2021 Vol.33(3)
Display Mode: |
2021, 33(3): 289-301.
doi: 10.21147/j.issn.1000-9604.2021.03.01
Abstract:
2021, 33(3): 302-307.
doi: 10.21147/j.issn.1000-9604.2021.03.02
Abstract:
2021, 33(3): 308-322.
doi: 10.21147/j.issn.1000-9604.2021.03.03
Abstract:
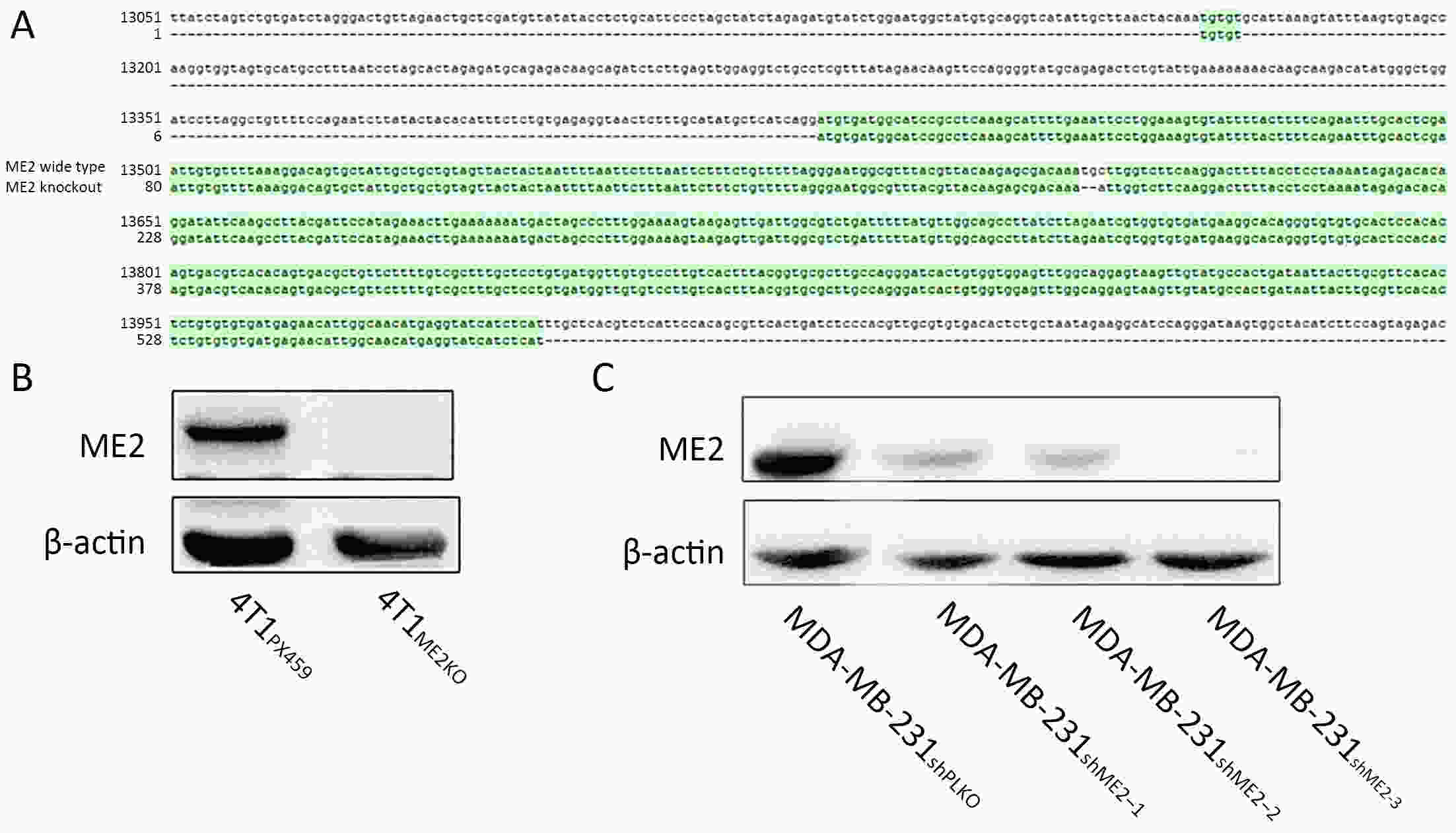
