2021 Vol.33(2)
Display Mode: |
2021, 33(2): 133-141.
doi: 10.21147/j.issn.1000-9604.2021.02.01
Abstract:
Gastric cancer is still a major cause of death worldwide. While laparoscopic gastrectomy (LG) has gained evidence as a standard treatment for early gastric cancer in the distal stomach, there are still concerns regarding its application for gastric cancer in the upper stomach and advanced gastric cancer. Nevertheless, LG has shown to have faster recovery, shorter hospital stay, less pain, and less blood loss in many retrospective and prospective studies. The application of LG has now extended from conventional radical gastrectomy to novel approaches such as function-preserving gastrectomy and sentinel-node navigated surgery. Studies on the use of laparoscopy in treatment for stage IV gastric cancer are rare, but show that there may be some roles of LG in selected cases. With the development of new laparoscopic tools that augment human ability, the future of LG should move on from proving non-inferiority to demonstrating superiority compared to the traditional open gastrectomy.
Gastric cancer is still a major cause of death worldwide. While laparoscopic gastrectomy (LG) has gained evidence as a standard treatment for early gastric cancer in the distal stomach, there are still concerns regarding its application for gastric cancer in the upper stomach and advanced gastric cancer. Nevertheless, LG has shown to have faster recovery, shorter hospital stay, less pain, and less blood loss in many retrospective and prospective studies. The application of LG has now extended from conventional radical gastrectomy to novel approaches such as function-preserving gastrectomy and sentinel-node navigated surgery. Studies on the use of laparoscopy in treatment for stage IV gastric cancer are rare, but show that there may be some roles of LG in selected cases. With the development of new laparoscopic tools that augment human ability, the future of LG should move on from proving non-inferiority to demonstrating superiority compared to the traditional open gastrectomy.
2021, 33(2): 142-149.
doi: 10.21147/j.issn.1000-9604.2021.02.02
Abstract:
With the increase in the incidence of early gastric cancer (EGC), several endoscopic and laparoscopic approaches, such as endoscopic submucosal dissection and function-preserving gastrectomy, have been accepted as standard treatments. Sentinel node navigation surgery (SNNS) is an ideal surgical option for preservation of most parts of the stomach and consequent maintenance of normal gastric function to improve quality of life in patients with EGC. Although many previous studies and clinical trials have demonstrated the safety and feasibility of the sentinel node concept in gastric cancer, the clinical application of SNNS is debatable. Several issues regarding technical standardization and oncological safety need to be resolved. Recently several studies to resolve these problems are being actively performed, and SNNS might be an important surgical option in the treatment of gastric cancer in the future.
With the increase in the incidence of early gastric cancer (EGC), several endoscopic and laparoscopic approaches, such as endoscopic submucosal dissection and function-preserving gastrectomy, have been accepted as standard treatments. Sentinel node navigation surgery (SNNS) is an ideal surgical option for preservation of most parts of the stomach and consequent maintenance of normal gastric function to improve quality of life in patients with EGC. Although many previous studies and clinical trials have demonstrated the safety and feasibility of the sentinel node concept in gastric cancer, the clinical application of SNNS is debatable. Several issues regarding technical standardization and oncological safety need to be resolved. Recently several studies to resolve these problems are being actively performed, and SNNS might be an important surgical option in the treatment of gastric cancer in the future.
2021, 33(2): 150-158.
doi: 10.21147/j.issn.1000-9604.2021.02.03
Abstract:
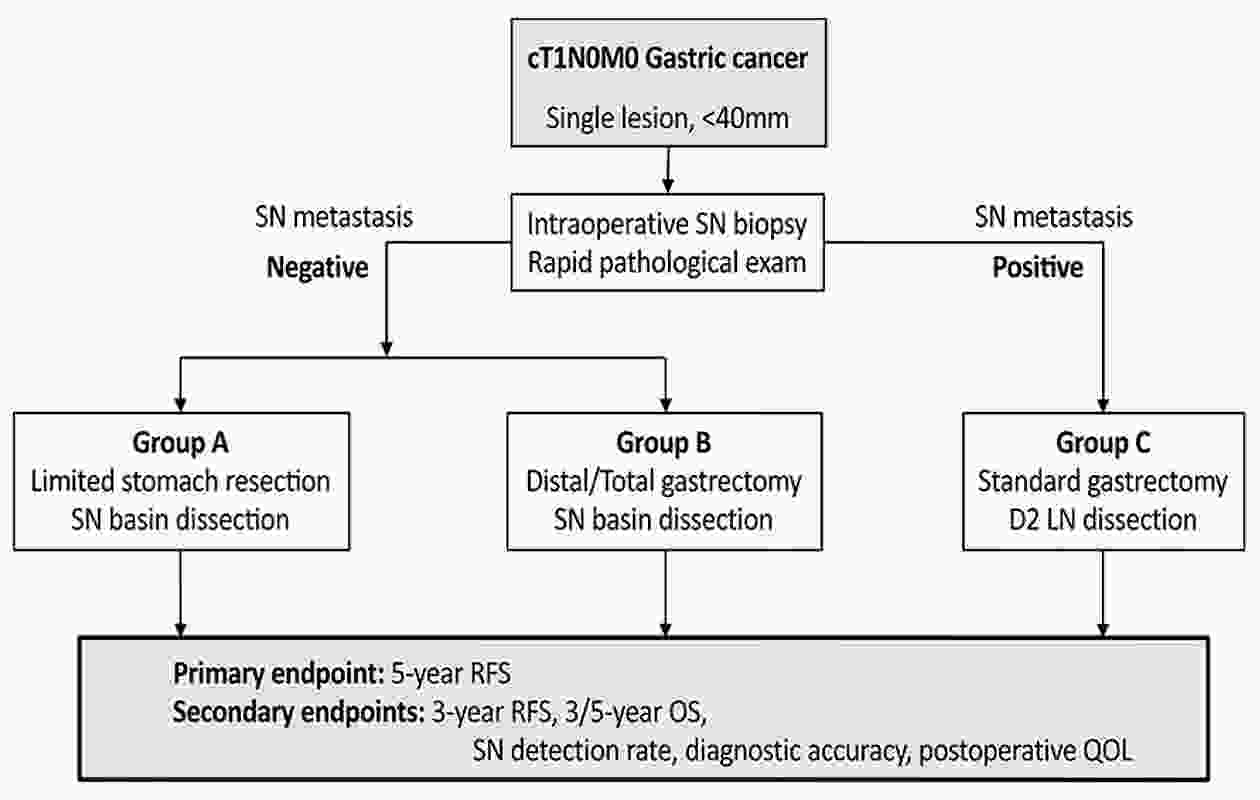
As an optimal surgical procedure to accurately evaluate lymph node (LN) metastasis during surgery with minimal surgical resection, we have been developing sentinel node (SN) biopsy for early gastric cancer since the 1990s. Twelve institutions from the Japanese Society of Sentinel Node Navigation Surgery (SNNS), including Keio University Hospital, conducted a multicenter prospective trial to validate the SN concept using the dual-tracer method with blue dye and a radioisotope. According to the results, 397 patients were included in the final analysis, and the overall accuracy in detecting LN metastasis using SN biopsy was 99% (383 of 387). Based on the validation study, we are targeting cT1N0 with a primary tumor of ≤4 cm in diameter as an indication for SN biopsy for gastric cancer. We are currently running a multicenter nonrandomized phase III trial to assess the safety and efficacy of SN navigation surgery. The Korean group has reported the result of a multicenter randomized phase III trial. Since meticulous gastric cancer in the remnant stomach was rescued by subsequent gastrectomy, the disease-specific survival was comparable between the two techniques, implying that SN navigation surgery can be an alternative to standard gastrectomy. With the development of SN biopsy procedure and treatment modalities, the application of SN biopsy will be expanded to achieve an individualized minimally invasive surgery.
As an optimal surgical procedure to accurately evaluate lymph node (LN) metastasis during surgery with minimal surgical resection, we have been developing sentinel node (SN) biopsy for early gastric cancer since the 1990s. Twelve institutions from the Japanese Society of Sentinel Node Navigation Surgery (SNNS), including Keio University Hospital, conducted a multicenter prospective trial to validate the SN concept using the dual-tracer method with blue dye and a radioisotope. According to the results, 397 patients were included in the final analysis, and the overall accuracy in detecting LN metastasis using SN biopsy was 99% (383 of 387). Based on the validation study, we are targeting cT1N0 with a primary tumor of ≤4 cm in diameter as an indication for SN biopsy for gastric cancer. We are currently running a multicenter nonrandomized phase III trial to assess the safety and efficacy of SN navigation surgery. The Korean group has reported the result of a multicenter randomized phase III trial. Since meticulous gastric cancer in the remnant stomach was rescued by subsequent gastrectomy, the disease-specific survival was comparable between the two techniques, implying that SN navigation surgery can be an alternative to standard gastrectomy. With the development of SN biopsy procedure and treatment modalities, the application of SN biopsy will be expanded to achieve an individualized minimally invasive surgery.
2021, 33(2): 159-167.
doi: 10.21147/j.issn.1000-9604.2021.02.04
Abstract:
Surgery is the most important and effective method for the treatment of gastric cancer. Since the first gastrectomy in the early 19th century, surgical treatment of gastric cancer has undergone more than 100 years of development. With the increasing understanding of gastric cancer and the promotion of a series of clinical trials, the concept of gastric cancer surgery has evolved from the initial “bigger is better” to today’s “standardized surgery” and is developing towards individualized surgery focusing on accurate resection and quality of life. This trend has had a tremendous impact on the development of surgical treatments, such as minimally invasive surgeries, function-preserving surgeries, and the optimal extent of lymph node dissection. Understanding the development and current status of gastric cancer surgery and exploring the remaining academic controversies are goals that every gastric surgeon should constantly pursue. However, how should gastric cancer surgery develop in the future? What opportunities and challenges will we encounter? In this review, we elaborate on the development and current status of gastric cancer surgery based on a series of clinical studies and discuss the controversy in the development of gastric cancer surgery.
Surgery is the most important and effective method for the treatment of gastric cancer. Since the first gastrectomy in the early 19th century, surgical treatment of gastric cancer has undergone more than 100 years of development. With the increasing understanding of gastric cancer and the promotion of a series of clinical trials, the concept of gastric cancer surgery has evolved from the initial “bigger is better” to today’s “standardized surgery” and is developing towards individualized surgery focusing on accurate resection and quality of life. This trend has had a tremendous impact on the development of surgical treatments, such as minimally invasive surgeries, function-preserving surgeries, and the optimal extent of lymph node dissection. Understanding the development and current status of gastric cancer surgery and exploring the remaining academic controversies are goals that every gastric surgeon should constantly pursue. However, how should gastric cancer surgery develop in the future? What opportunities and challenges will we encounter? In this review, we elaborate on the development and current status of gastric cancer surgery based on a series of clinical studies and discuss the controversy in the development of gastric cancer surgery.
2021, 33(2): 168-180.
doi: 10.21147/j.issn.1000-9604.2021.02.05
Abstract:
Gastric cancer (GC) is one of the major cancers in China and all over the world. Most GCs are diagnosed at an advanced stage with unfavorable prognosis. Along with some other countries, China has developed the government-funded national screening programs for GC and other major cancers. GC screening has been shown to effectively decrease the incidence of and mortality from GC in countries adopting nationwide screening programs (Japan and Korea) and in studies based on selected Chinese populations. The screening of GC relies mostly on gastroendoscopy, the accuracy, reliability and safety of which have been indicated by previous studies. However, considering its invasive screening approach, requirements on skilled endoscopists and pathologists, and a high cost, developing noninvasive methods to amend endoscopic screening would be highly needed. Numerous studies have examined biomarkers for GC screening and the combination of biomarkers involving pepsinogen, gastrin, and Helicobacter pylori antibodies has been proposed for risk stratification, seeking to narrow down the high-risk populations for further endoscopy. Despite all the achievements of endoscopic screening, evidence on appropriate screening age, intervals for repeated screening, novel biomarkers promoting precision prevention, and health economics need to be accumulated to inform policymakers on endoscopic screening in China. With the guide of Health China 2030 Planning Outline, we have golden opportunities to promote prevention and control of GC. In this review, we summarize the characteristics of screening programs in China and other East Asian countries and introduce the past and current approaches and strategies for GC screening, aiming for featuring the latest advances and key challenges, and illustrating future visions of GC screening.
Gastric cancer (GC) is one of the major cancers in China and all over the world. Most GCs are diagnosed at an advanced stage with unfavorable prognosis. Along with some other countries, China has developed the government-funded national screening programs for GC and other major cancers. GC screening has been shown to effectively decrease the incidence of and mortality from GC in countries adopting nationwide screening programs (Japan and Korea) and in studies based on selected Chinese populations. The screening of GC relies mostly on gastroendoscopy, the accuracy, reliability and safety of which have been indicated by previous studies. However, considering its invasive screening approach, requirements on skilled endoscopists and pathologists, and a high cost, developing noninvasive methods to amend endoscopic screening would be highly needed. Numerous studies have examined biomarkers for GC screening and the combination of biomarkers involving pepsinogen, gastrin, and Helicobacter pylori antibodies has been proposed for risk stratification, seeking to narrow down the high-risk populations for further endoscopy. Despite all the achievements of endoscopic screening, evidence on appropriate screening age, intervals for repeated screening, novel biomarkers promoting precision prevention, and health economics need to be accumulated to inform policymakers on endoscopic screening in China. With the guide of Health China 2030 Planning Outline, we have golden opportunities to promote prevention and control of GC. In this review, we summarize the characteristics of screening programs in China and other East Asian countries and introduce the past and current approaches and strategies for GC screening, aiming for featuring the latest advances and key challenges, and illustrating future visions of GC screening.
2021, 33(2): 181-192.
doi: 10.21147/j.issn.1000-9604.2021.02.06
Abstract:
Gastric cancer, with high morbidity and mortality rates, is one of the most heterogeneous tumors. Radical gastrectomy and postoperative chemotherapy are the standard treatments. However, the safety and efficacy of neoadjuvant therapy (NAT) need to be confirmed by many trials before implementation, creating a bottleneck in development. Although clinical benefits of NAT have been observed, a series of problems remain to be solved. Before therapy, more contributing factors should be offered for choice in the intended population and ideal regimens. Enhanced computed tomography (CT) scanning is usually applied to evaluate effectiveness according to Response Evaluation Criteria in Solid Tumors (RECIST), yet CT scanning results sometimes differ from pathological responses. After NAT, the appropriate time for surgery is still empirically defined. Our review aims to discuss the abovementioned issues regarding NAT for GC, including indications, selection of regimens, lesion assessment and NAT-surgery interval time.
Gastric cancer, with high morbidity and mortality rates, is one of the most heterogeneous tumors. Radical gastrectomy and postoperative chemotherapy are the standard treatments. However, the safety and efficacy of neoadjuvant therapy (NAT) need to be confirmed by many trials before implementation, creating a bottleneck in development. Although clinical benefits of NAT have been observed, a series of problems remain to be solved. Before therapy, more contributing factors should be offered for choice in the intended population and ideal regimens. Enhanced computed tomography (CT) scanning is usually applied to evaluate effectiveness according to Response Evaluation Criteria in Solid Tumors (RECIST), yet CT scanning results sometimes differ from pathological responses. After NAT, the appropriate time for surgery is still empirically defined. Our review aims to discuss the abovementioned issues regarding NAT for GC, including indications, selection of regimens, lesion assessment and NAT-surgery interval time.
2021, 33(2): 193-202.
doi: 10.21147/j.issn.1000-9604.2021.02.07
Abstract:
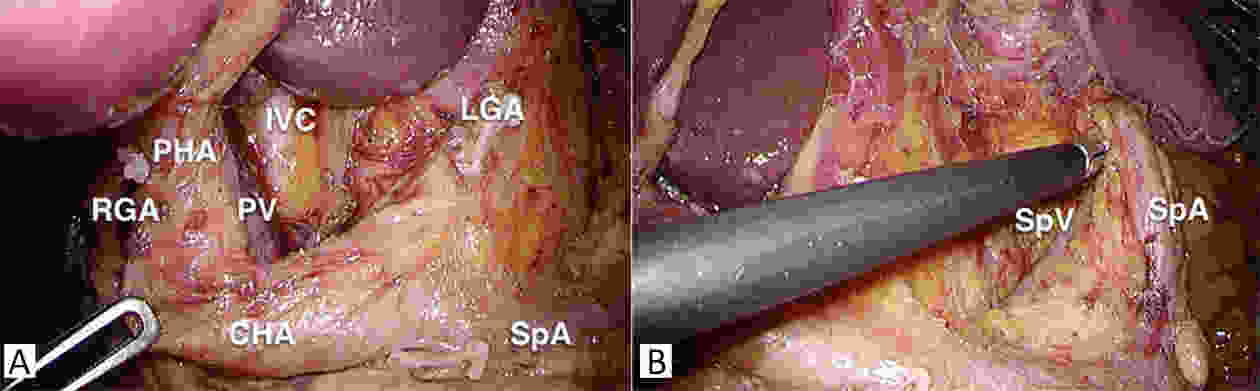
Gastrectomy with lymph node (LN) dissection has been regarded as the standard surgery for gastric cancer (GC), however, the rational extent of lymphadenectomy remains controversial. Though gastrectomy with extended lymphadenectomy beyond D2 is classified as a non-standard gastrectomy, its clinical significance has been evaluated in many studies. Although hard evidence is lacking, D2 plus superior mesenteric vein (No. 14v) LN dissection is recommended when harbor metastasis to No. 6 nodes is suspected in the lower stomach, and dissection of splenic hilar (No. 10) LN can be performed for advanced GC invading the greater curvature of the upper stomach, and D2 plus posterior surface of the pancreatic head (No. 13) LN dissection may be an option in a potentially curative gastrectomy for cancer invading the duodenum. Prophylactic D2+ para-aortic nodal dissection (PAND) was not routinely recommended for advanced GC patients, but therapeutic D2 plus PAND may offer a chance of cure in selected patients, preoperative chemotherapy was considered as the standard treatment for GC with para-aortic node metastasis. There has been no consensus on the extent of lymphadenectomy for the adenocarcinoma of the esophagogastric junction (AEG) so far. The length of esophageal invasion can be used as a reference point for mediastinal LN metastases, and the distance from the esophagogastric junction to the distal end of the tumor is essential for determining the optimal extent of resection. The quality of lymphadenectomy may influence prognosis in GC patients. Both hospital volume and surgeon volume were important factors for the quality of radical gastrectomy. Centralization of GC surgery may be needed to improve prognosis.
