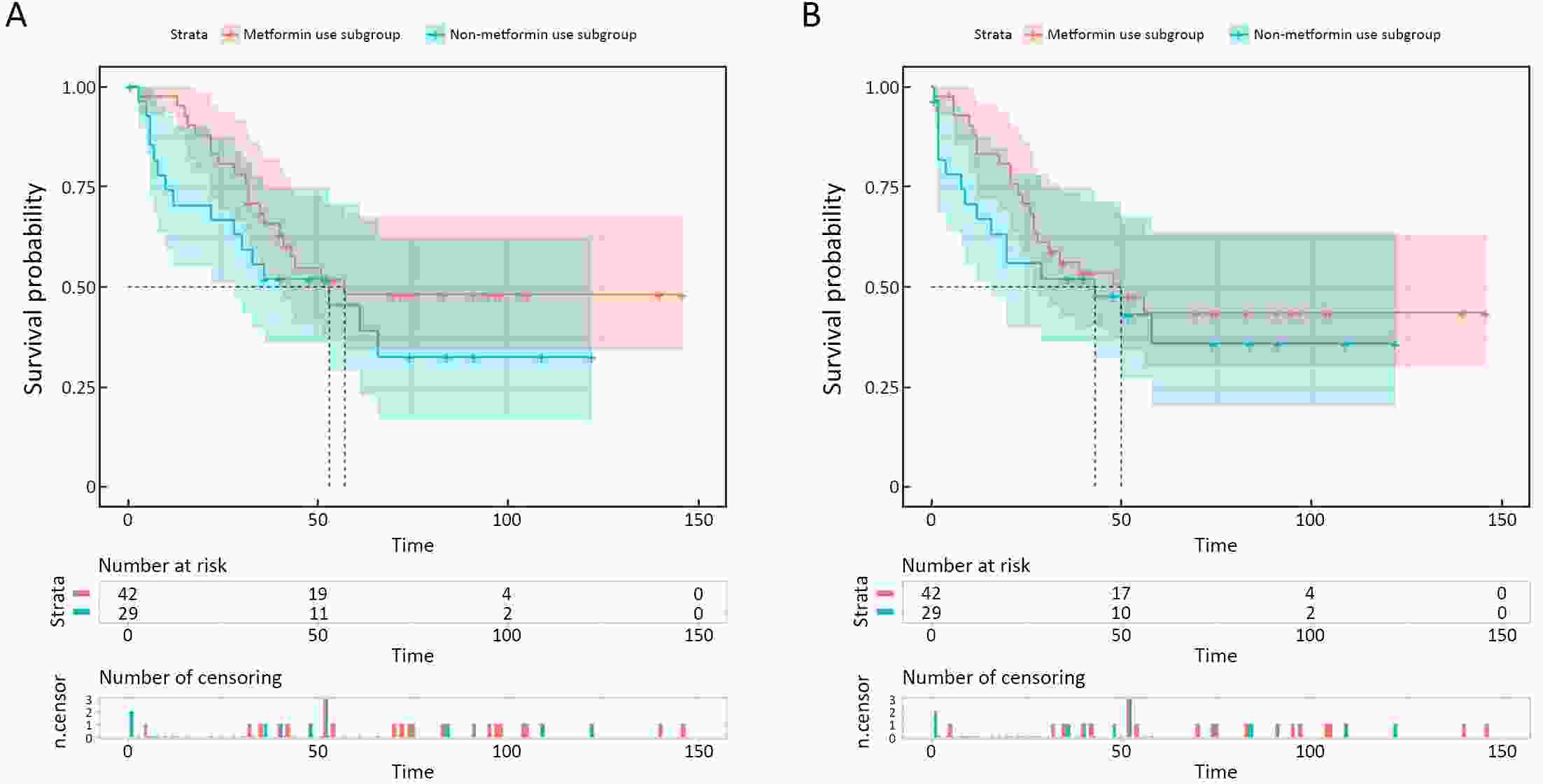
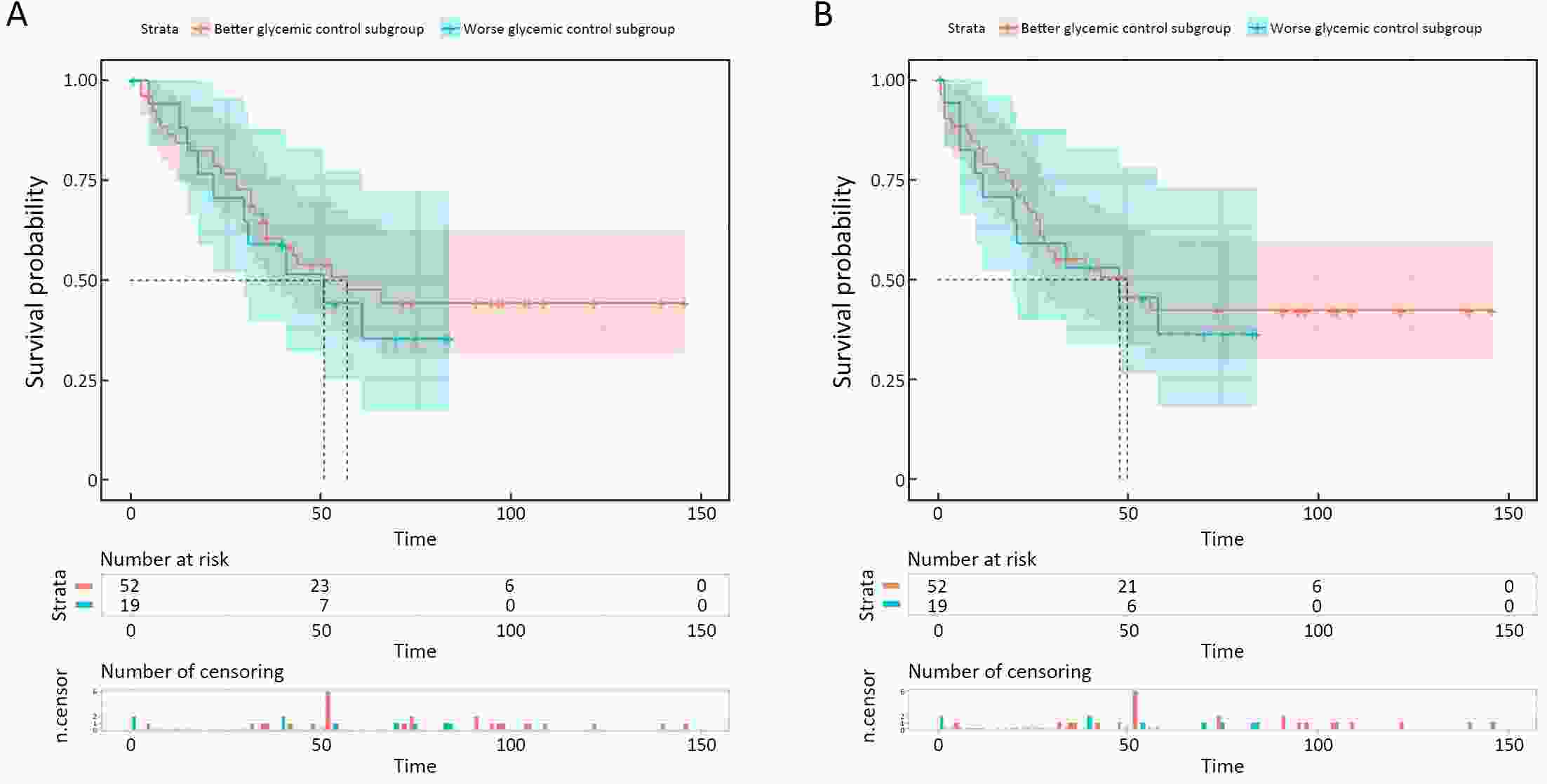
ObjectiveThis study aimed to determine the impact of type 2 diabetes mellitus (T2DM) on clinical outcomes of gastric cancer (GC) patients and explore whether metformin use and good glycemic control could reverse it. MethodsClinicopathologic data of consecutive GC patients who underwent gastrectomy at Nanfang Hospital between October 2004 and December 2015 were included. Propensity score matching (PSM) was performed to balance the important factors of the disease status between non-T2DM and T2DM group. The last follow-up time was January 2019. ResultsA total of 1,692 eligible patients (1,621 non-T2DM vs. 71 T2DM) were included. After PSM, non-T2DM group (n=139) and T2DM group (n=71) were more balanced in baseline variables. The 5-year cancer-specific survival (CSS) rate in T2DM group (47.0%) was inferior to that in non-T2DM group (58.0%), but did not reach statistical significance [hazard ratio (HR)=1.319, 95% confidence interval (95% CI): 0.868−2.005, P=0.192]. While the 5-year progress-free survival (PFS) rate of T2DM group (40.6%) is significantly worse than that in non-T2DM group (56.3%) (HR=1.516, 95% CI: 1.004−2.290, P=0.045). Univariate and multivariate analyses showed that T2DM was an independent risk factor for PFS but not for CSS. In T2DM group, metformin use subgroup was associated with superior 5-year CSS and PFS in compared with non-metformin use subgroup, although the difference was not statistically significant (5-year CSS: 48.0% vs. 45.4%, HR=0.680, 95% CI: 0.352−1.313, P=0.246; 5-year PFS: 43.5% vs. 35.7%, HR=0.763, 95% CI: 0.400−1.454, P=0.406). The 5-year CSS rate was 47.5% in good glycemic control subgroup and 44.1% in poor glycemic control subgroup (HR=0.826, 95% CI: 0.398−1.713, P=0.605). And both two subgroups yielded a similar 5-year PFS rate (42.2% vs. 36.3%, HR=0.908, 95% CI: 0.441−1.871, P=0.792). ConclusionsDM promoted disease progress of GC after gastrectomy but had not yet led to the significant discrepancy of CSS. For GC patients with T2DM, metformin use was associated with superior survival but without statistical significance, while better glycemic control could not improve the prognosis.
2020, 32(5): 645-653.