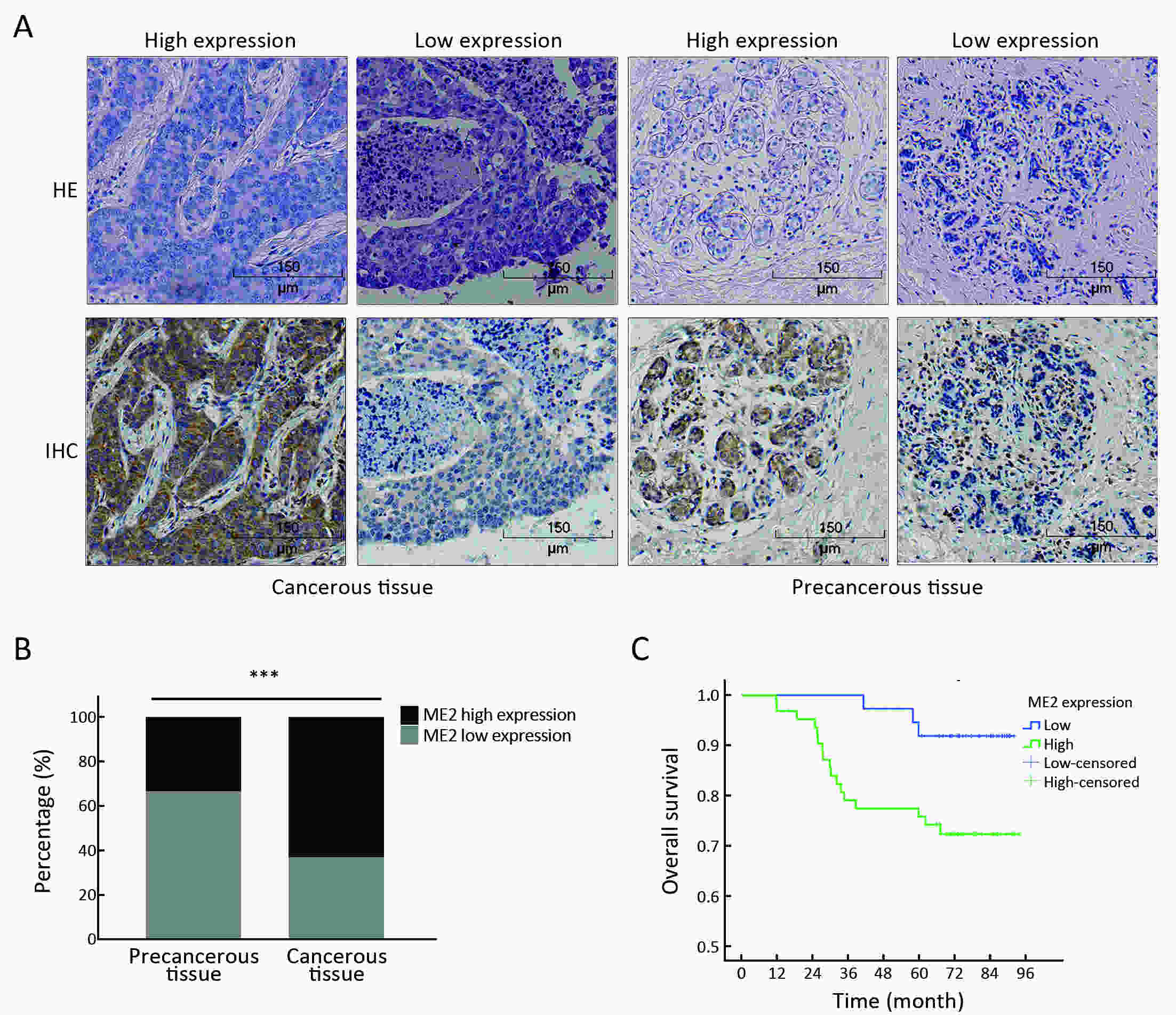
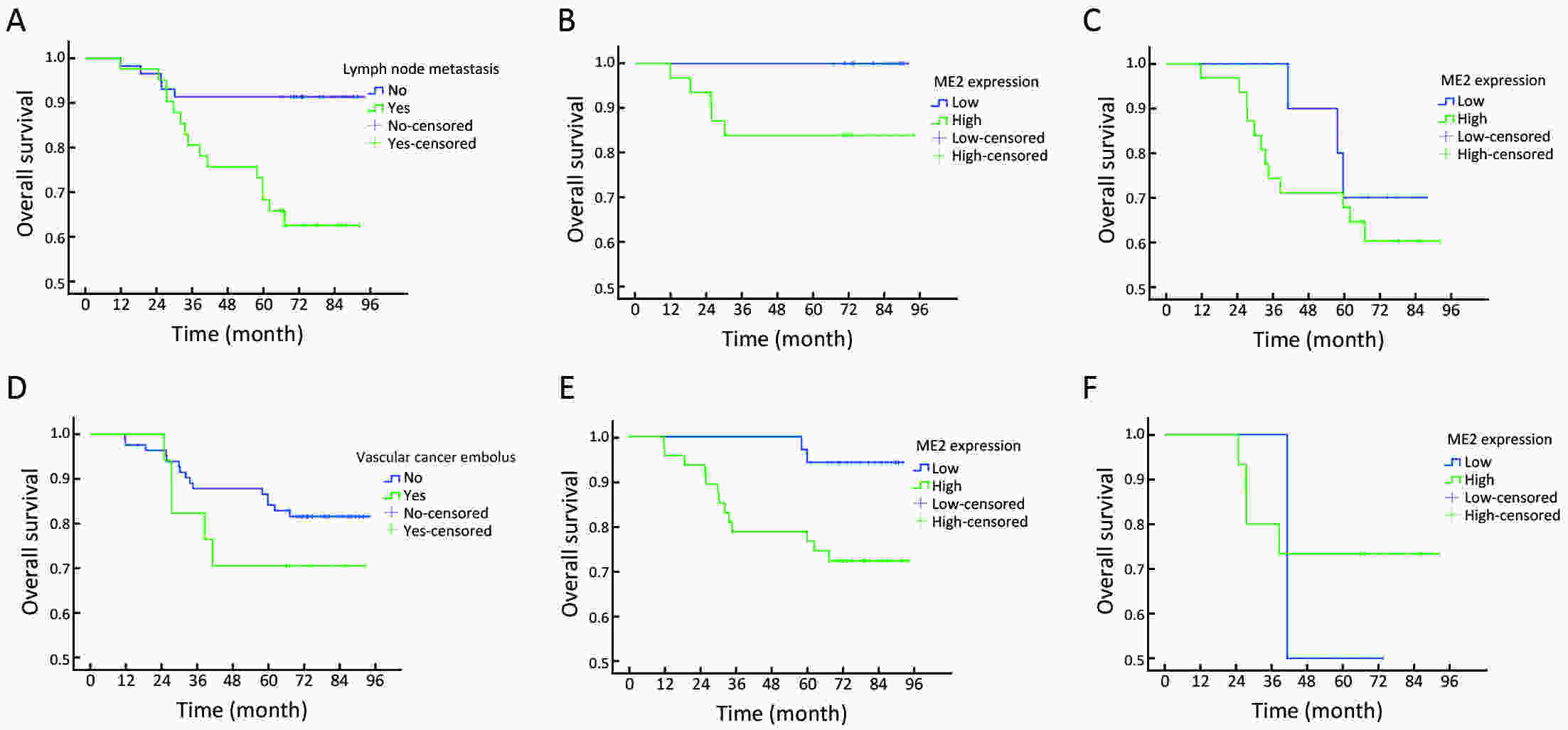