Gastrectomy with lymph node (LN) dissection has been regarded as the standard surgery for gastric cancer (GC), however, the rational extent of lymphadenectomy remains controversial. Though gastrectomy with extended lymphadenectomy beyond D2 is classified as a non-standard gastrectomy, its clinical significance has been evaluated in many studies. Although hard evidence is lacking, D2 plus superior mesenteric vein (No. 14v) LN dissection is recommended when harbor metastasis to No. 6 nodes is suspected in the lower stomach, and dissection of splenic hilar (No. 10) LN can be performed for advanced GC invading the greater curvature of the upper stomach, and D2 plus posterior surface of the pancreatic head (No. 13) LN dissection may be an option in a potentially curative gastrectomy for cancer invading the duodenum. Prophylactic D2+ para-aortic nodal dissection (PAND) was not routinely recommended for advanced GC patients, but therapeutic D2 plus PAND may offer a chance of cure in selected patients, preoperative chemotherapy was considered as the standard treatment for GC with para-aortic node metastasis. There has been no consensus on the extent of lymphadenectomy for the adenocarcinoma of the esophagogastric junction (AEG) so far. The length of esophageal invasion can be used as a reference point for mediastinal LN metastases, and the distance from the esophagogastric junction to the distal end of the tumor is essential for determining the optimal extent of resection. The quality of lymphadenectomy may influence prognosis in GC patients. Both hospital volume and surgeon volume were important factors for the quality of radical gastrectomy. Centralization of GC surgery may be needed to improve prognosis.
2021, 33(2): 203-215.
doi: 10.21147/j.issn.1000-9604.2021.02.08
Abstract:
Immune checkpoint inhibitors (ICIs), a type of immunotherapy, have become one of the most important therapeutic options for first- and second-line treatment of advanced non-small cell lung cancer (NSCLC). Recent clinical studies have shown that immunotherapy can offer substantial survival benefits to patients with early-stage or resectable advanced NSCLC. However, considering the importance of timing when using ICIs and their associated adverse events (AEs), the advantages and disadvantages of using these agents need to be weighed carefully when deciding the use of a combined treatment. In addition, the inconsistency between imaging assessment and pathological results poses further challenges to the evaluation of efficacy of neoadjuvant immunotherapy. It is also important to develop new methodologies and discover suitable biomarkers that can be used to evaluate survival outcomes of immunotherapy and identify patients who would benefit the most from this treatment. In this review, we aimed to summarize previous results of ongoing clinical trials on neoadjuvant immunotherapy for lung cancer and discuss the challenges and future perspectives of this therapeutic approach in the treatment of resectable NSCLC.
Immune checkpoint inhibitors (ICIs), a type of immunotherapy, have become one of the most important therapeutic options for first- and second-line treatment of advanced non-small cell lung cancer (NSCLC). Recent clinical studies have shown that immunotherapy can offer substantial survival benefits to patients with early-stage or resectable advanced NSCLC. However, considering the importance of timing when using ICIs and their associated adverse events (AEs), the advantages and disadvantages of using these agents need to be weighed carefully when deciding the use of a combined treatment. In addition, the inconsistency between imaging assessment and pathological results poses further challenges to the evaluation of efficacy of neoadjuvant immunotherapy. It is also important to develop new methodologies and discover suitable biomarkers that can be used to evaluate survival outcomes of immunotherapy and identify patients who would benefit the most from this treatment. In this review, we aimed to summarize previous results of ongoing clinical trials on neoadjuvant immunotherapy for lung cancer and discuss the challenges and future perspectives of this therapeutic approach in the treatment of resectable NSCLC.
2021, 33(2): 216-231.
doi: 10.21147/j.issn.1000-9604.2021.02.09
Abstract:
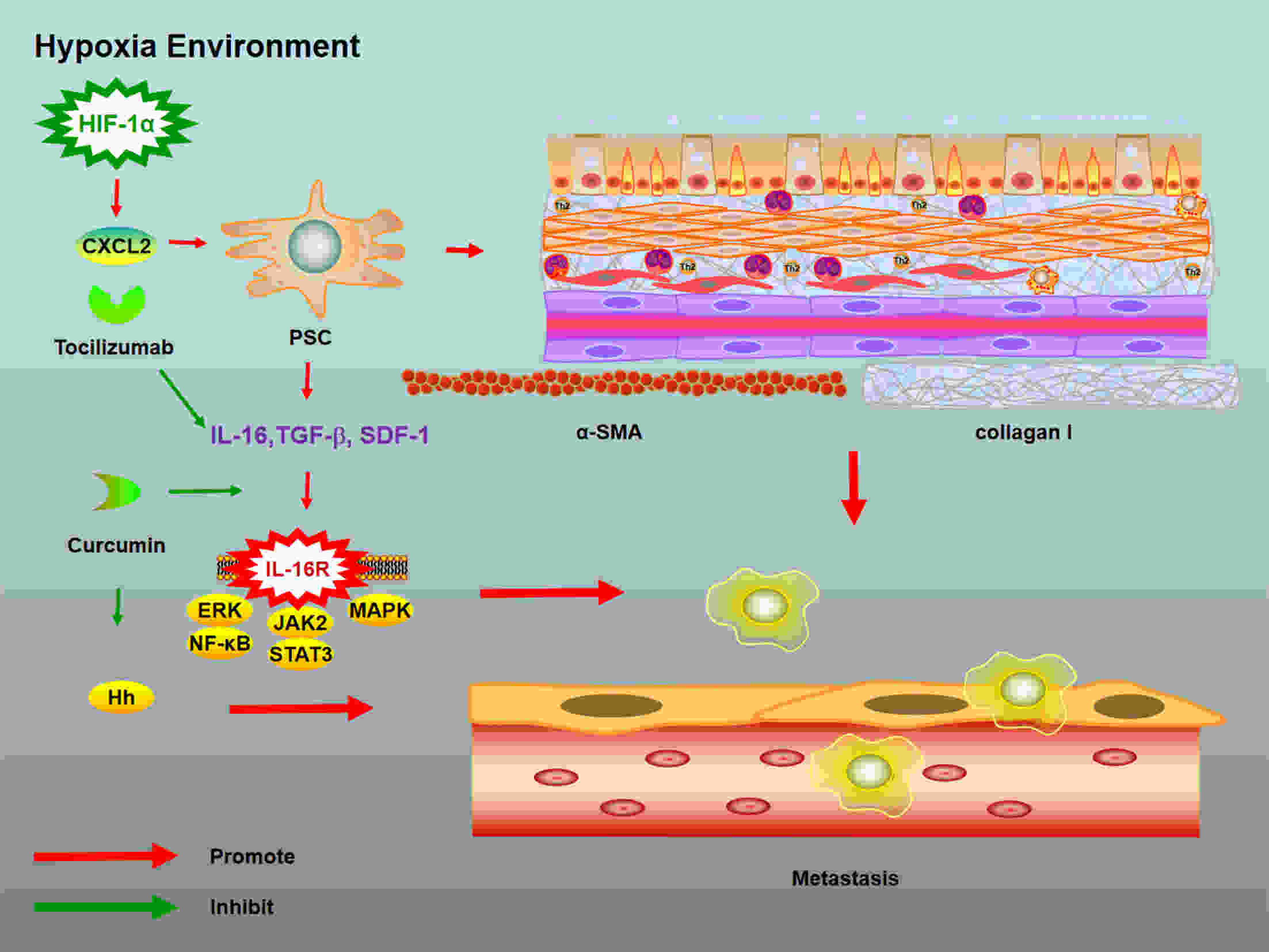
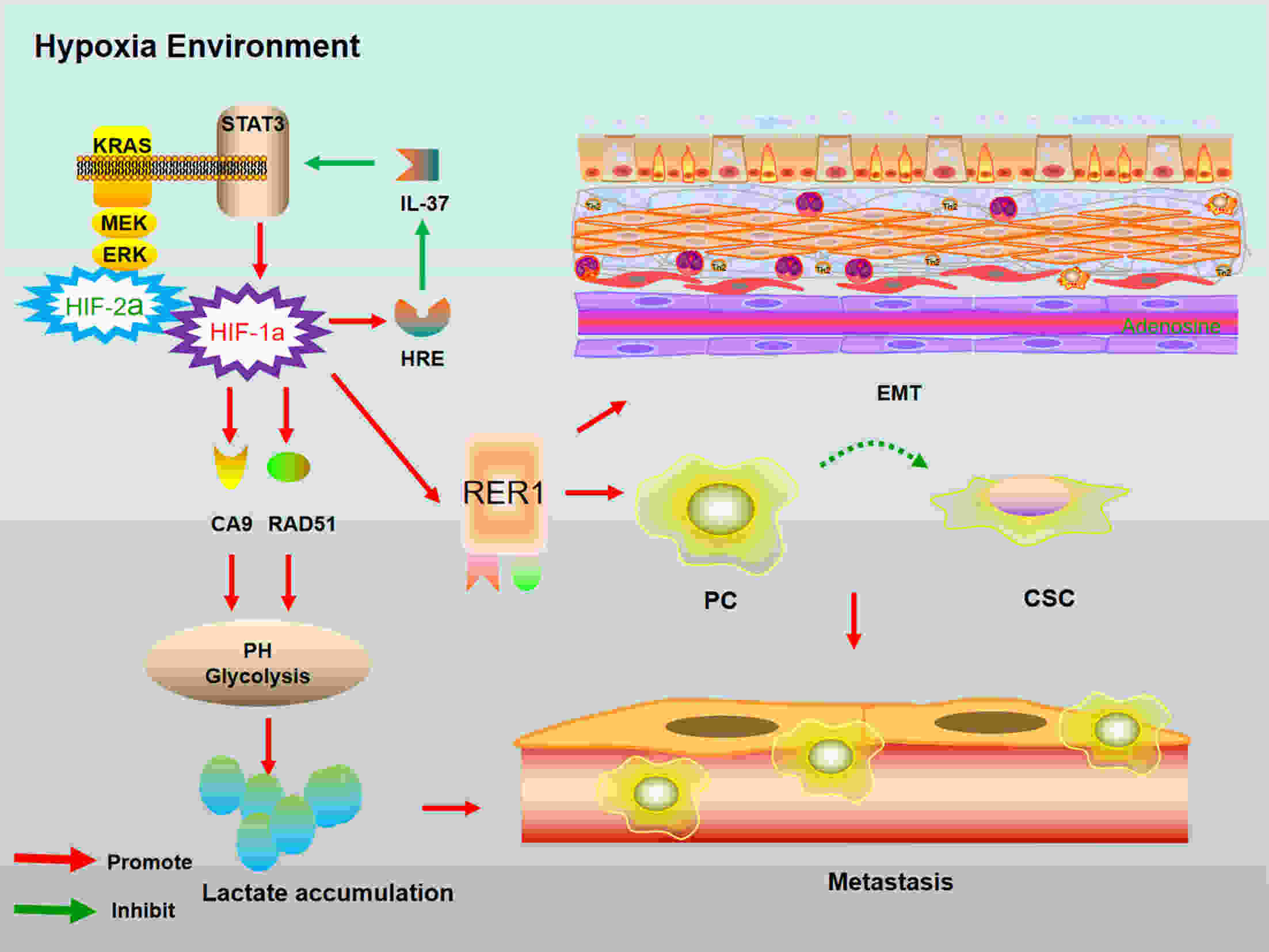
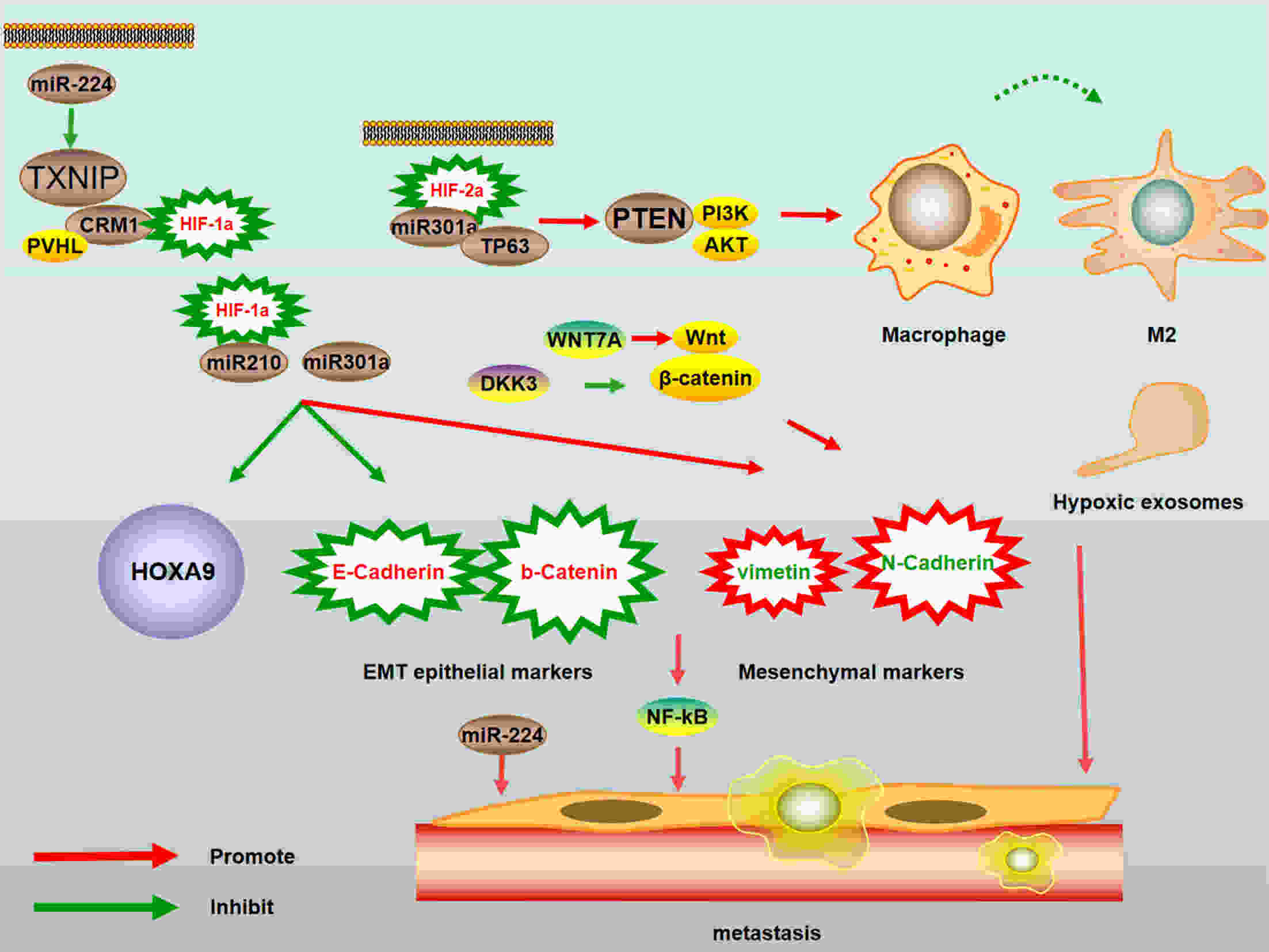
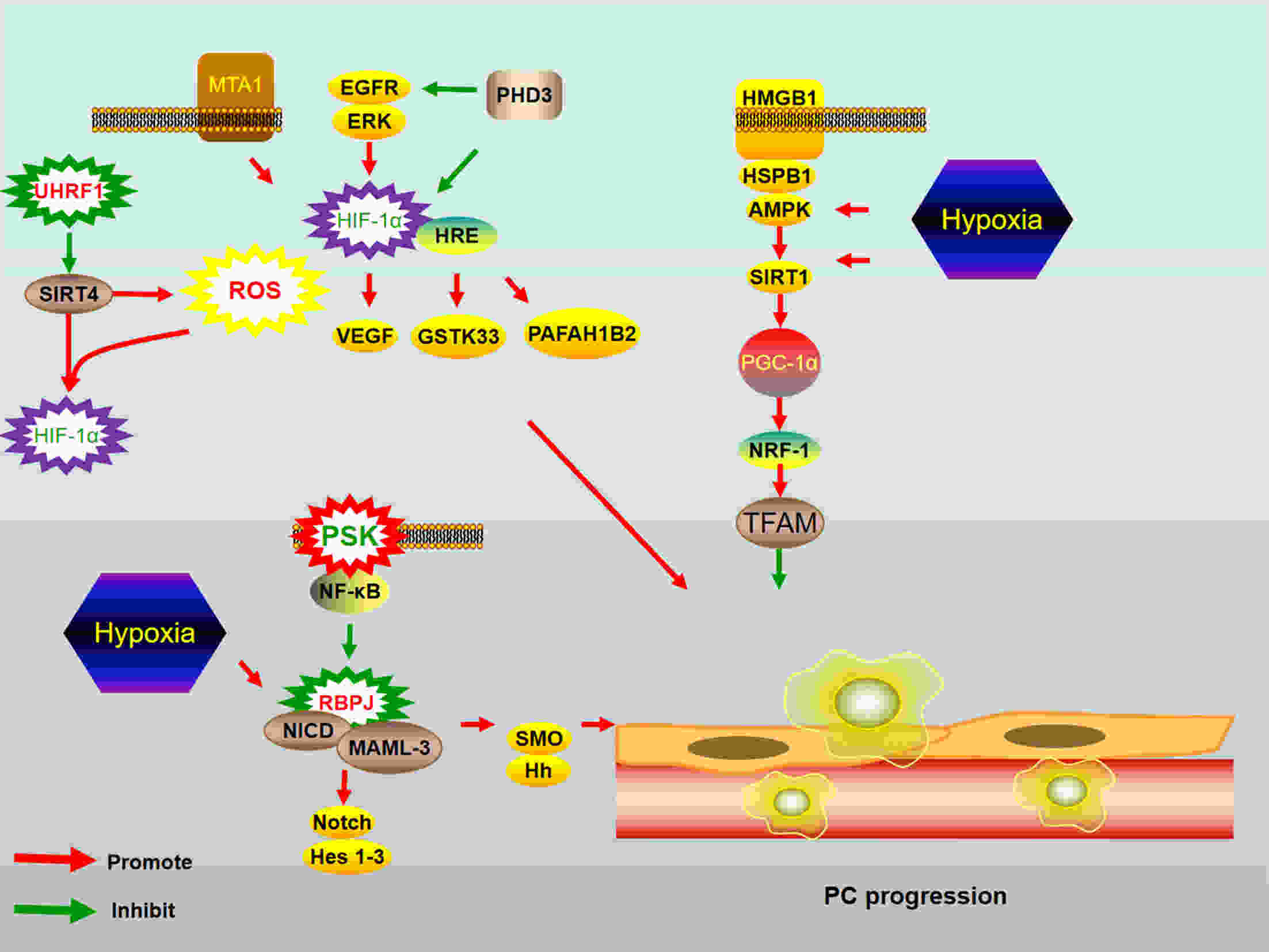
Pancreatic cancer (PC) is one of the deadliest malignancies. The high mortality rate of PC largely results from delayed diagnosis and early metastasis. Therefore, identifying novel treatment targets for patients with PC is urgently required to improve survival rates. A major barrier to successful treatment of PC is the presence of a hypoxic tumor microenvironment, which is associated with poor prognosis, treatment resistance, increased invasion and metastasis. Recent studies have identified a number of novel molecules and pathways in PC cells that promote cancer cells progression under hypoxic conditions, which may provide new therapy strategies to inhibit the development and metastasis of PC. This review summarizes the latest research of hypoxia in PC and provides an overview of how the current therapies have the capacity to overcome hypoxia and improve PC patient treatment. These findings will eventually provide guidance for future PC management and clinical trials and hopefully improve the survival of patients with PC.