doi: 10.21147/j.issn.1000-9604.2020.05.09
Abstract:
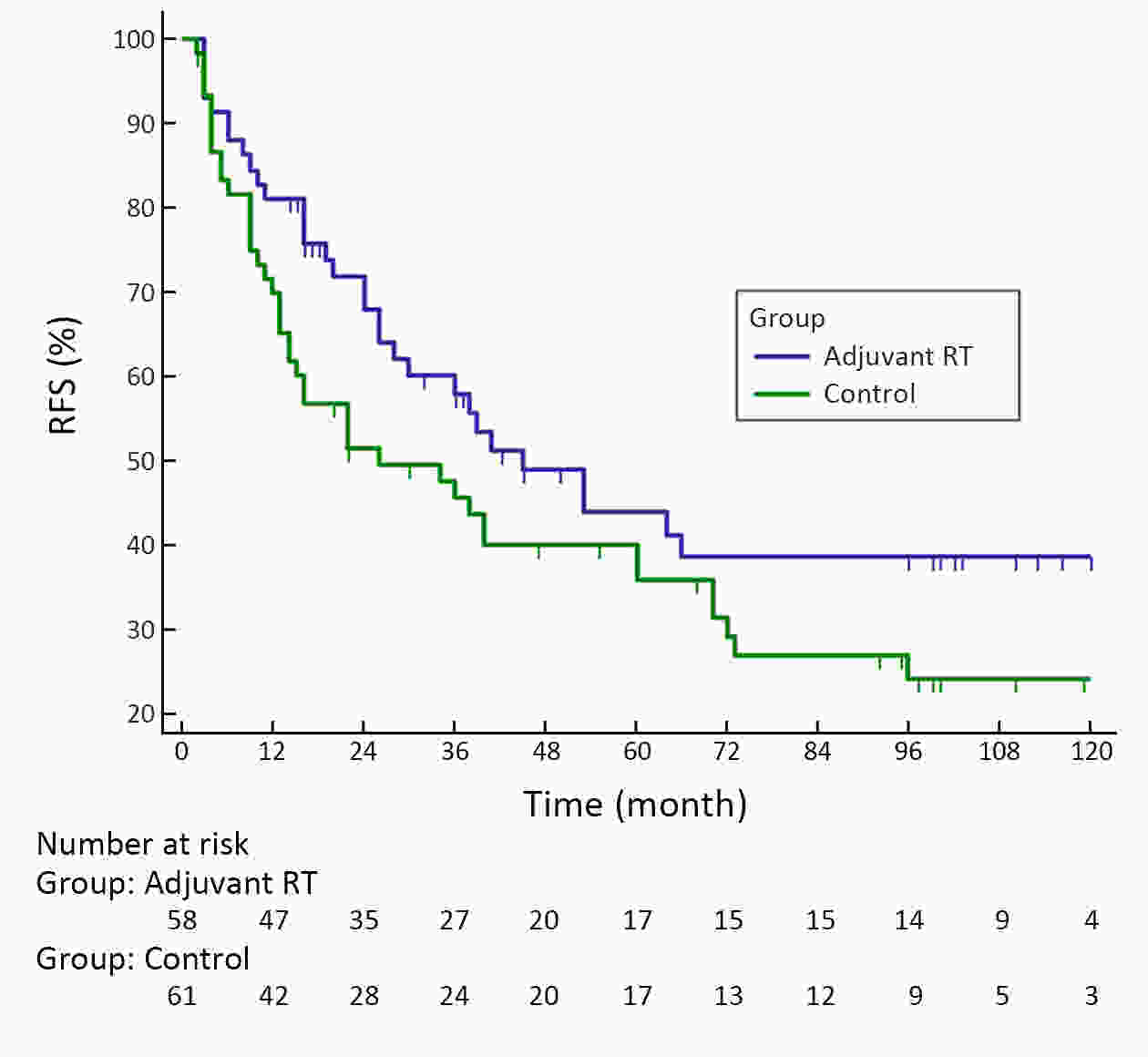
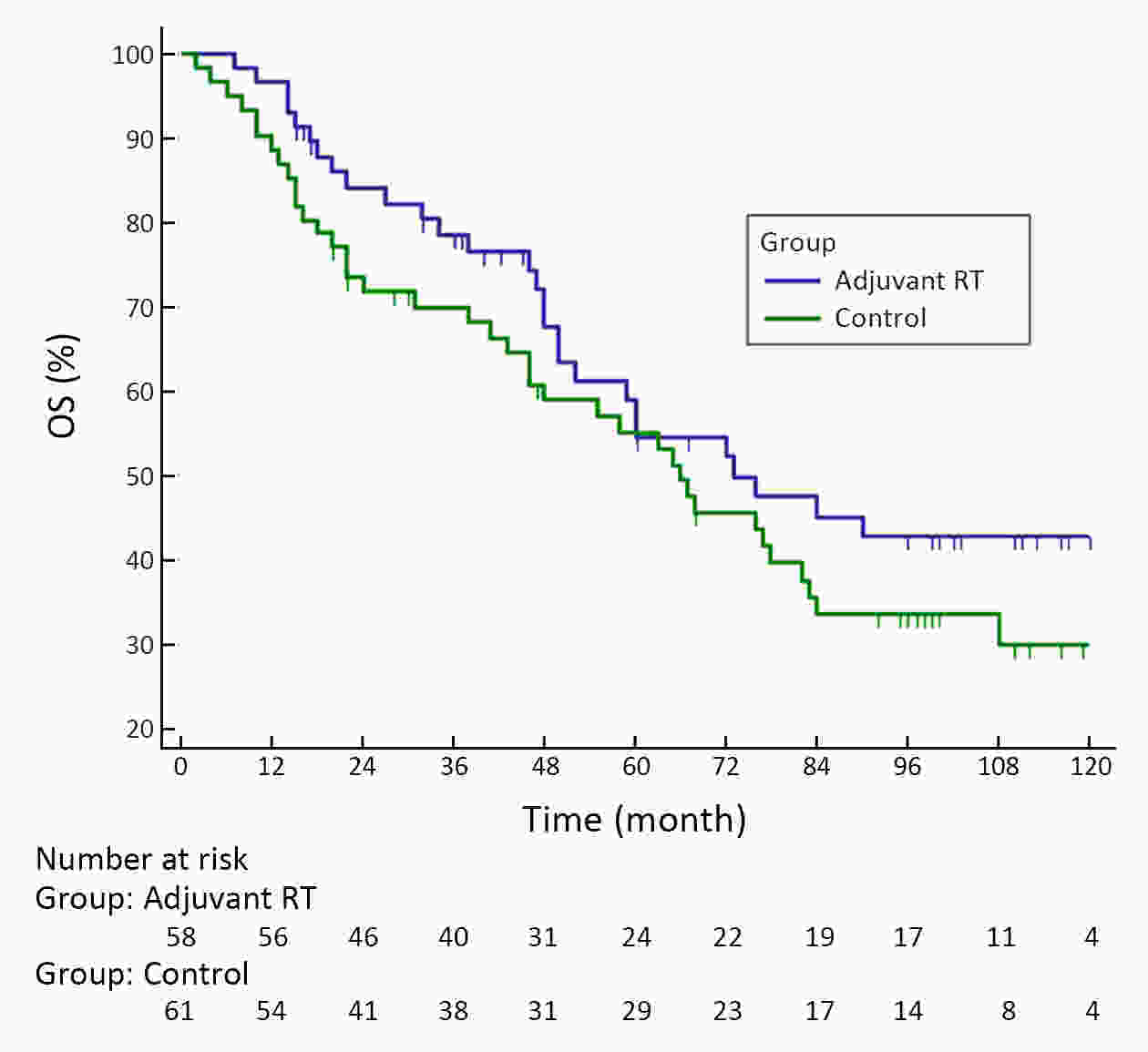
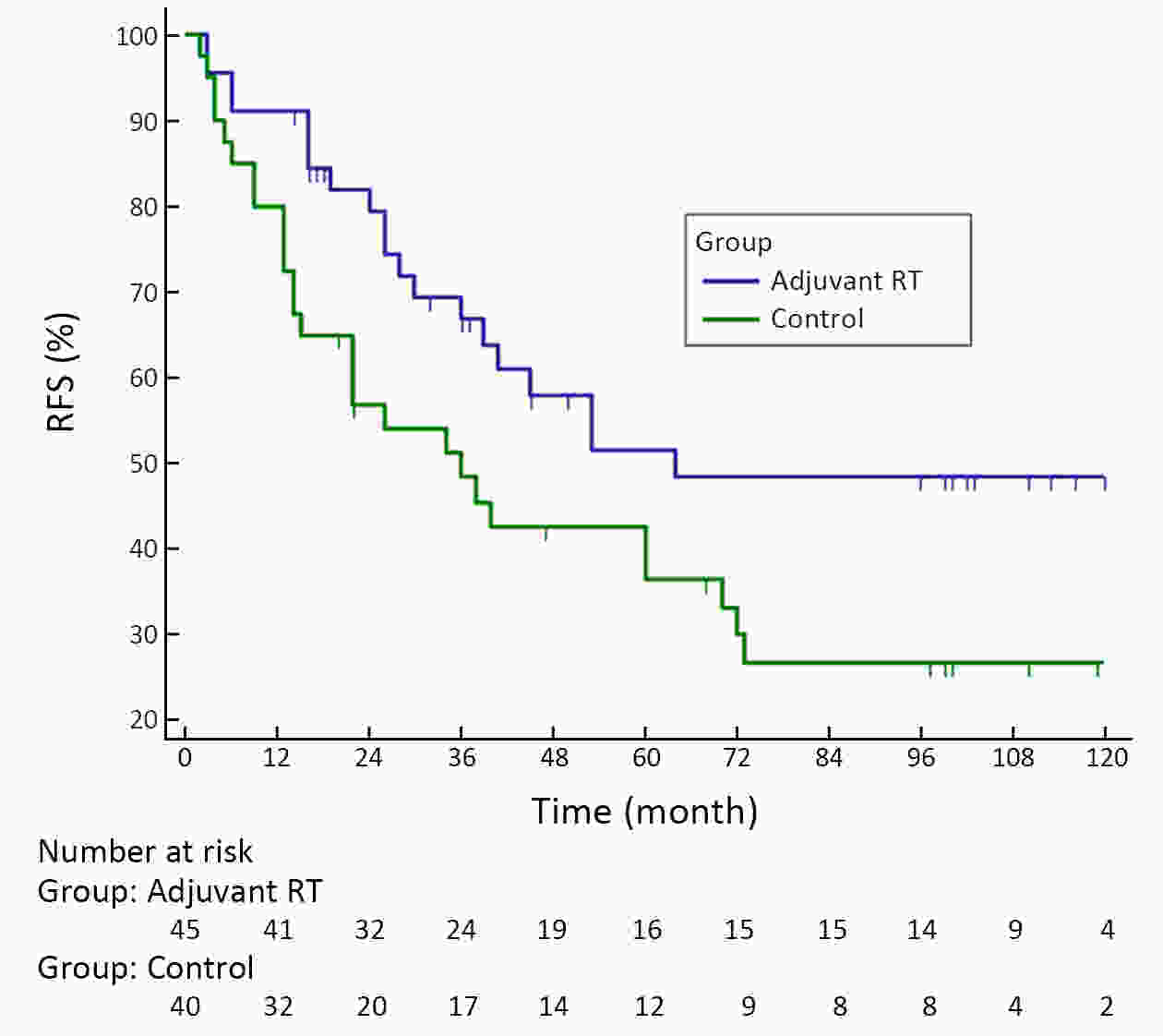
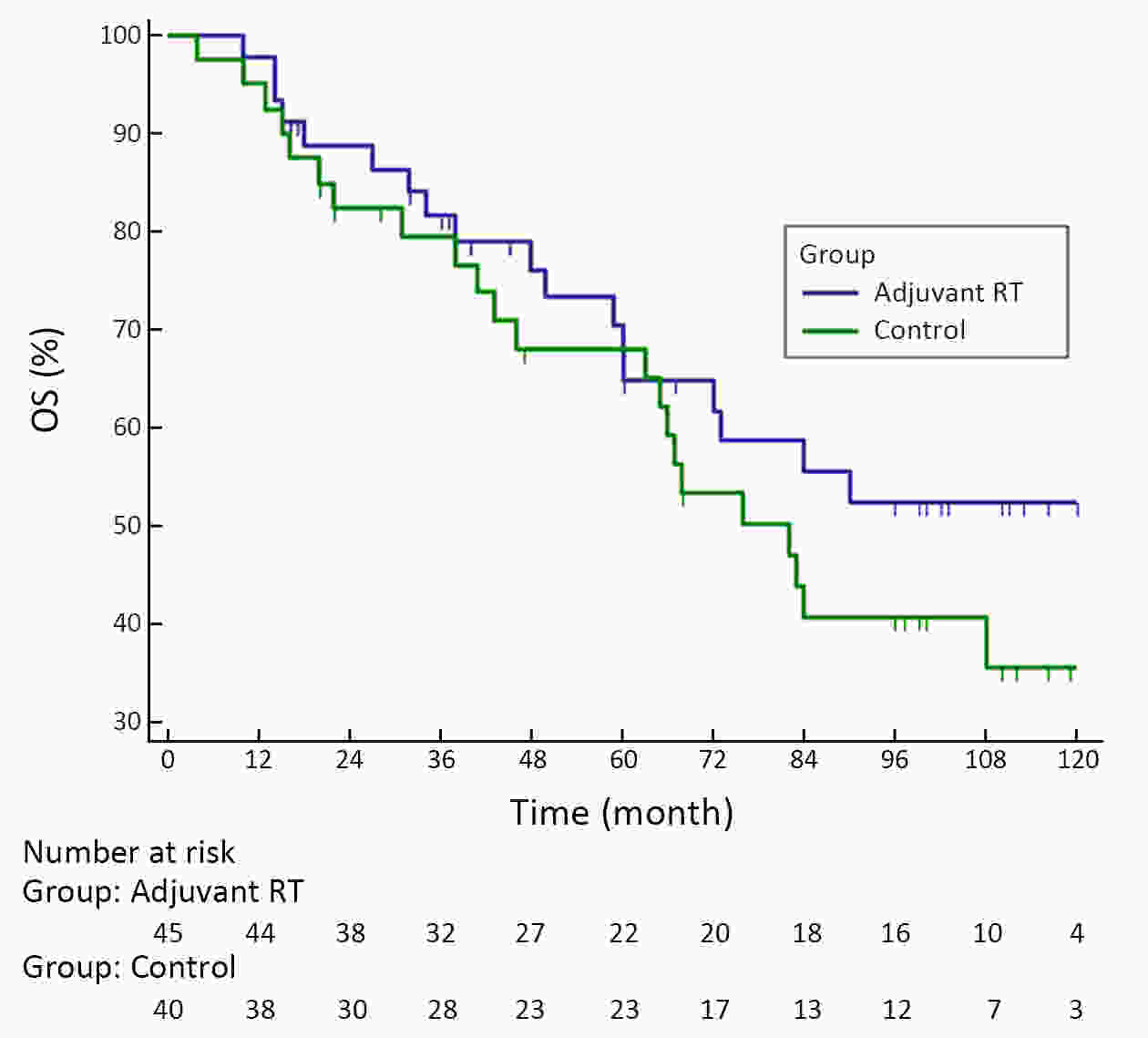
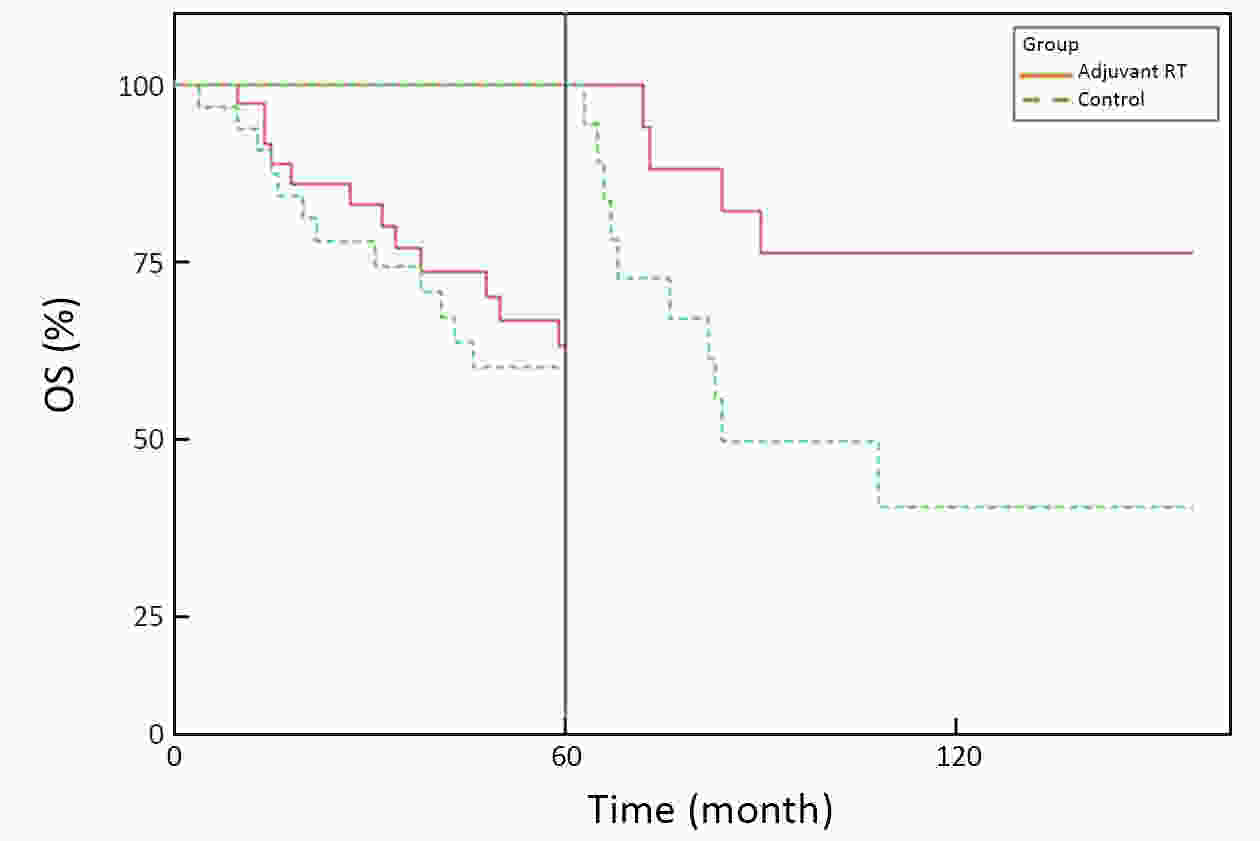
ObjectiveA prospective randomized control study investigated the feasibility and efficacy of adjuvant radiotherapy on patients with central hepatocellular carcinoma (HCC) after narrow-margin hepatectomy (<1 cm). This study presents an updated 10-year real-world evidence to further characterize the role of adjuvant radiotherapy. MethodsPatients with central HCC after narrow-margin hepatectomy (<1 cm) were prospectively assigned to adjuvant radiotherapy group and control group. Patients’ outcome, adverse events, long-term recurrence and survival rates were investigated. ResultsThe 1-, 5-, and 10-year recurrence-free survival (RFS) rates were 81.0%, 43.9%, and 38.7%, respectively in adjuvant radiotherapy group and 71.7%, 