Pancreatic cancer (PC) is one of the deadliest malignancies. The high mortality rate of PC largely results from delayed diagnosis and early metastasis. Therefore, identifying novel treatment targets for patients with PC is urgently required to improve survival rates. A major barrier to successful treatment of PC is the presence of a hypoxic tumor microenvironment, which is associated with poor prognosis, treatment resistance, increased invasion and metastasis. Recent studies have identified a number of novel molecules and pathways in PC cells that promote cancer cells progression under hypoxic conditions, which may provide new therapy strategies to inhibit the development and metastasis of PC. This review summarizes the latest research of hypoxia in PC and provides an overview of how the current therapies have the capacity to overcome hypoxia and improve PC patient treatment. These findings will eventually provide guidance for future PC management and clinical trials and hopefully improve the survival of patients with PC.
2021, 33(2): 232-242.
doi: 10.21147/j.issn.1000-9604.2021.02.10
Abstract:
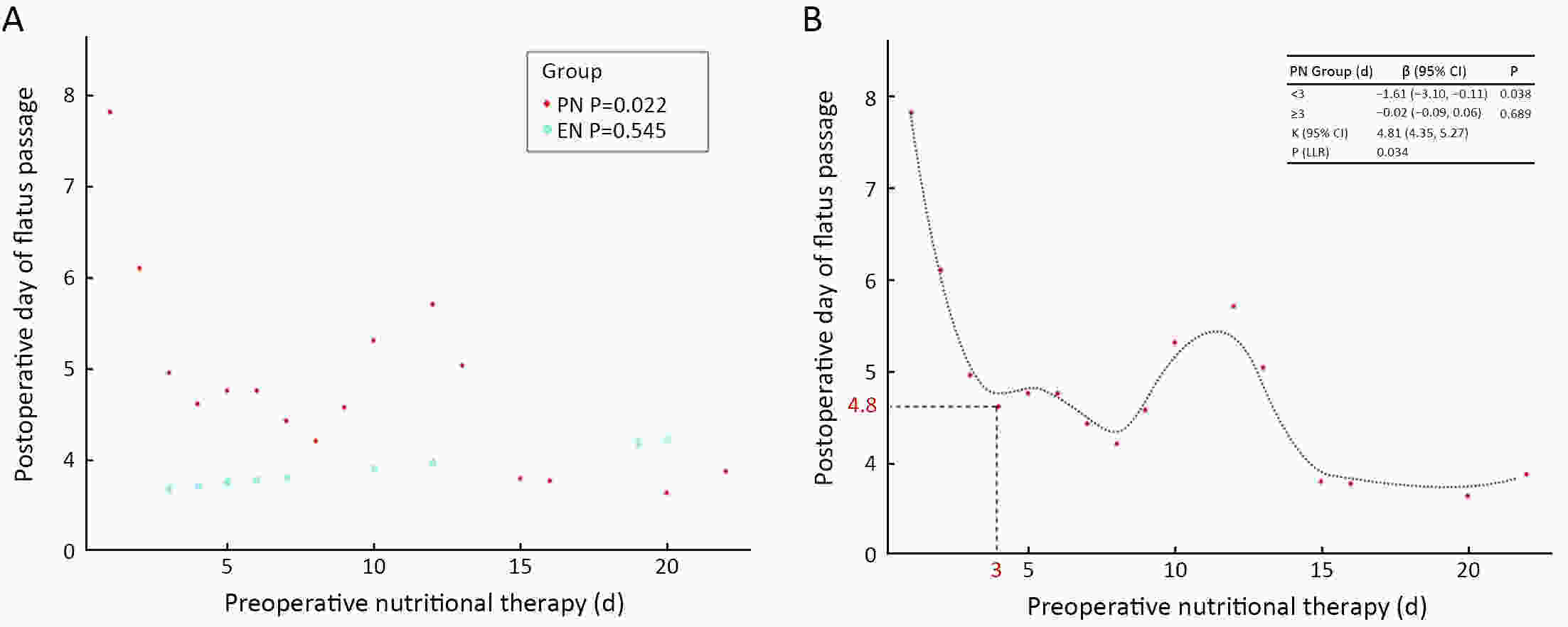
ObjectiveTo avoid perioperative complications caused malnutrition, nutrition therapy is necessary in gastric outlet obstruction (GOO) patients. Compared to parenteral nutrition (PN), enteral nutrition (EN) is associated with many advantages. This study aimed to investigate whether preoperative EN has beneficial clinical effects compared to preoperative PN in gastric cancer patients with GOO undergoing surgery. MethodsAccording to the methods of preoperative nutrition therapy, 143 patients were divided into EN group (n=42) and PN group (n=101) between January 2013 and December 2017 at the Chinese People’s Liberation Army General Hospital. Multiple logistic regression models were used to assess the association between the methods of preoperative nutrition therapy and postoperative day of flatus passage. The generalized additive model and two-piecewise linear regression model were used to calculate the inflection point of the preoperative nutritional therapy time on the postoperative day of flatus passage in the PN group. ResultsEN shortened the postoperative day of flatus passage in gastric cancer patients with GOO, which is a protective factor, especially in patients who underwent non-radical operations and the postoperative day of flatus passage reduced