35.8%, and 24.2%, respectively in control group (log-rank test, P=0.09). The 1-, 5-, and 10-year overall survival (OS) rates were 96.6%, 54.7%, and 42.8%, respectively in adjuvant radiotherapy group and 90.2%, 55.1%, and 30.0%, respectively in control group (log-rank test, P=0.20). The 1-, 5-, and 10-year RFS rates for patients with small HCC (≤5 cm) were 91.1%, 51.6%, and 48.4%, respectively in adjuvant radiotherapy group and 80.0%, 36.6%, and 26.6%, respectively in control group (log-rank test, P=0.03). Landmark analysis demonstrated that patients with small HCC in adjuvant radiotherapy group had a significantly improved OS in second five years after treatment in comparison to patients in control group (log-rank test, P=0.05). ConclusionsOur updated results showed a sustained clinical benefit on reducing recurrence, improving long-term survival for small central HCC by adjuvant radiotherapy after narrow-margin hepatectomy. Long-term survival data also indicated that hepatectomy is an optimal treatment for selected patients with central HCC.
2020, 32(5): 654-664.
doi: 10.21147/j.issn.1000-9604.2020.05.10
Abstract:
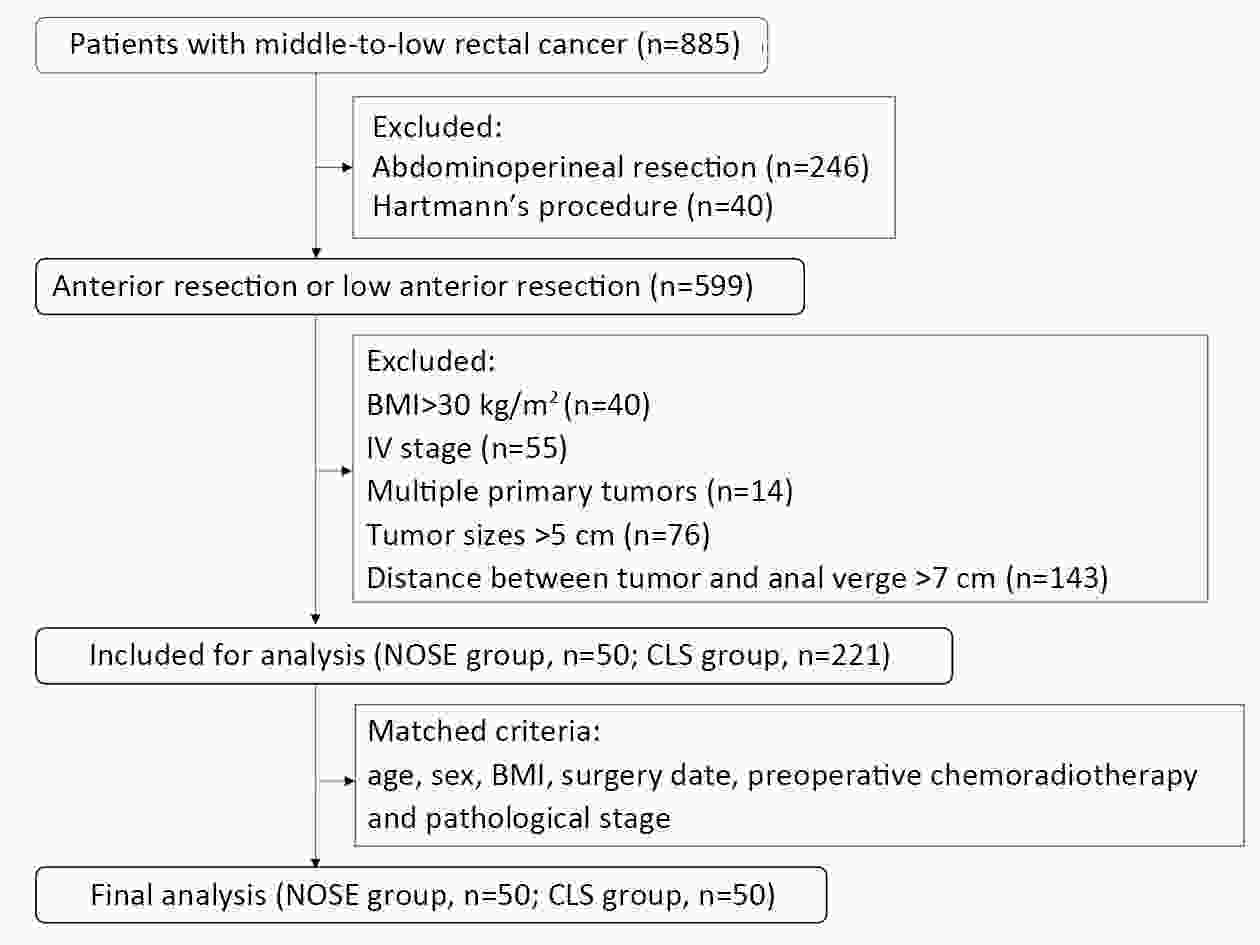
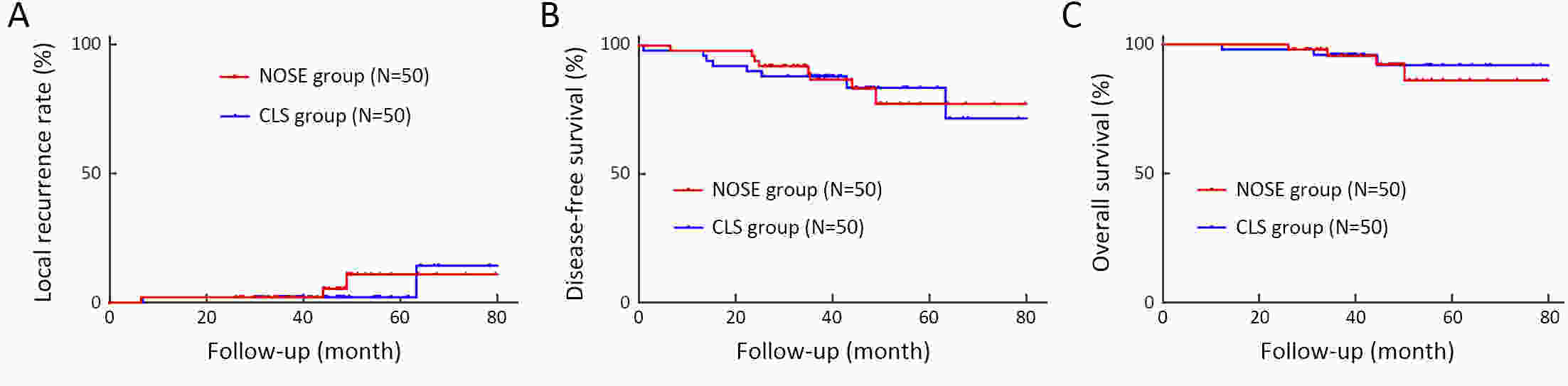
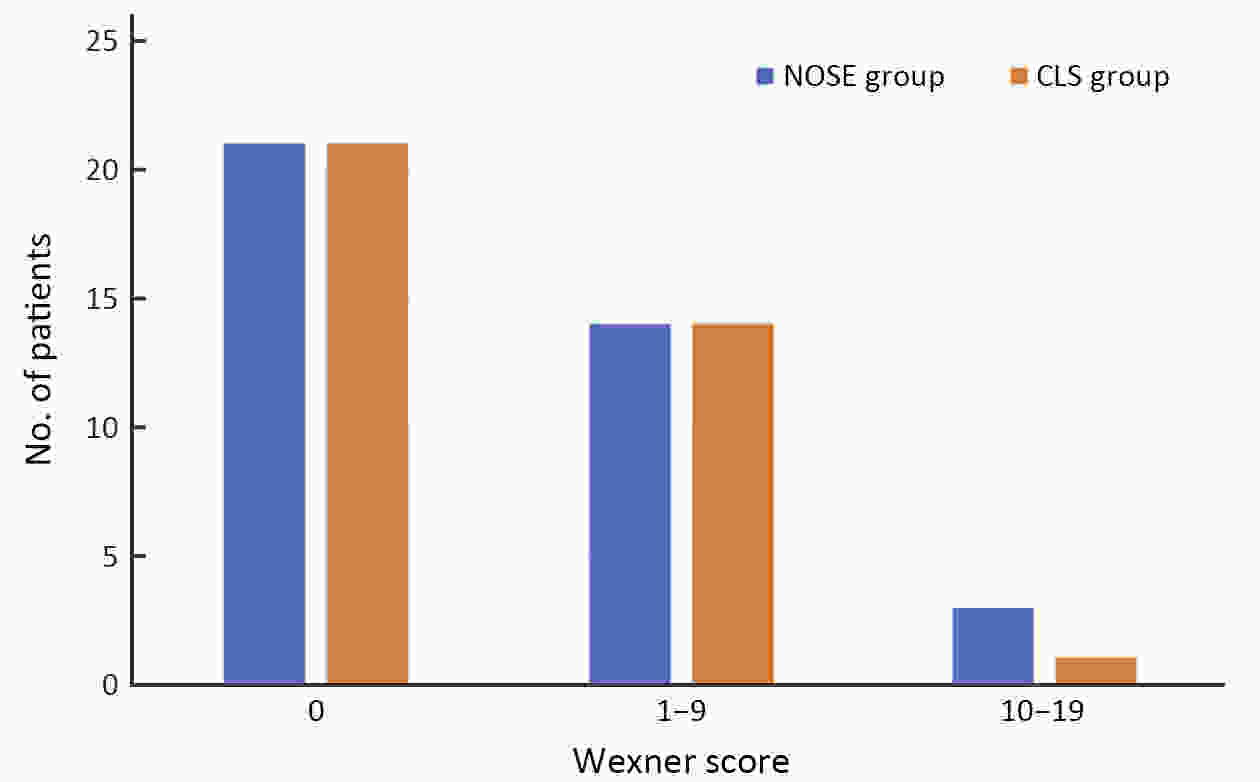
ObjectiveThe transanal approach to specimen collection, combined with the prolapsing technique, is a well-established and minimally invasive surgery for treating rectal cancer. However, reports on outcomes for this approach are sparse. We compared short- and long-term outcomes of conventional laparoscopic surgery (CLS) vs. transanal natural orifice specimen extraction (NOSE) using the prolapsing technique for patients with middle- to low-rectal cancer. MethodsFrom January 2013 to December 2017, we enrolled consecutive patients with middle- to low-rectal cancer undergoing laparoscopic anterior resection. Totally, 50 patients who underwent transanal NOSE using the prolapsing technique were matched with 50 patients who received CLS. Clinical parameters and survival outcomes between the two groups were compared. ResultsEstimated blood loss (29.70±29.28 vs. 52.80±45.09 mL, P=0.003), time to first flatus (2.50±0.79 vs. 2.86±0.76, P=0.022), time to liquid diet (3.62±0.64 vs. 4.20±0.76 d, P<0.001), and the need for analgesics (22% vs. 48%, P=0.006) were significantly lower for the NOSE group compared to the CLS group. The incidences of overall complications and fecal incontinence were comparable in both groups. After a median follow-up of 44.52 months, the overall local recurrence rate (6% vs. 5%, P=0.670), 3-year disease-free survival (86.7% vs. 88.0%, P=0.945) and 3-year overall survival (95.6% vs. 96.0%, P=0.708), were not significantly different. ConclusionsFor total laparoscopic rectal resection, transanal NOSE using the prolapsing technique is effective and safe, and associated with less trauma and pain, a faster recovery, and similar survival outcomes compared to CLS.
2020, 32(5): 665-672.
doi: 10.21147/j.issn.1000-9604.2020.05.11
Abstract:
ObjectiveFor locally advanced nasopharyngeal carcinoma (LA-NPC) patients, high incidences of distant metastases and severe treatment related toxicities are the main obstacles needed to be overcome. Raltitrexed, a specific thymidylate synthase inhibitor with a convenient administration schedule, has an acceptable and manageable toxicity, and possesses radio-sensitizing properties. To investigate the efficacy and safety of raltitrexed and cisplatin induction chemotherapy and concurrent chemoradiotherapy (IC+CCRT) in patients with LA-NPC, a phase II clinical study was conducted. MethodsSixty eligible patients with LA-NPC were enrolled into this study. A raltitrexed-cisplatin combination was used as part of an IC+CCRT regimen. Raltitrexed-cisplatin IC was given once every 3 weeks (q3w) for two cycles, followed by raltitrexed-cisplatin based CCRT q3w for two cycles. Intensity-modulated radiotherapy (IMRT) was given for all enrolled patients. ResultsAll patients were included in survival analysis according to the intent-to-treat principle. The objective response rate (ORR) 3 months after treatment was 98%. The 2-year overall survival (OS) rate was 92%. The median relapse-free survival (RFS) time was 30.5 [95% confidence interval (95% CI), 28.4−32.3] months. The 2-year RFS rate was 85%. The 2-year local failure-free survival (LFFS) rate was 97% and the 2-year distant metastasis-free survival (DMFS) rate was 88%. Acute toxicities were mostly grade 2 and 3 reactions in bone marrow suppression, gastrointestinal side effect and oropharyngeal mucositis. Only two patients occurred grade 4 acute toxicities, one was bone marrow suppression and the other was dermatitis radiation. ConclusionsThe combination of raltitrexed and cisplatin has a comparable efficacy to those in standard first-line therapy.

 Abstract
Abstract FullText HTML
FullText HTML PDF 5702KB
PDF 5702KB