when the preoperative PN therapy was up to 3 d and a longer PN therapy (>3 d) did not accelerate the postoperative recovery of gastrointestinal functions. ConclusionsPreoperative EN therapy would benefit gastric cancer patients with GOO by accelerating postoperative recovery. For patients with absolute obstruction, no more than 3-day PN therapy is recommended if patients can tolerate general anesthesia and surgery.
2021, 33(2): 243-255.
doi: 10.21147/j.issn.1000-9604.2021.02.11
Abstract:
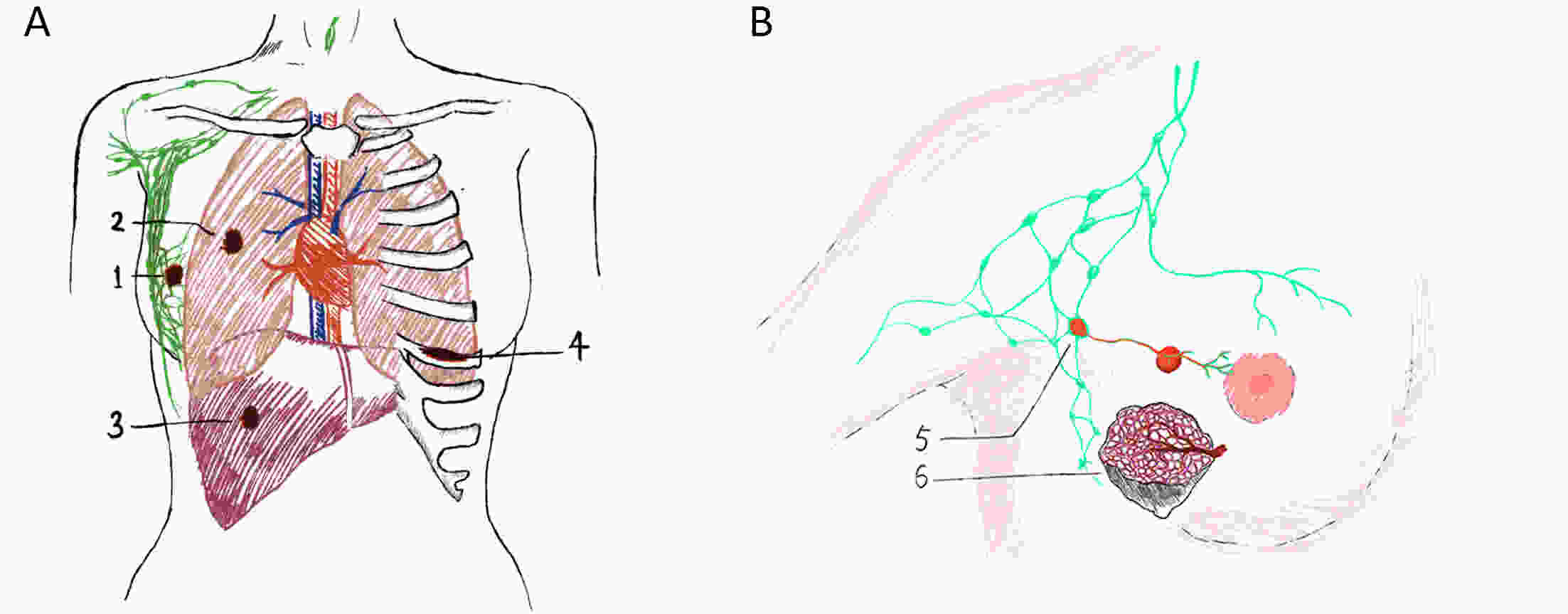
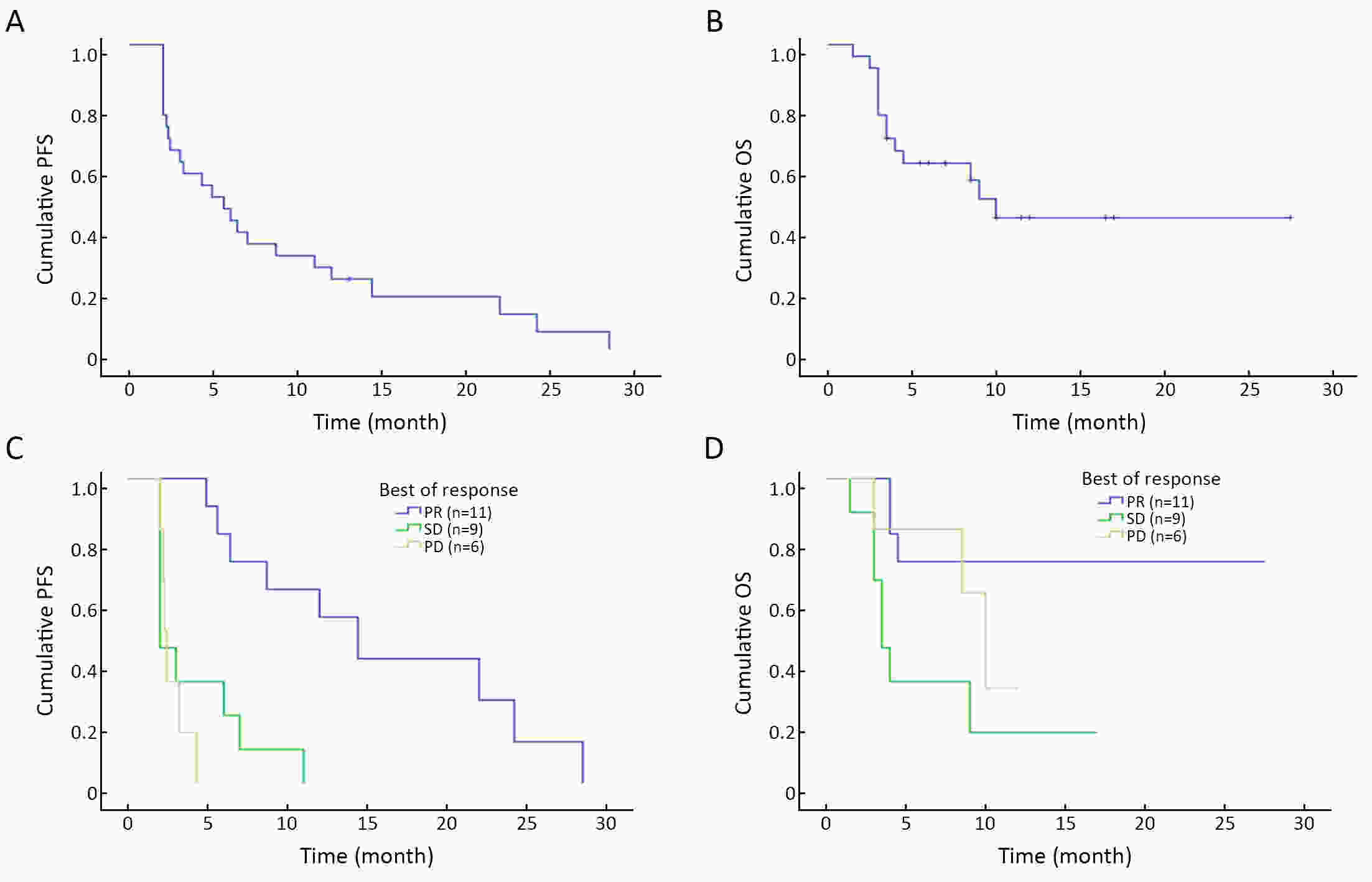
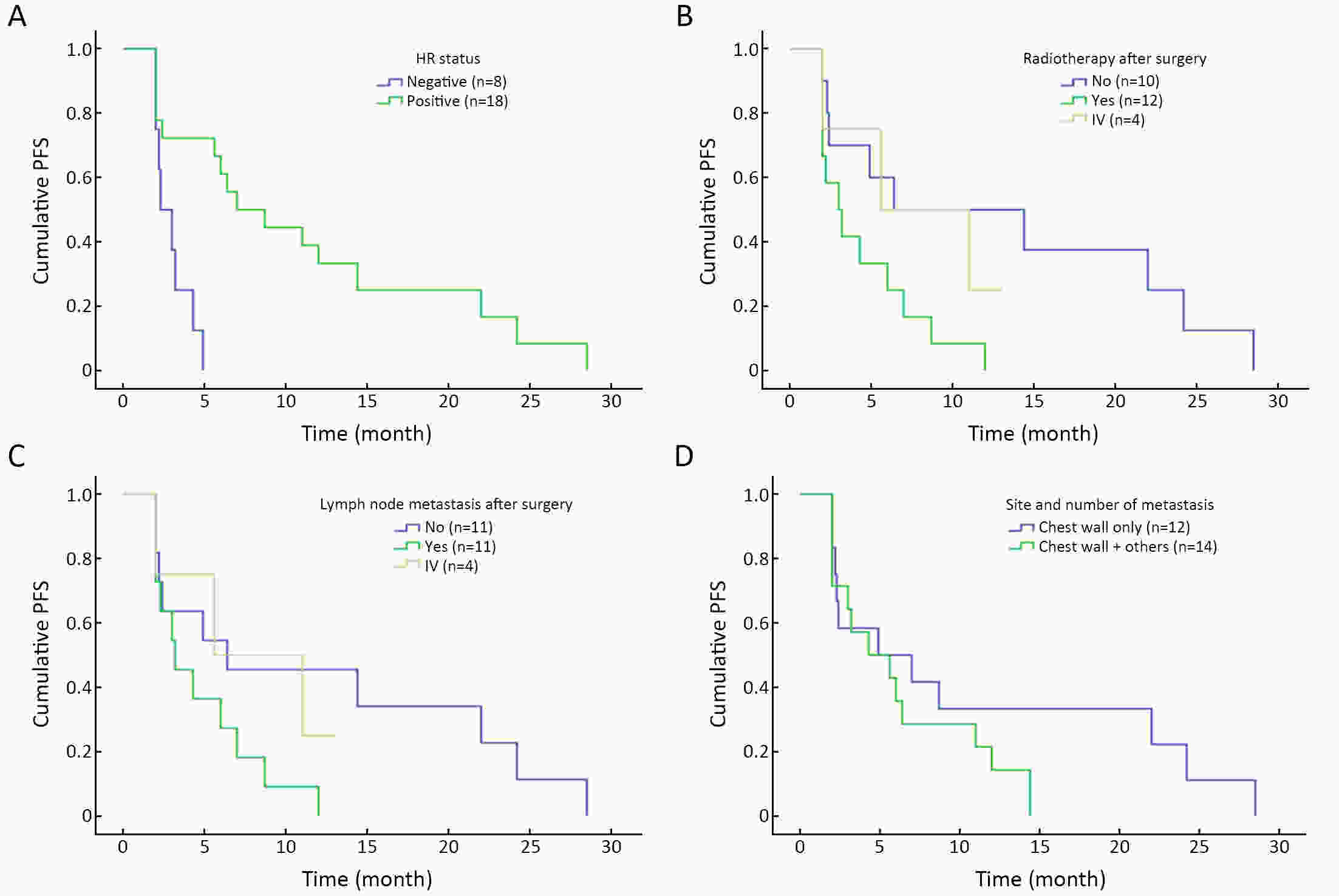
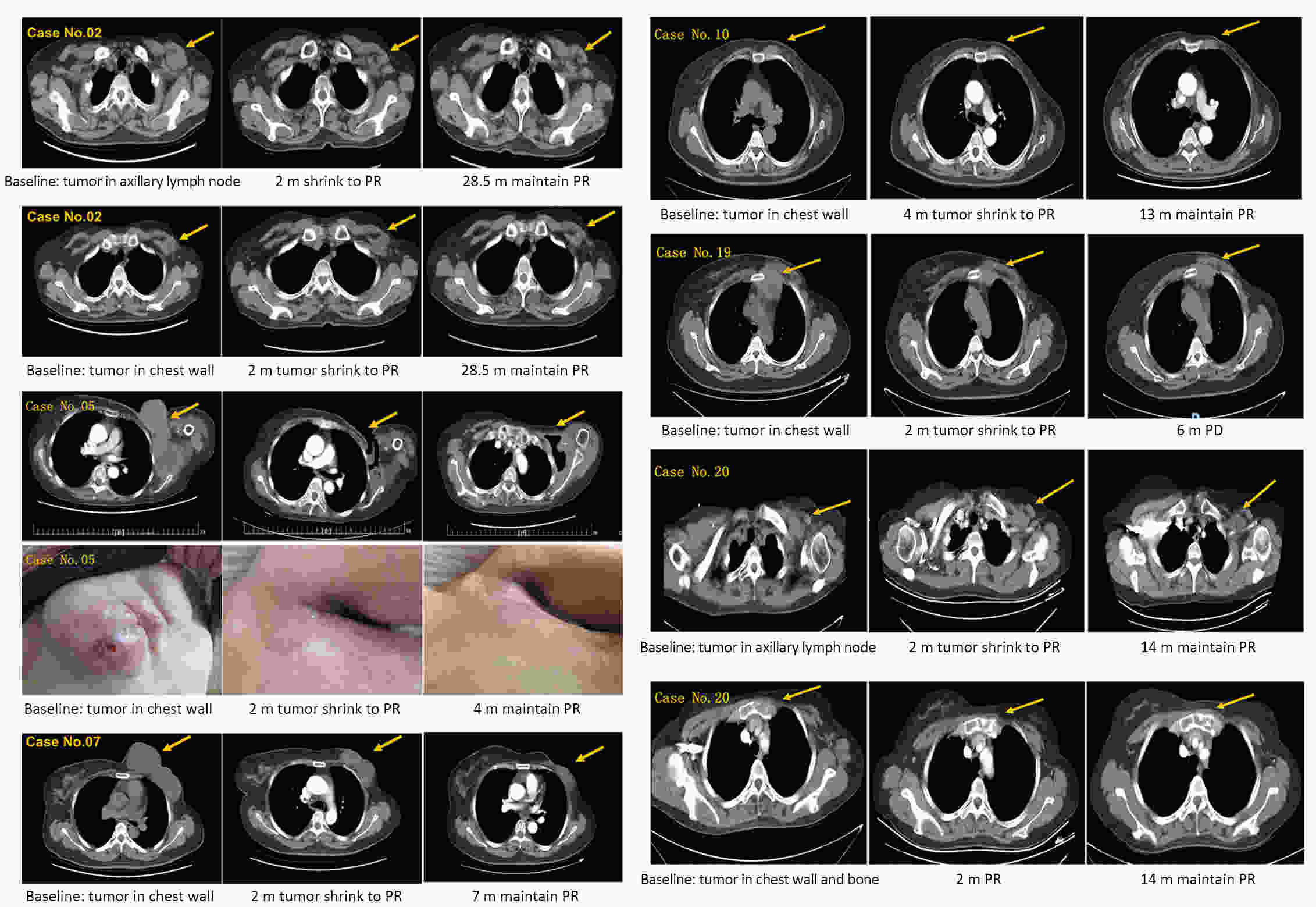
ObjectiveBreast cancer (BC) with chest wall metastasis (CWM) usually shows rich neovascularization. This trial explored the clinical effect of apatinib on human epidermal growth factor receptor 2 (HER2)-negative advanced BC involving CWM. MethodsThis trial involved four centers in China and was conducted from September 2016 to March 2020. Patients received apatinib 500 mg/d [either alone or with endocrine therapy if hormone receptor-positive (HR+)] until disease progression or unacceptable toxicity. Progression-free survival (PFS) was the primary endpoint. ResultsWe evaluated 26 patients for efficacy. The median PFS (mPFS) and median overall survival (mOS) were 4.9 [range: 2.0−28.5; 95% confidence interval (95% CI): 2.1−8.3] months and 18 (range: 3−55; 95% CI: 12.9−23.1) months, respectively. The objective response rate (ORR) was 42.3% (11/26), and the disease-control rate was 76.9% (20/26). In the subgroup analysis, HR+ patients compared with HR-negative patients had significantly improved mPFS of 7.0 (95% CI: 2.2−11.8) months vs. 2.3 (95% CI: 1.2−3.4) months, respectively (P=0.001); and mPFS in patients without or with chest wall radiotherapy was 6.4 (95% CI: 1.6−19.5) months vs. 3.0 (95% CI: 1.3−4.6) months, respectively (P=0.041). In the multivariate analysis, HR+ status was the only independent predictive factor for favorable PFS (P=0.014). ConclusionsApatinib was highly effective for BC patients with CWM, especially when combined with endocrine therapy. PFS improved significantly in patients with HR+ status who did not receive chest wall radiotherapy. However, adverse events were serious and should be carefully monitored from the beginning of apatinib treatment.
2021, 33(2): 256-270.
doi: 10.21147/j.issn.1000-9604.2021.02.12
Abstract:
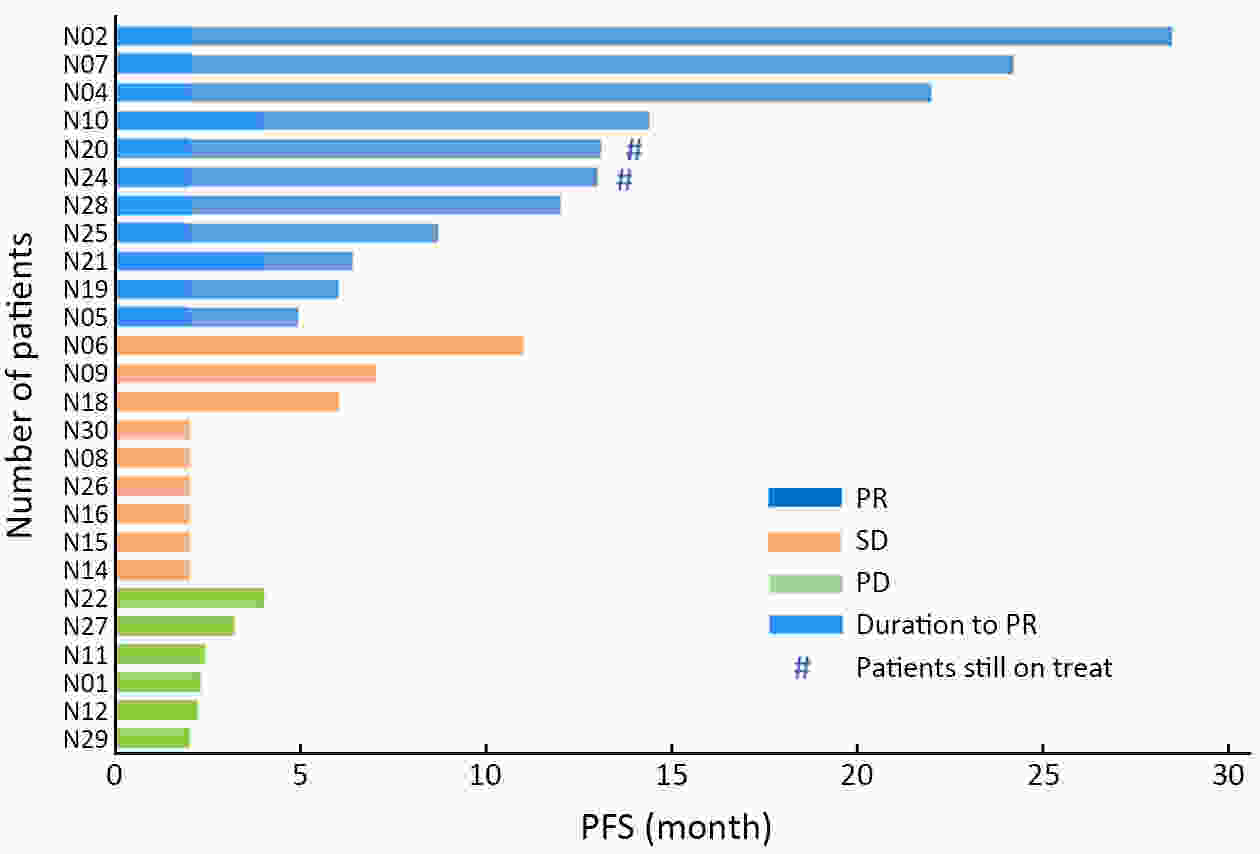
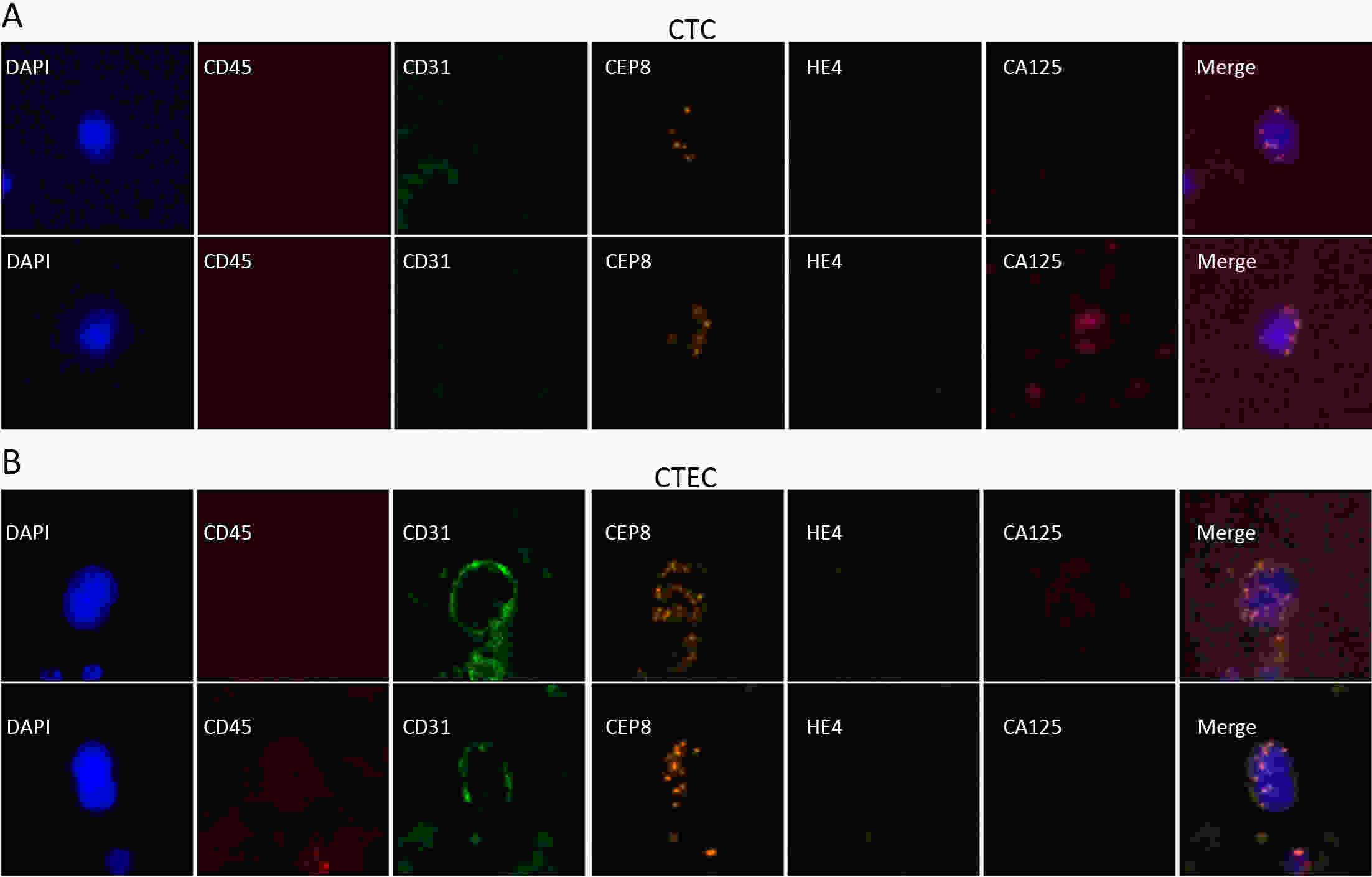
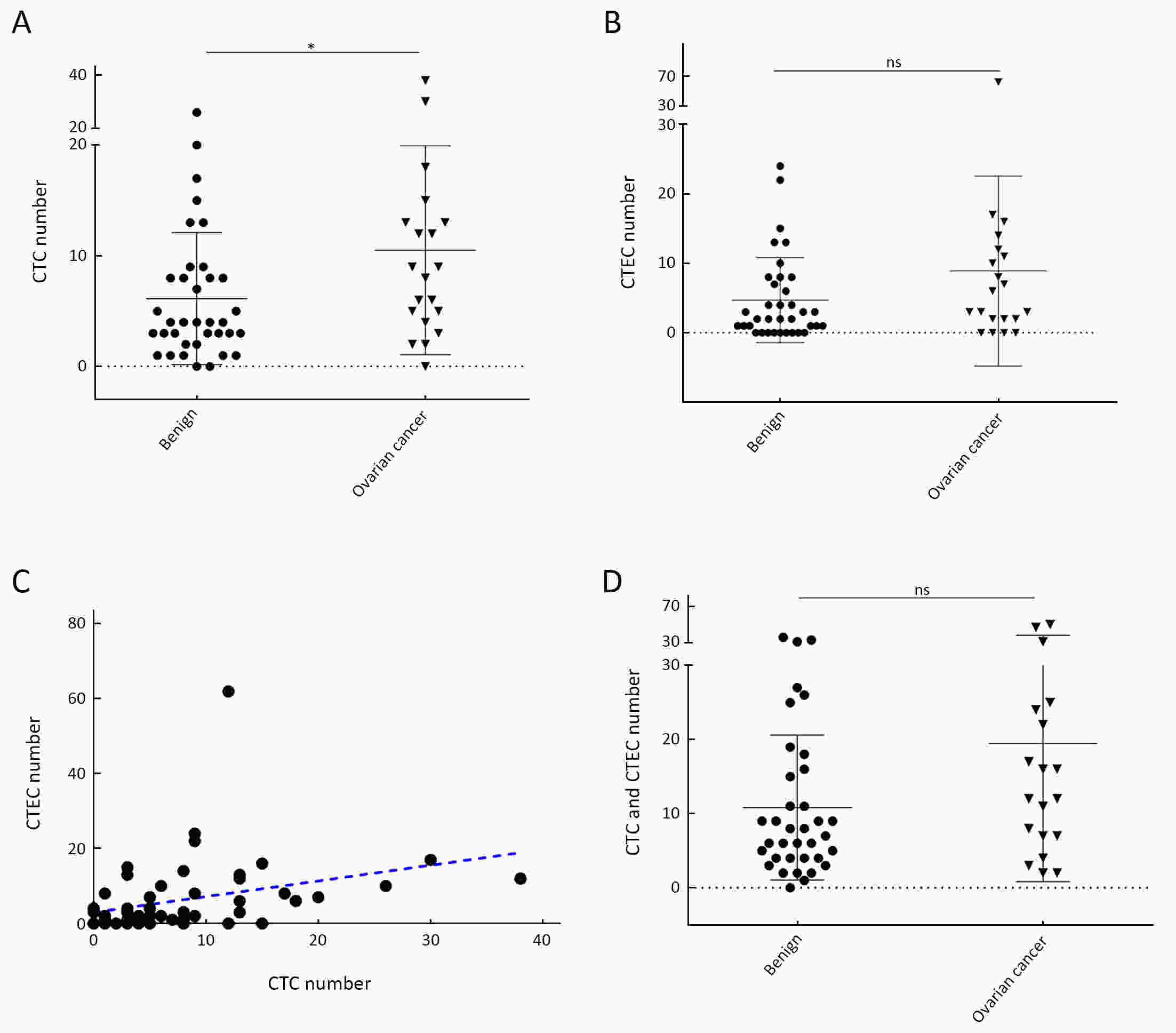
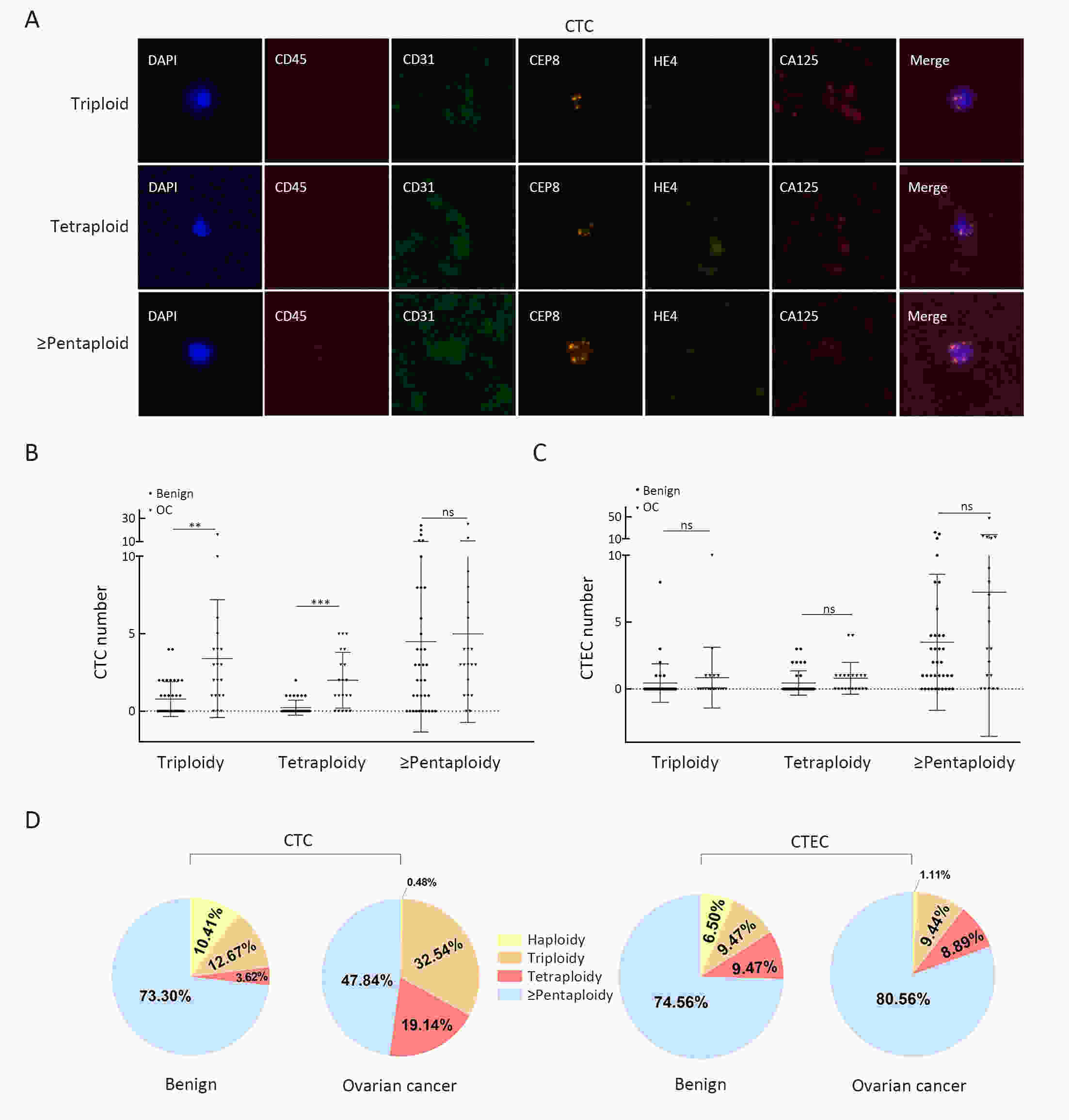
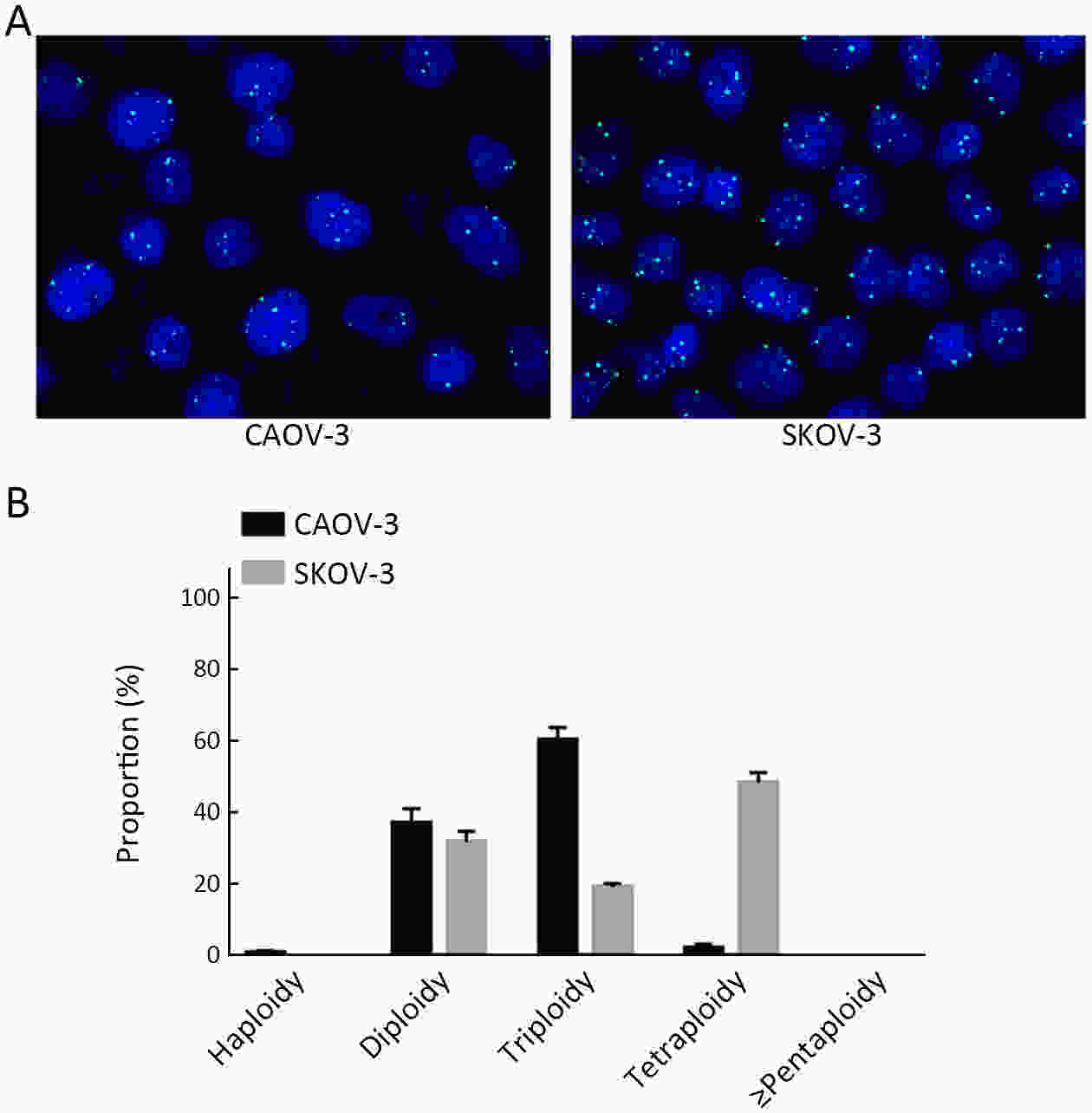
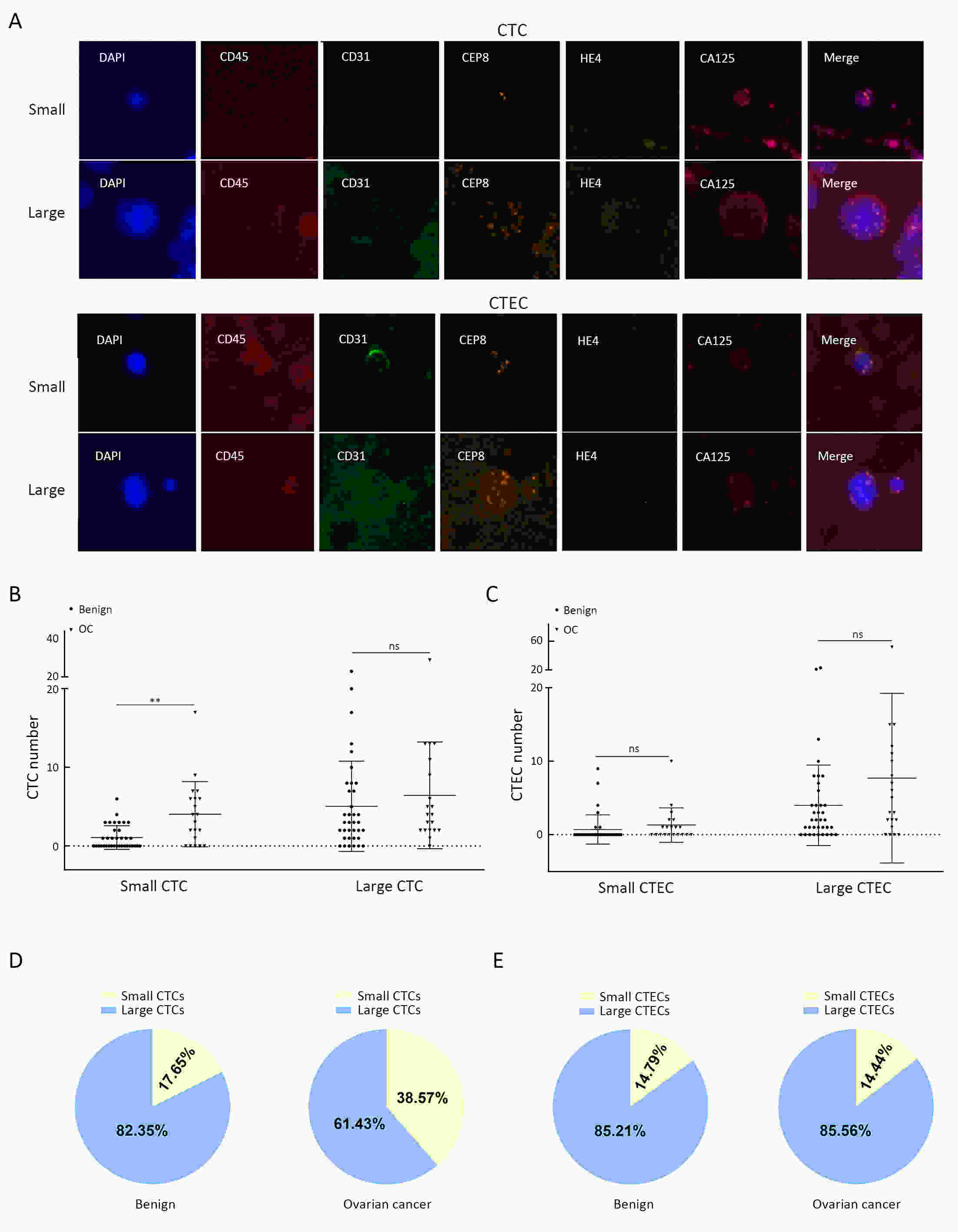
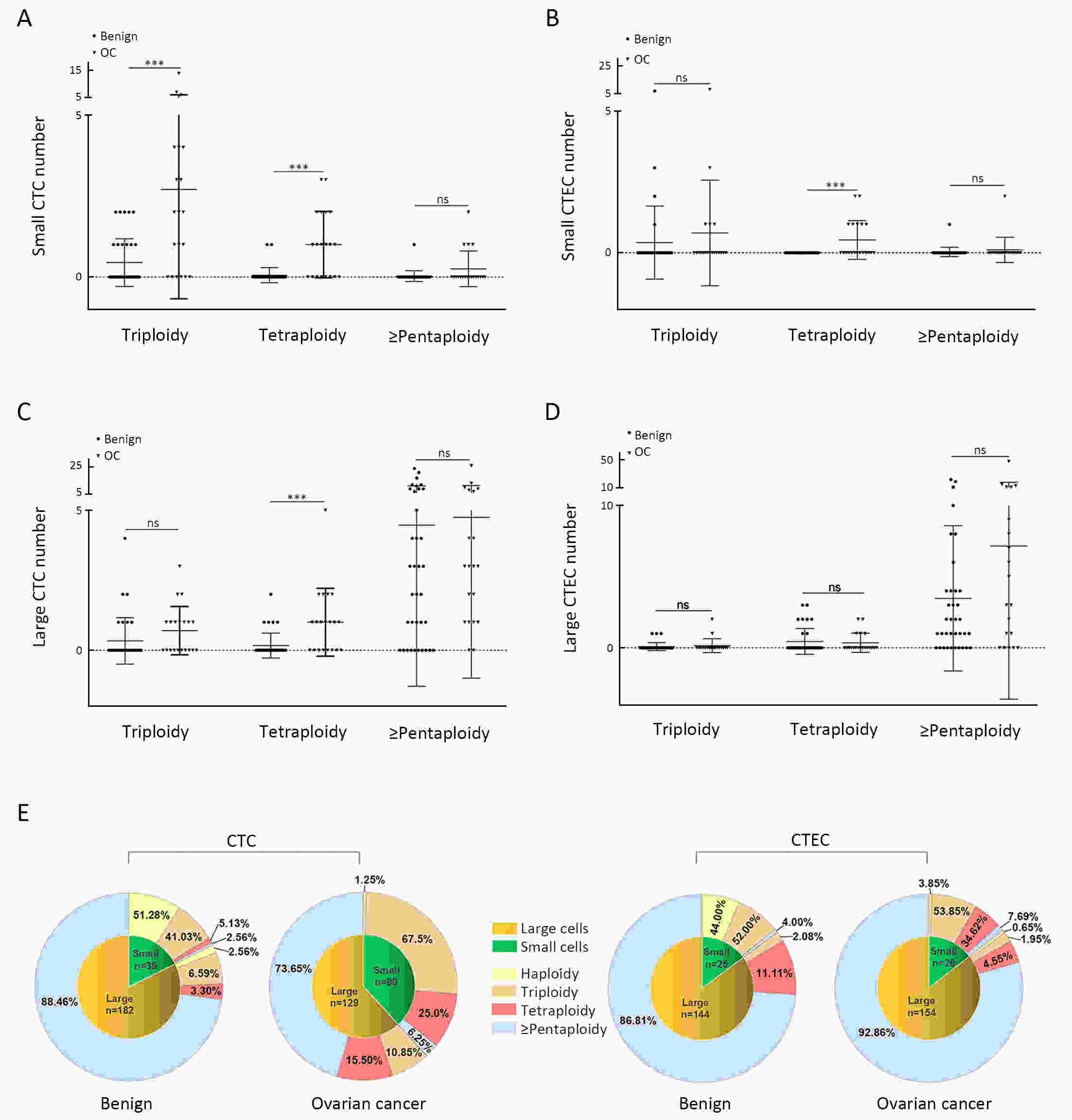
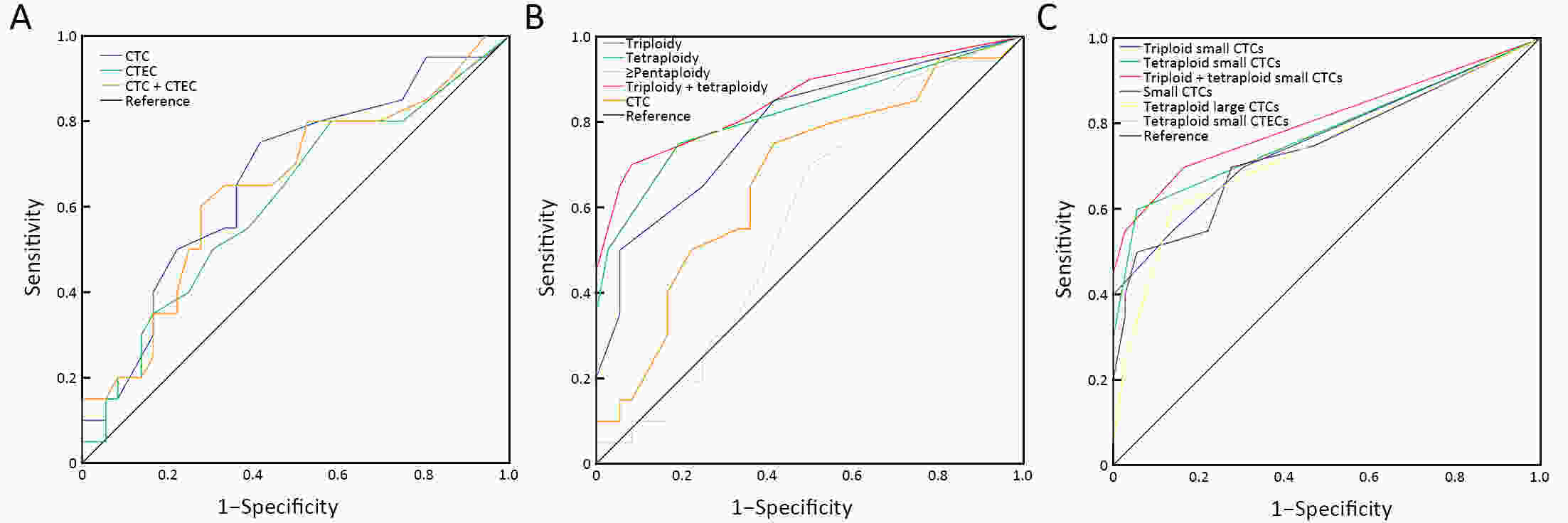
ObjectiveHematogenous metastasis is essential for the progression of ovarian cancer (OC), and circulating tumor cells (CTCs) are part of the metastatic cascade. However, the detection rate of CTC is low due to the use of less sensitive detection methods. Therefore, this study aimed to detect CTCs and circulating tumorigenic endothelial cells (CTECs) in patients with OC using subtraction enrichment and immunostaining and fluorescence in situ hybridization (SE-iFISH). MethodsWe enrolled a total of 56 subjects, including 20 OC patients and 36 ovarian benign tumor patients. CTCs and CTECs were captured by subtraction enrichment (SE) and counted and classified according to immunofluorescence staining of tumor markers (TMs) carbohydrate antigen 125 (CA125) and human epididymis protein 4 (HE4) combined with fluorescence in situ hybridization (iFISH) of chromosome 8 (Chr8) aneuploidy. The diagnostic value and subtype characteristics of CTCs and CTECs were investigated. ResultsThe detection rate of CTCs by SE-iFISH was high. Compared with CA125 and HE4, Chr8 aneuploidy was the major identification feature of CTC. CTC counts in OC were statistically higher than those in benign groups. CTC and CTEC with ≥pentaploidy were detected in both groups, illustrating the poor diagnostic value of CTC or CTEC. Distributions of triploid and tetraploid CTC subtypes were significantly different, and combined detection of triploid and tetraploid CTCs showed the best diagnostic value. In contrast, the distribution of CTECs in the OC and benign groups had no statistically significant difference. Small CTCs accounted for over 1/3 of the total CTC count. We also found that small CTCs and CTECs primarily comprised triploid cells, while large CTCs and CTECs mainly comprised pentaploidy and beyond. ConclusionsThe application of SE-iFISH offered a more comprehensive understanding of heterogeneous CTCs and CTECs in OC. Analysis of subclass characteristics of the CTCs and CTECs according to Chr8 aneuploidy and cell size may broaden their potential clinical utility and deepen mechanistic studies in OC.
2021, 33(2): 271-288.
doi: 10.21147/j.issn.1000-9604.2021.02.13
Abstract:
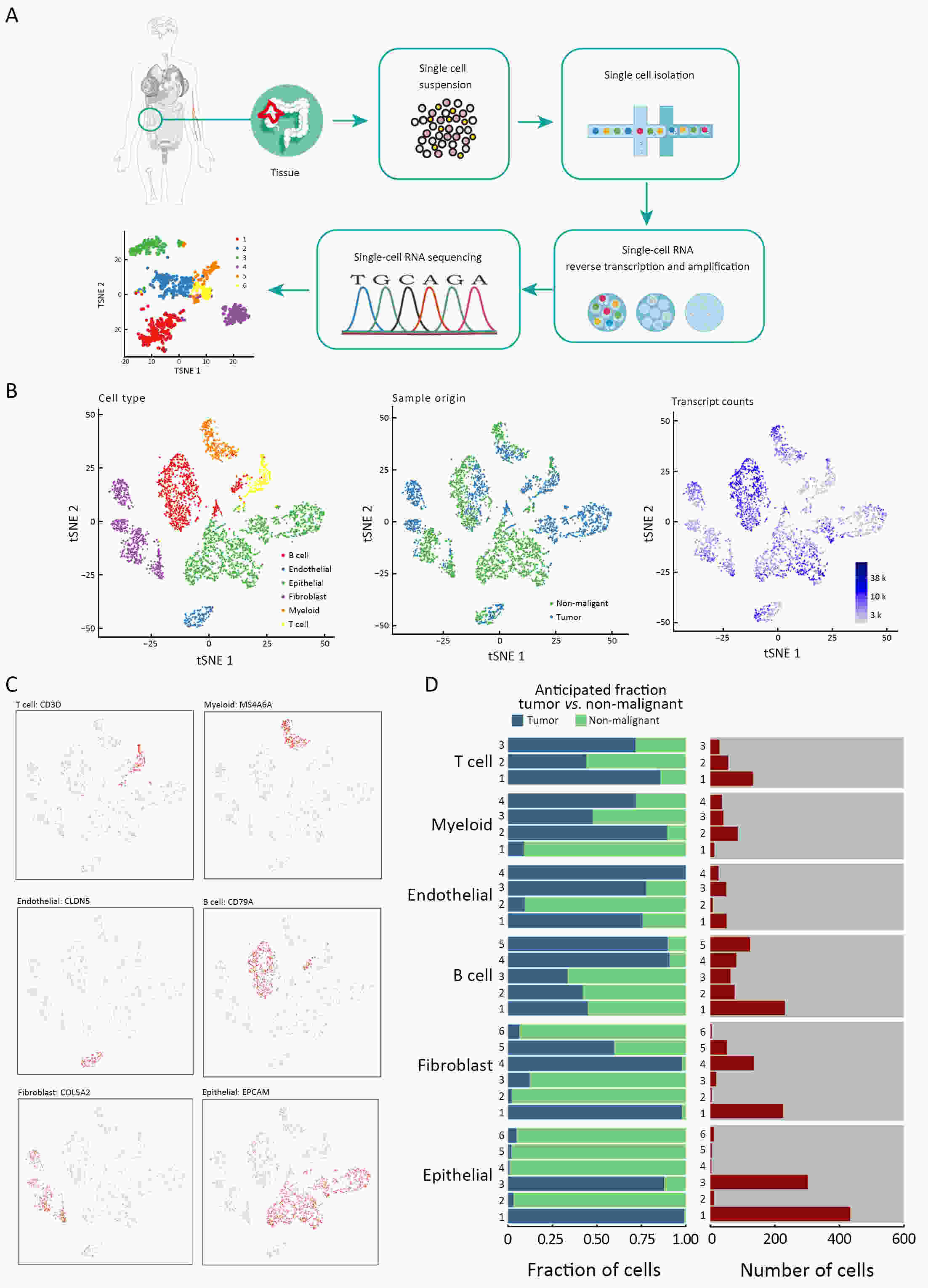
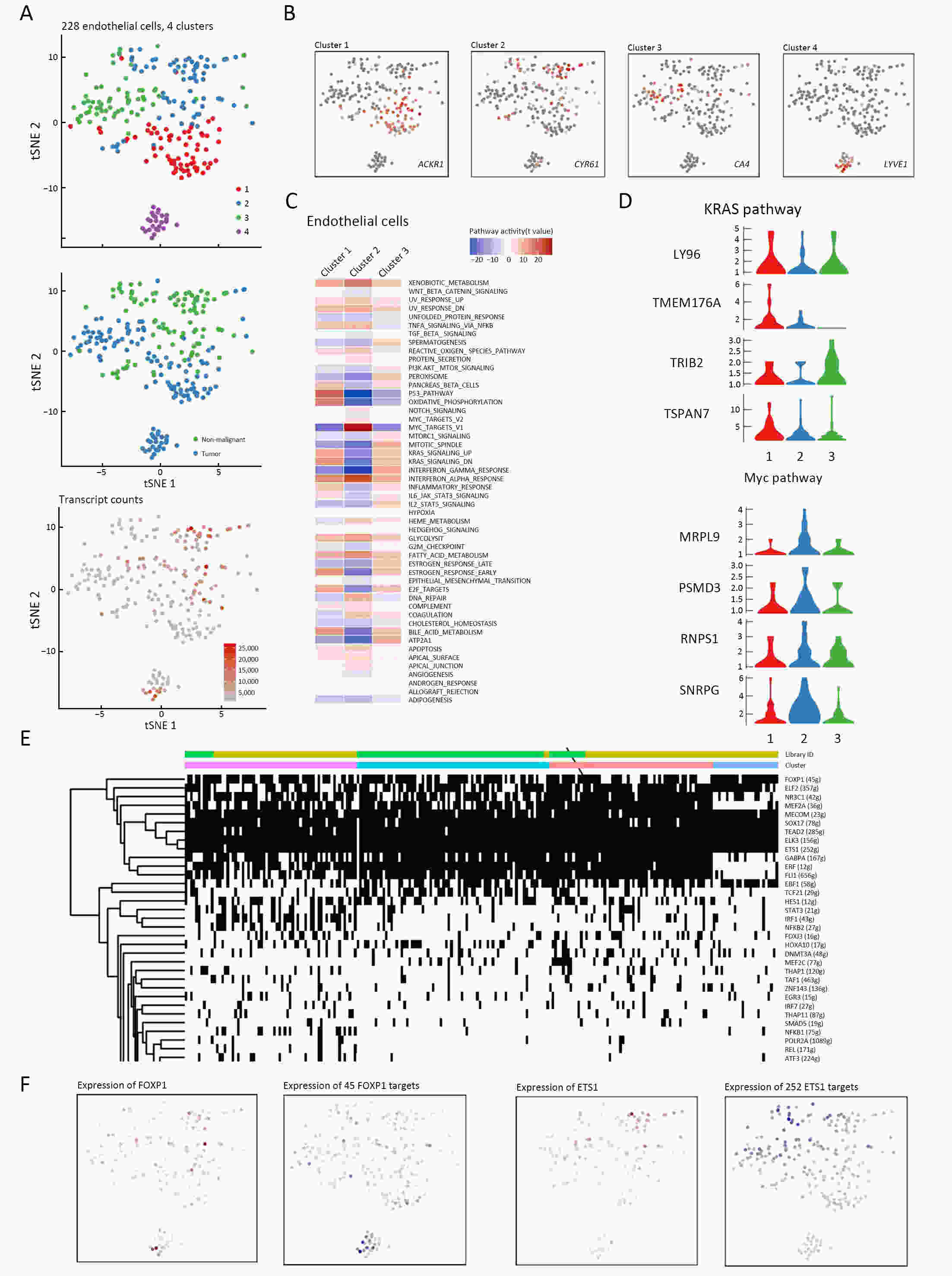
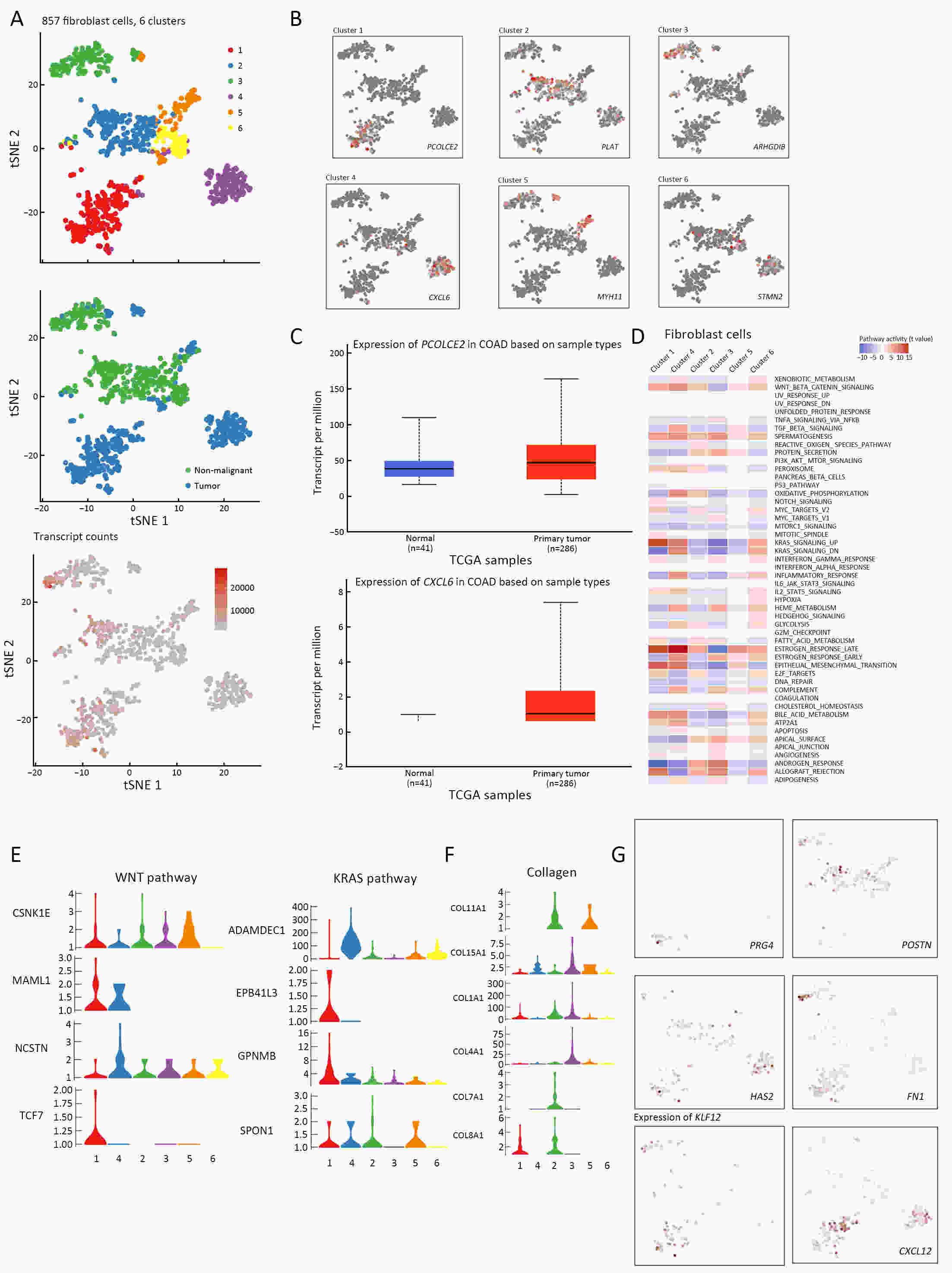
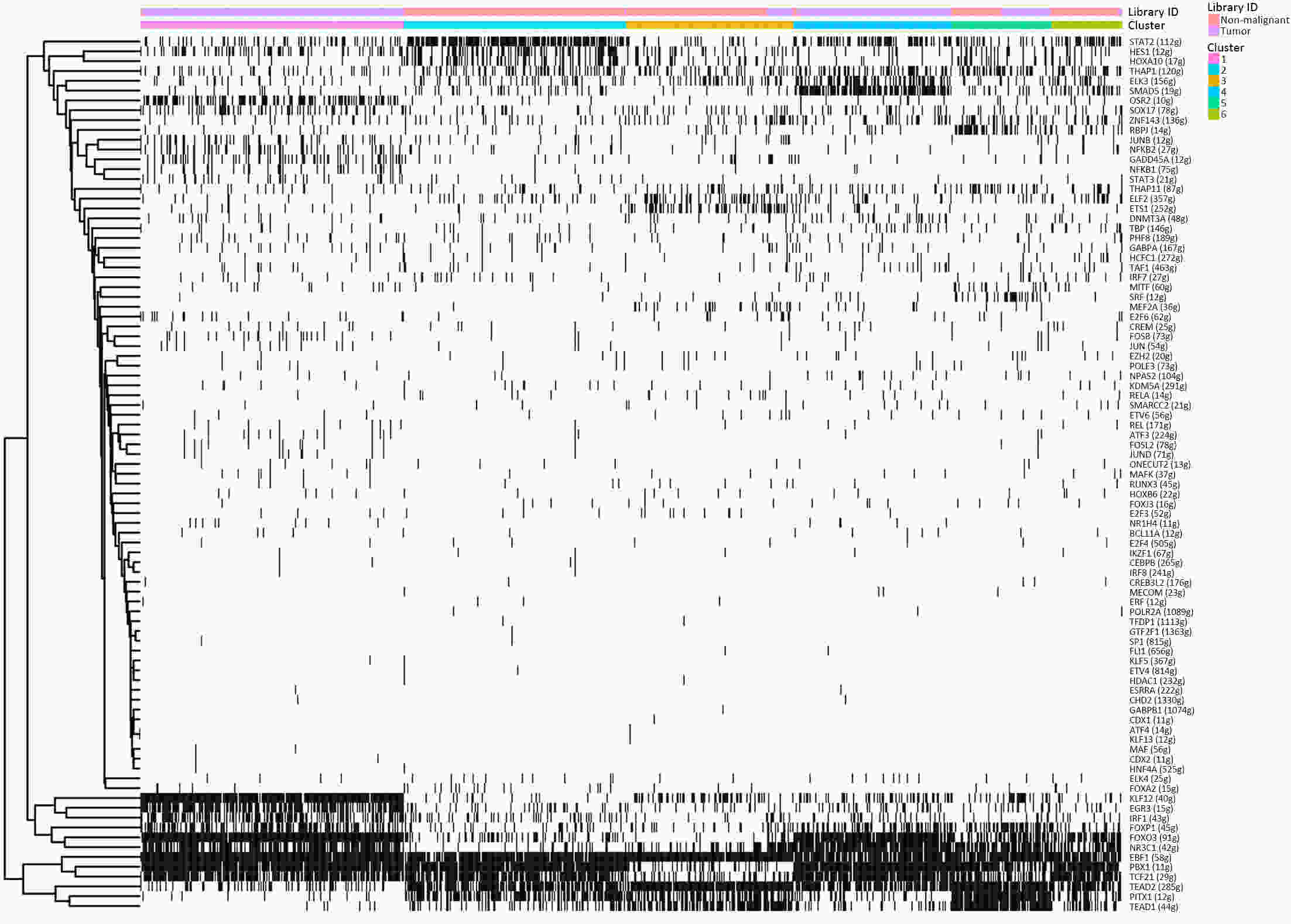
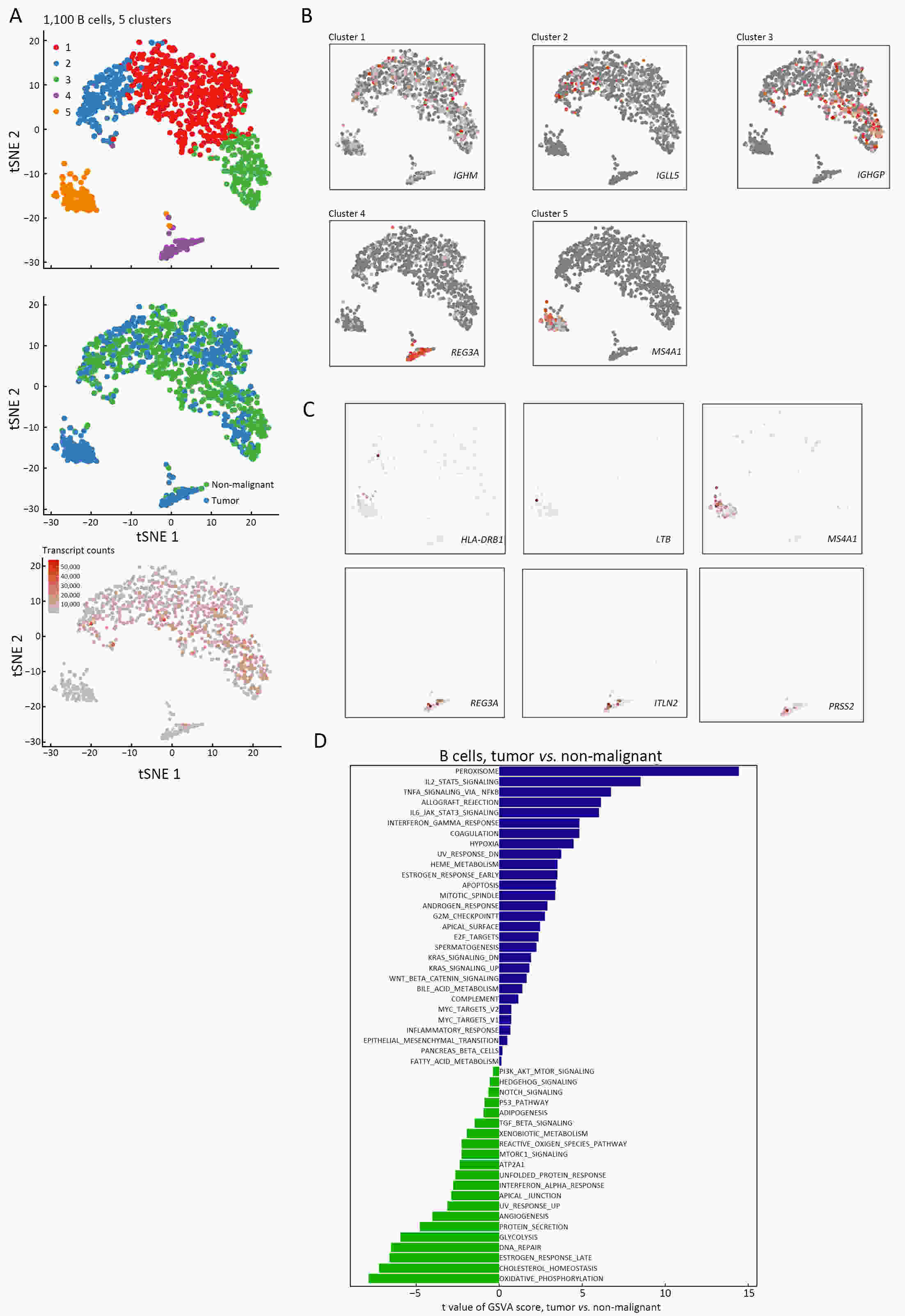
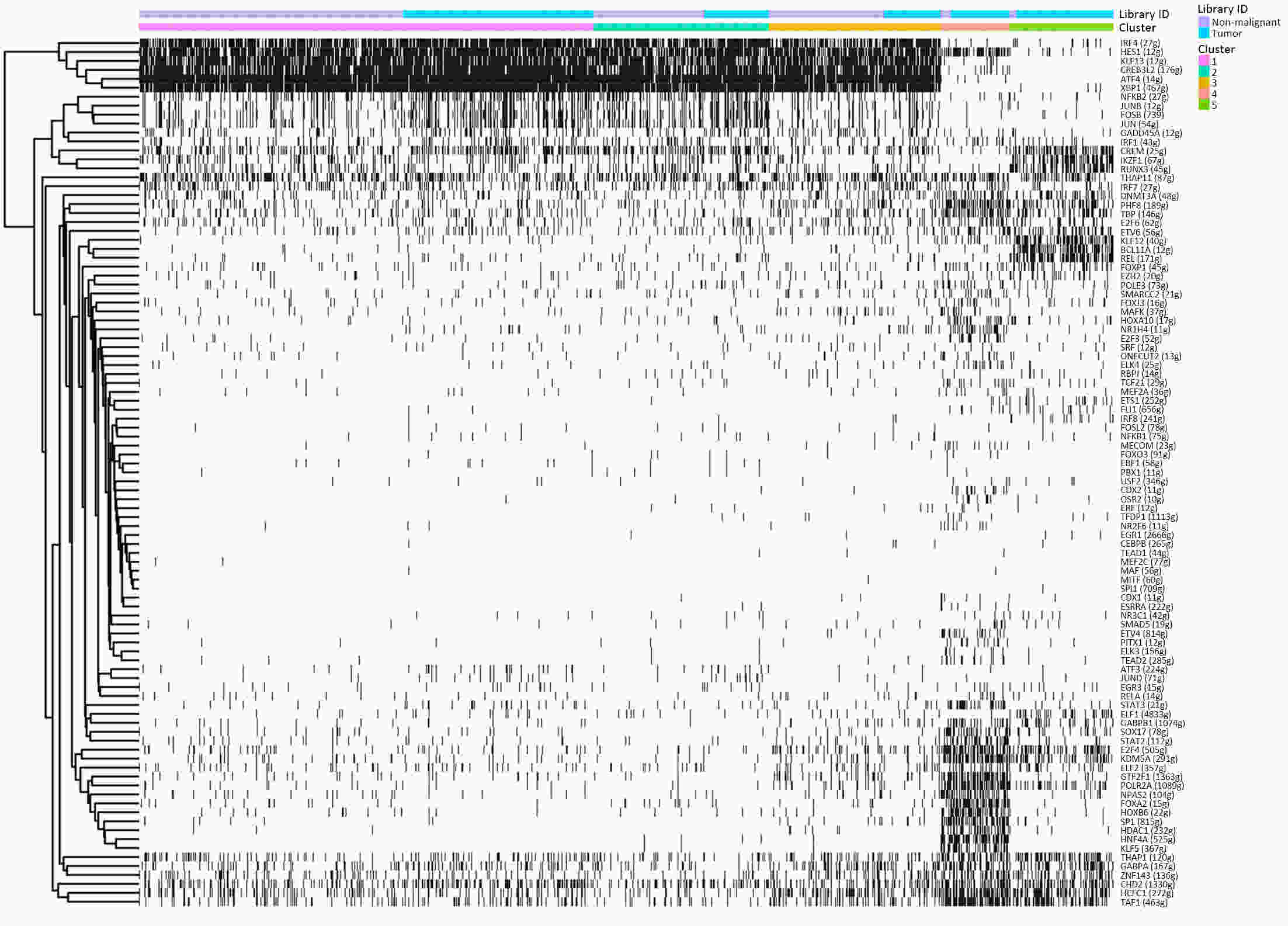
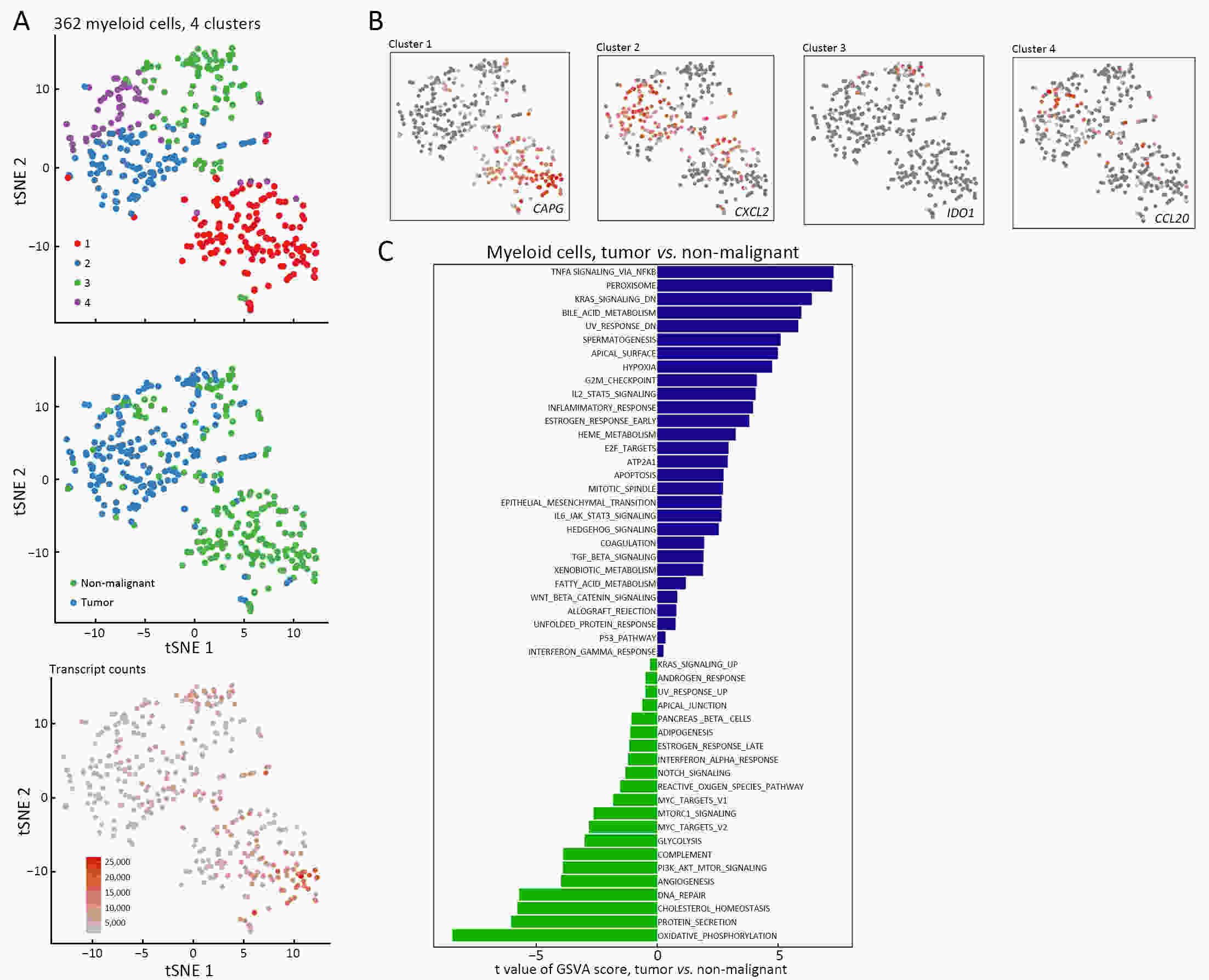
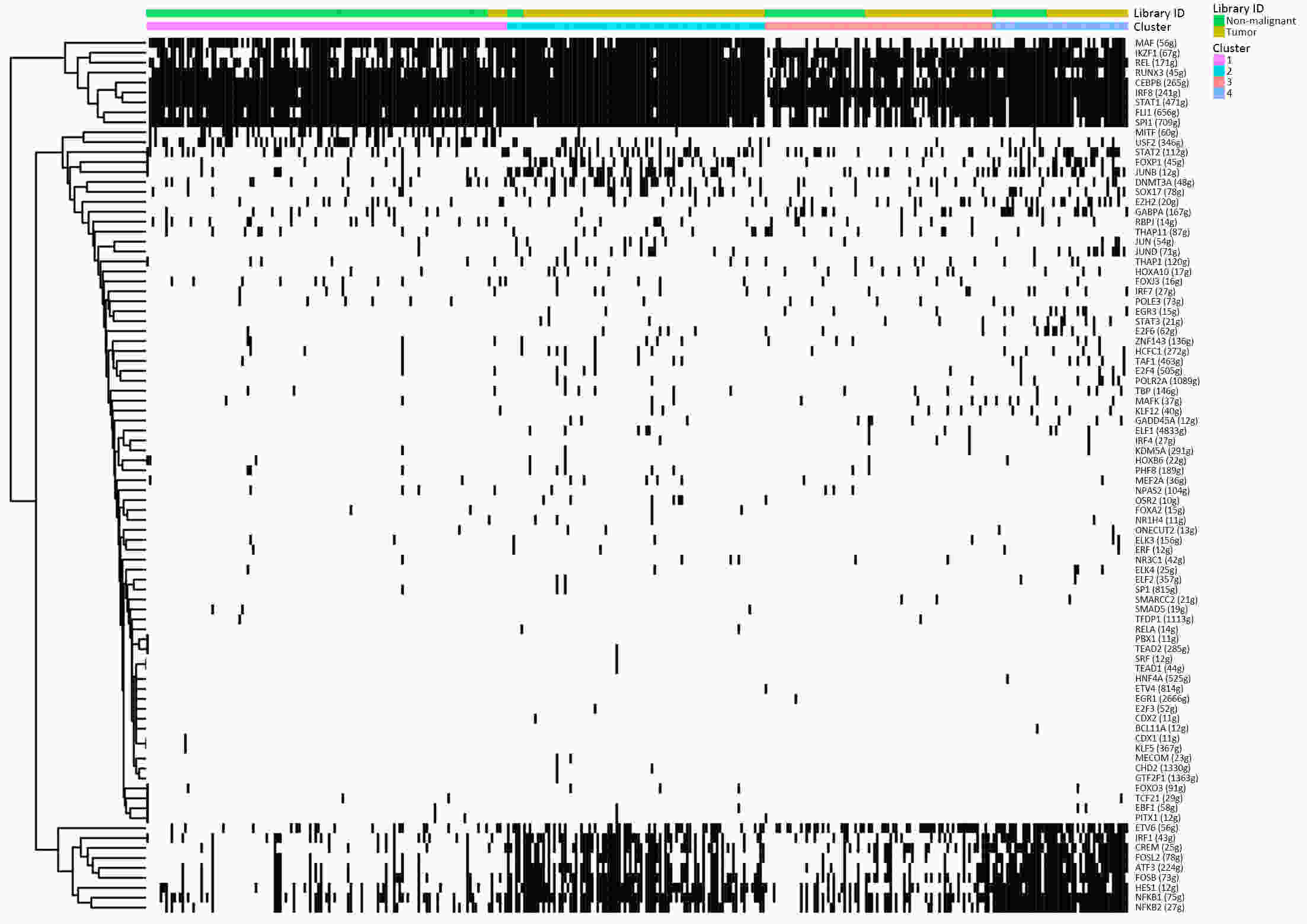
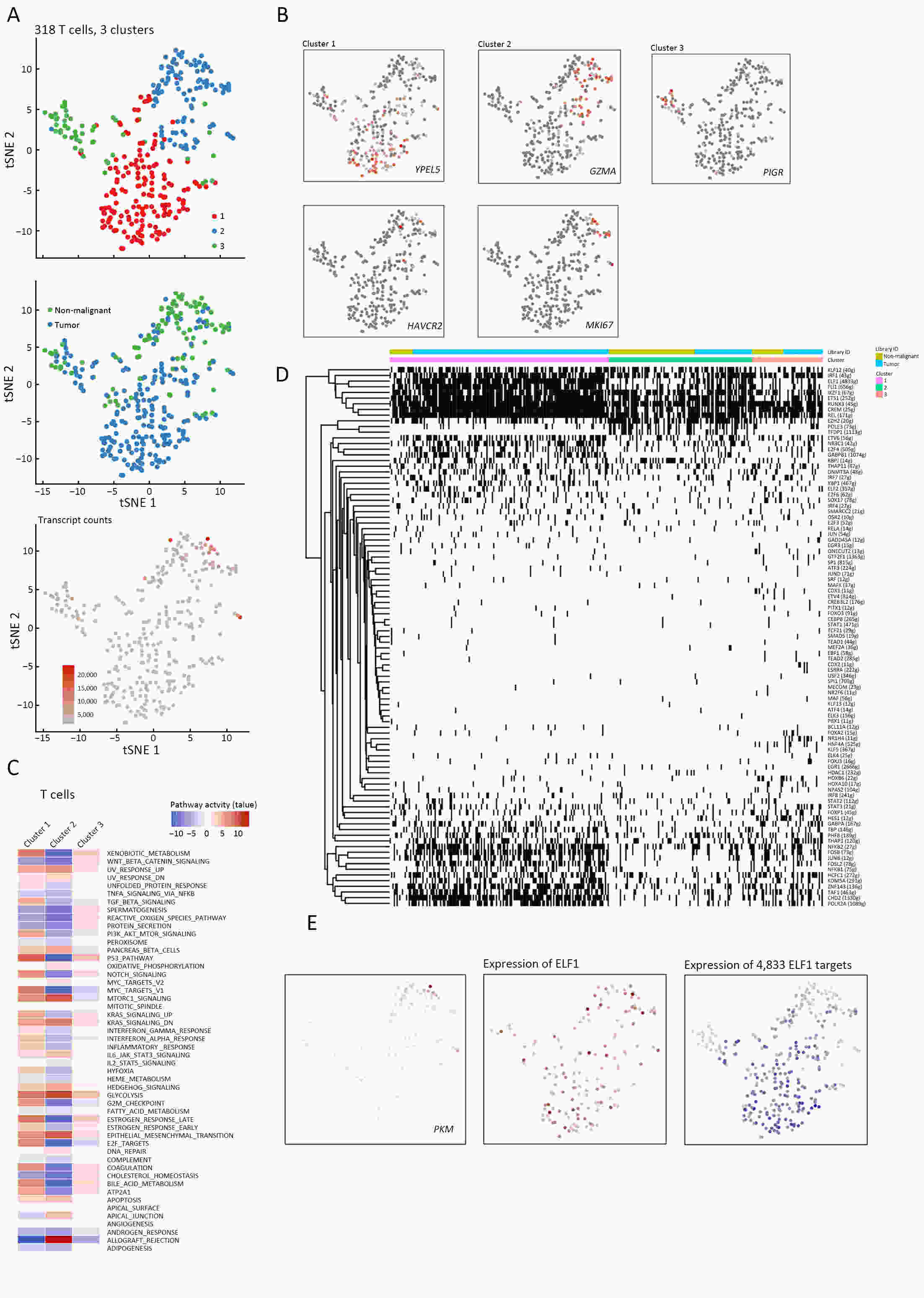
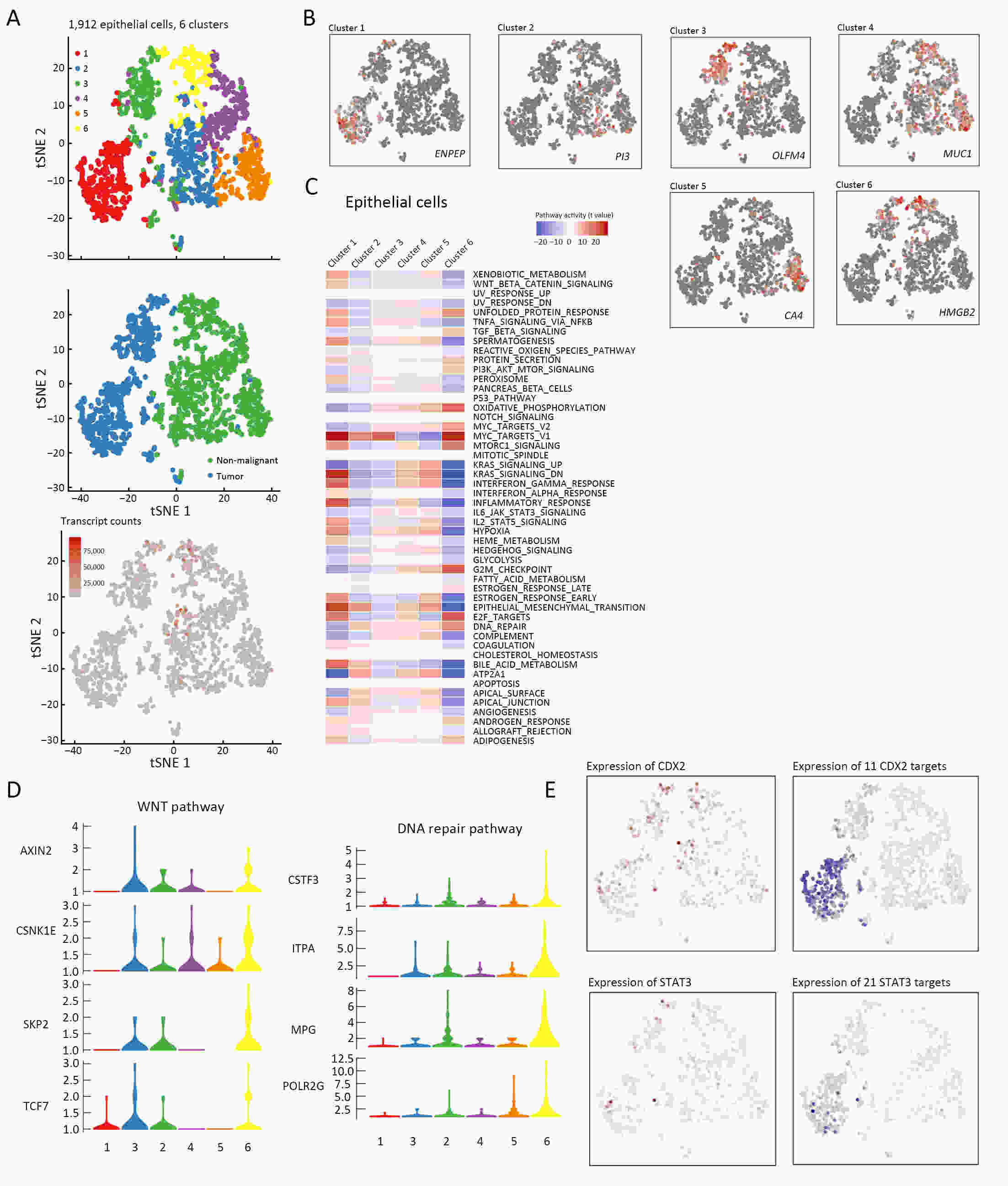
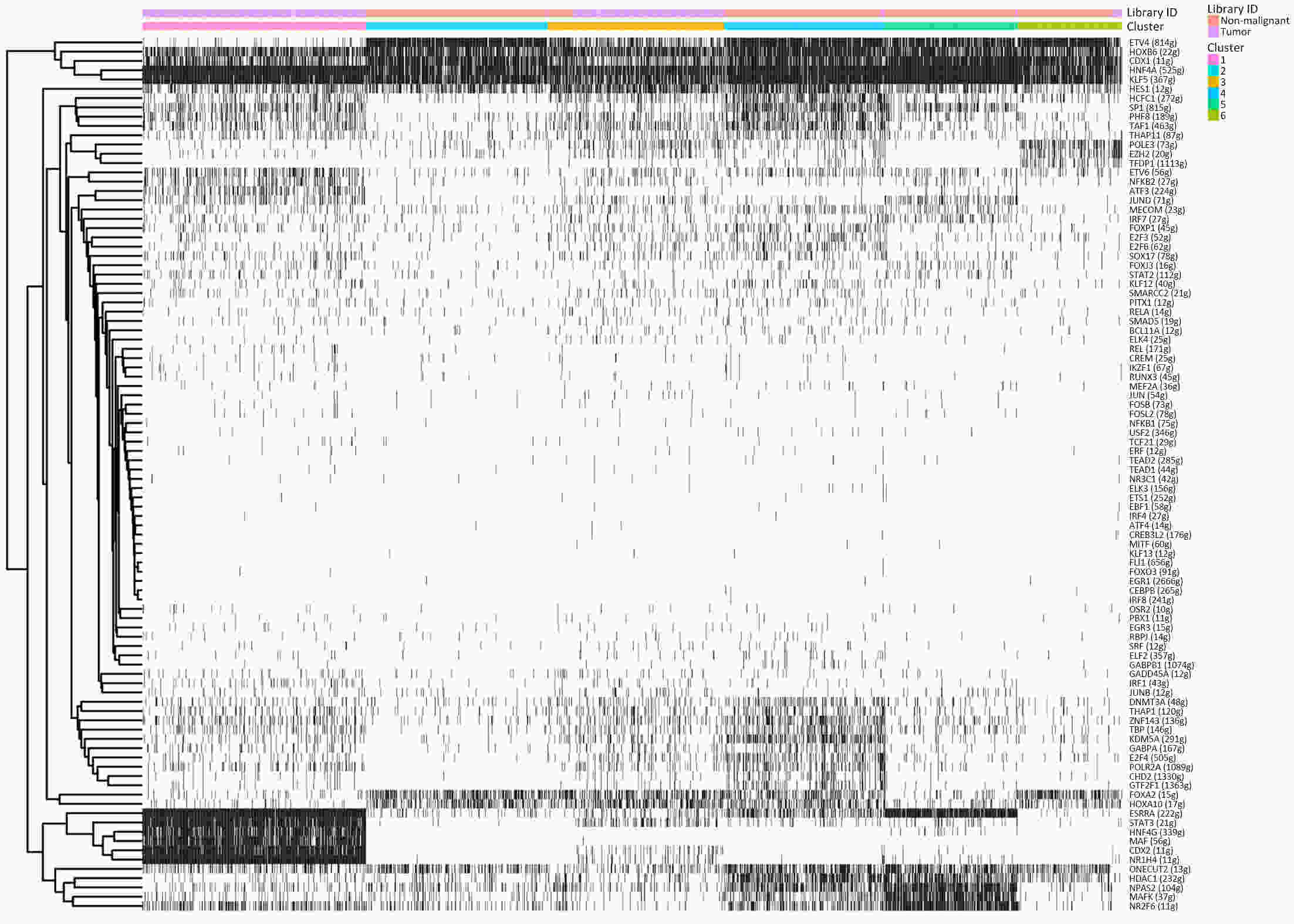
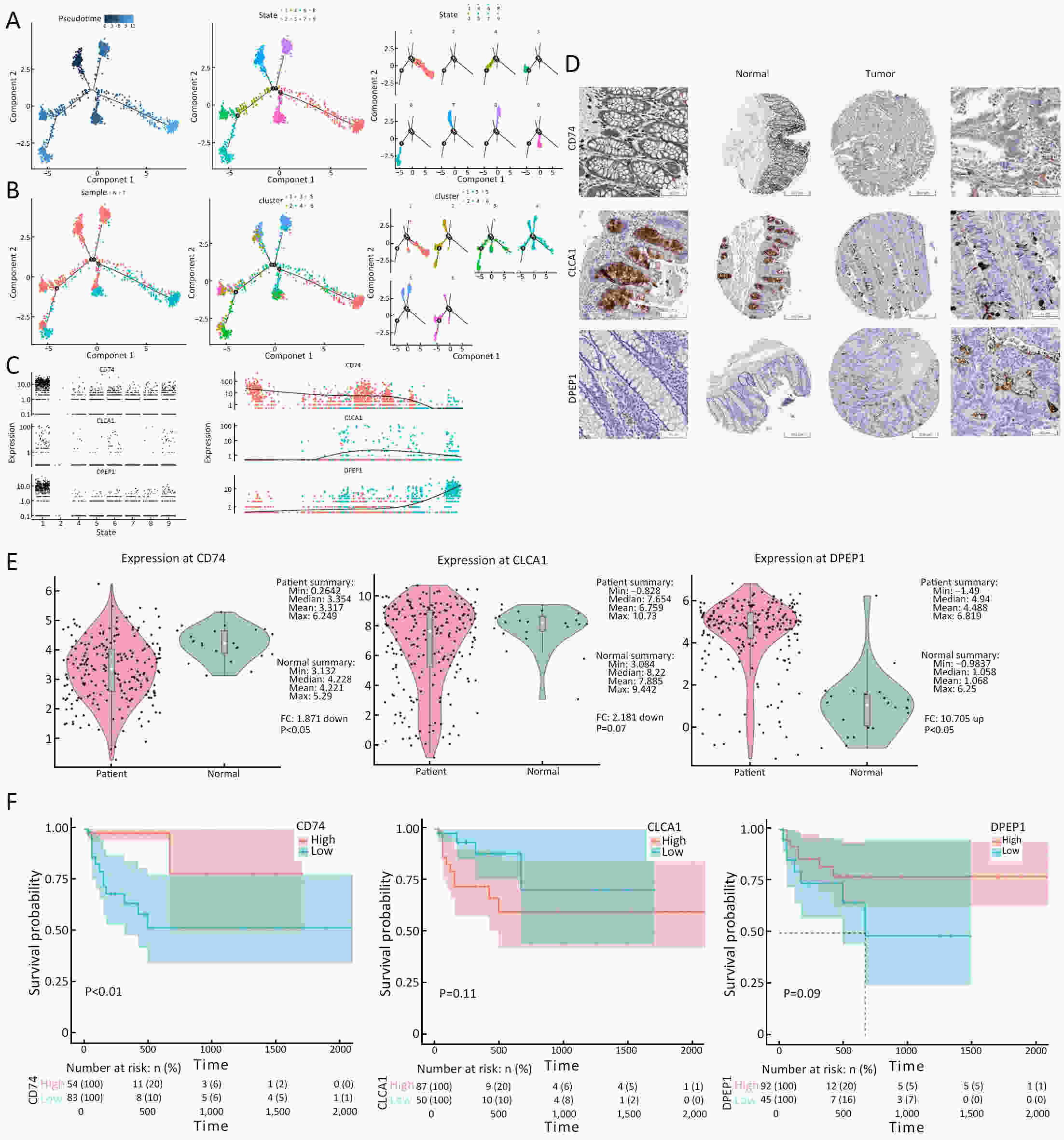
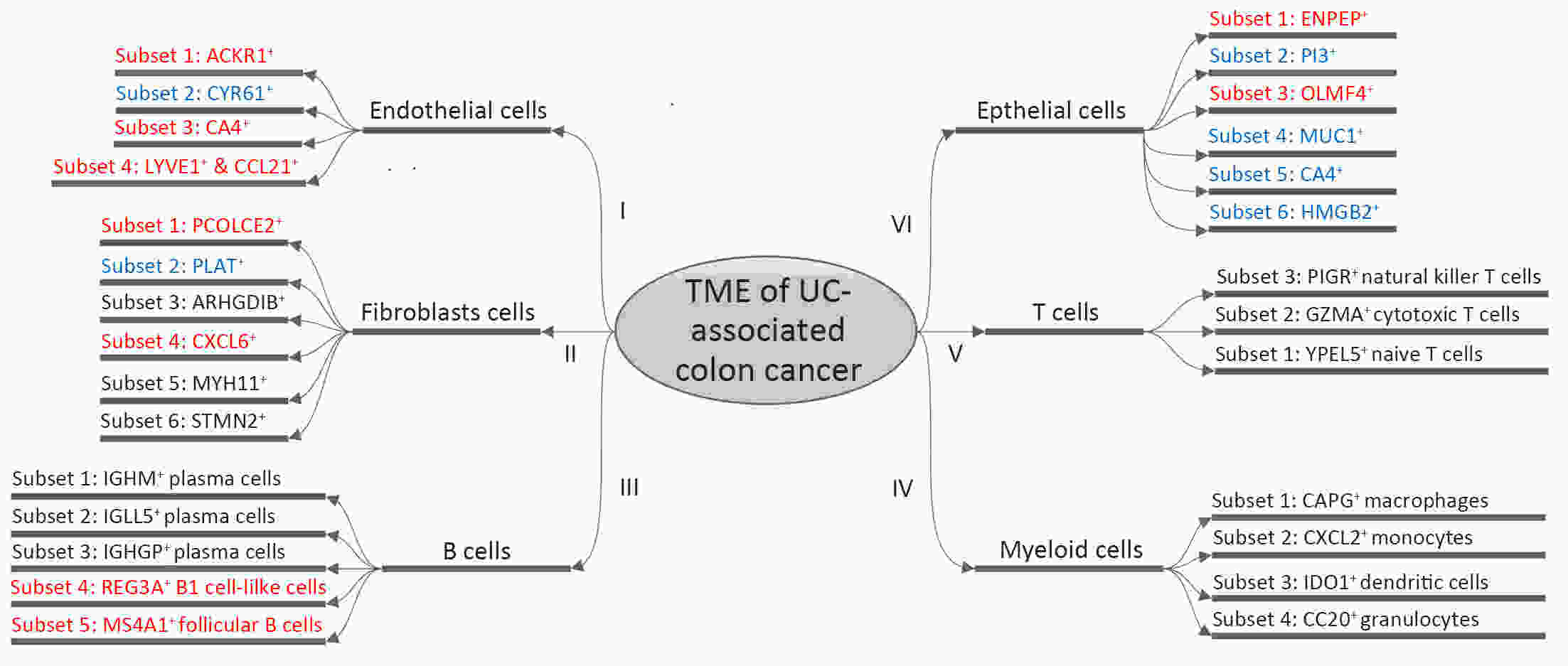
ObjectiveThe goal of this study was to get preliminary insight on the intra-tumor heterogeneity in colitis-associated cancer (CAC) and to reveal a potential evolutionary trajectory from ulcerative colitis (UC) to CAC at the single-cell level. MethodsFresh samples of tumor tissues and adjacent UC tissues from a CAC patient with pT3N1M0 stage cancer were examined by single-cell RNA sequencing (scRNA-seq). Data from The Cancer Genome Atlas (TCGA) and The Human Protein Atlas were used to confirm the different expression levels in normal and tumor tissues and to determine their relationships with patient prognosis. ResultsUltimately, 4,777 single-cell transcriptomes (1,220 genes per cell) were examined, of which 2,250 (47%) and 2,527 (53%) originated from tumor and adjacent UC tissues, respectively. We defined the composition of cancer-associated stromal cells and identified six cell clusters, including myeloid, T and B cells, fibroblasts, endothelial and epithelial cells. Notable pathways and transcription factors involved in these cell clusters were analyzed and described. Moreover, the precise cellular composition and developmental trajectory from UC to UC-associated colon cancer were graphed, and it was predicted that CD74, CLCA1, and DPEP1 played a potential role in disease progression. ConclusionsscRNA-seq technology revealed intra-tumor cell heterogeneity in UC-associated colon cancer, and might provide a promising direction to identify novel potential therapeutic targets in the evolution from UC to CAC.

 Abstract
Abstract FullText HTML
FullText HTML PDF 356KB
PDF 356KB