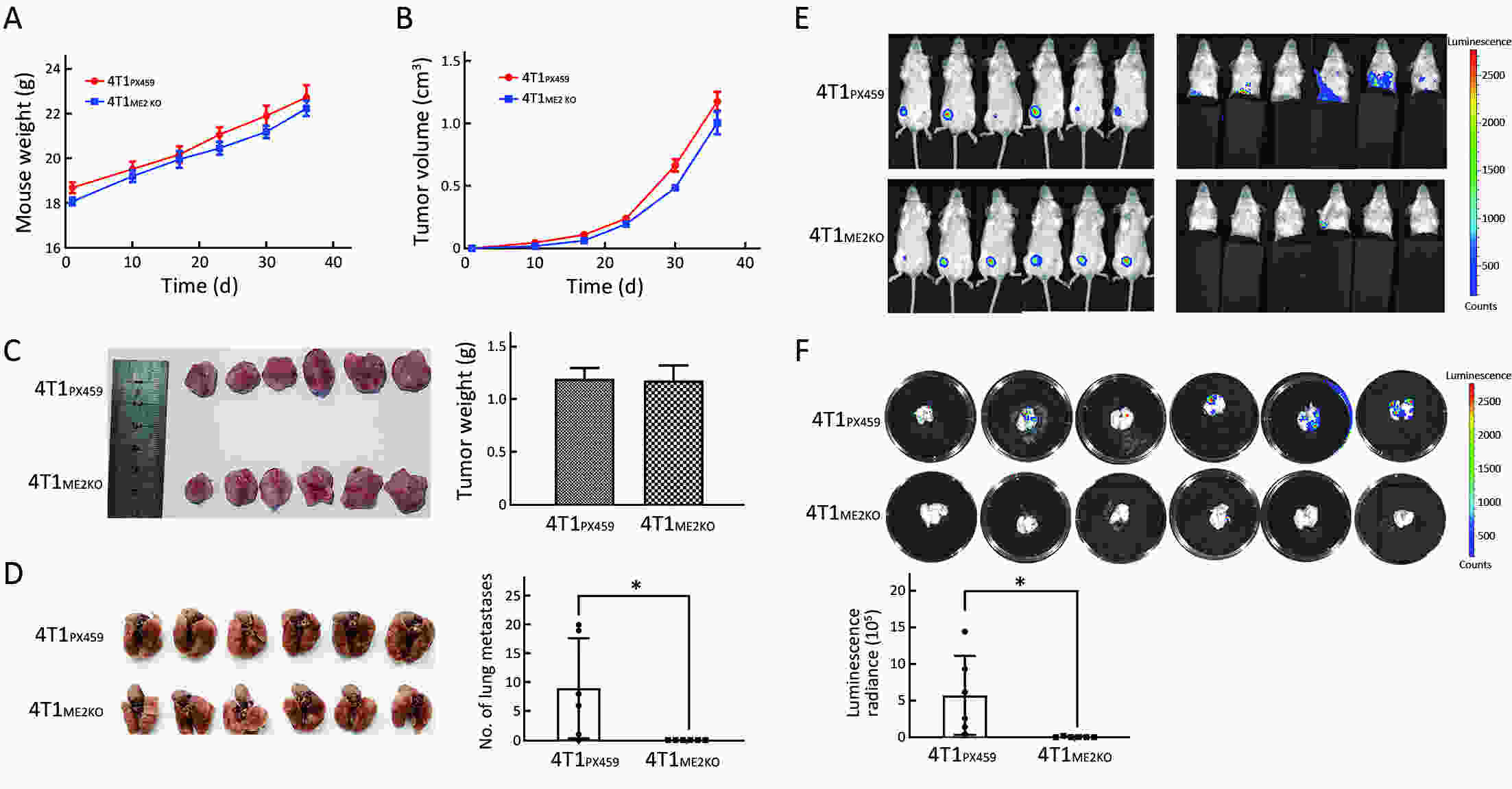
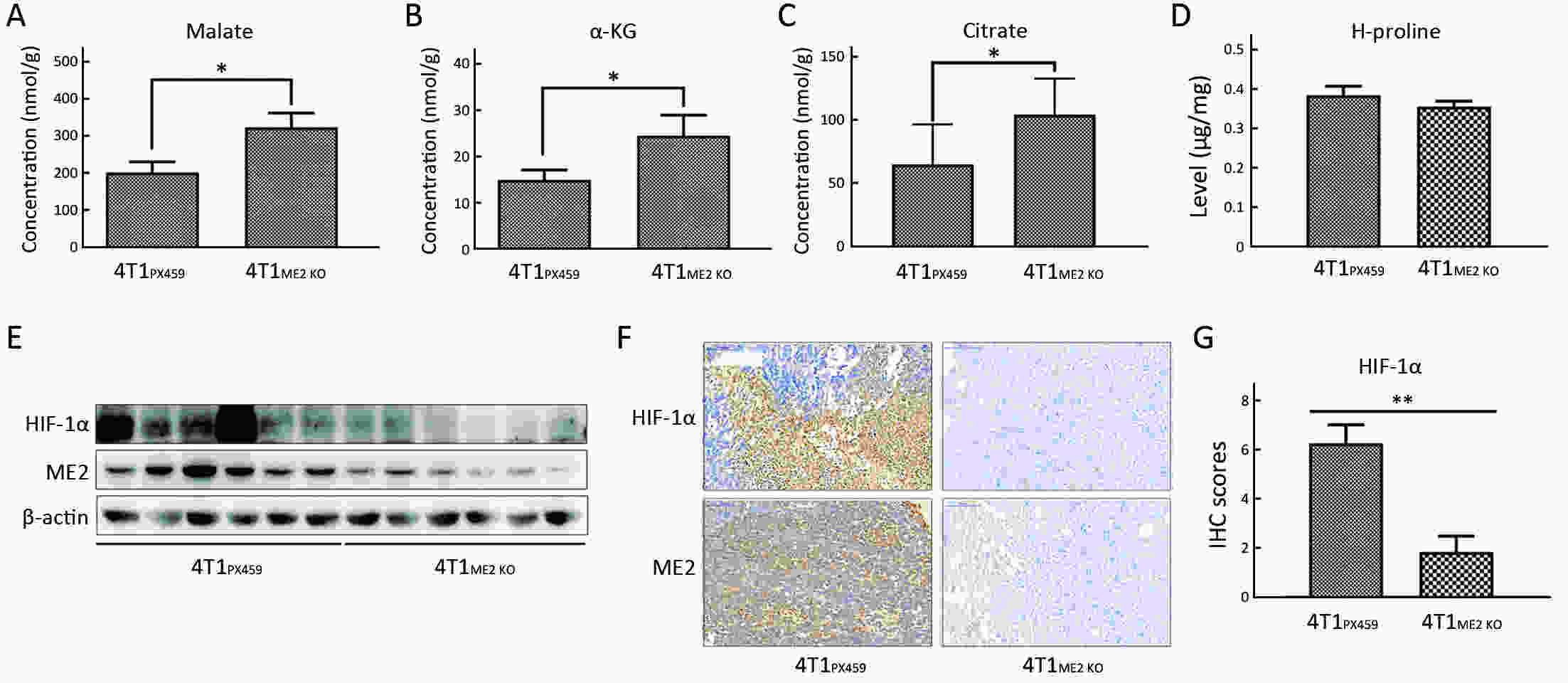
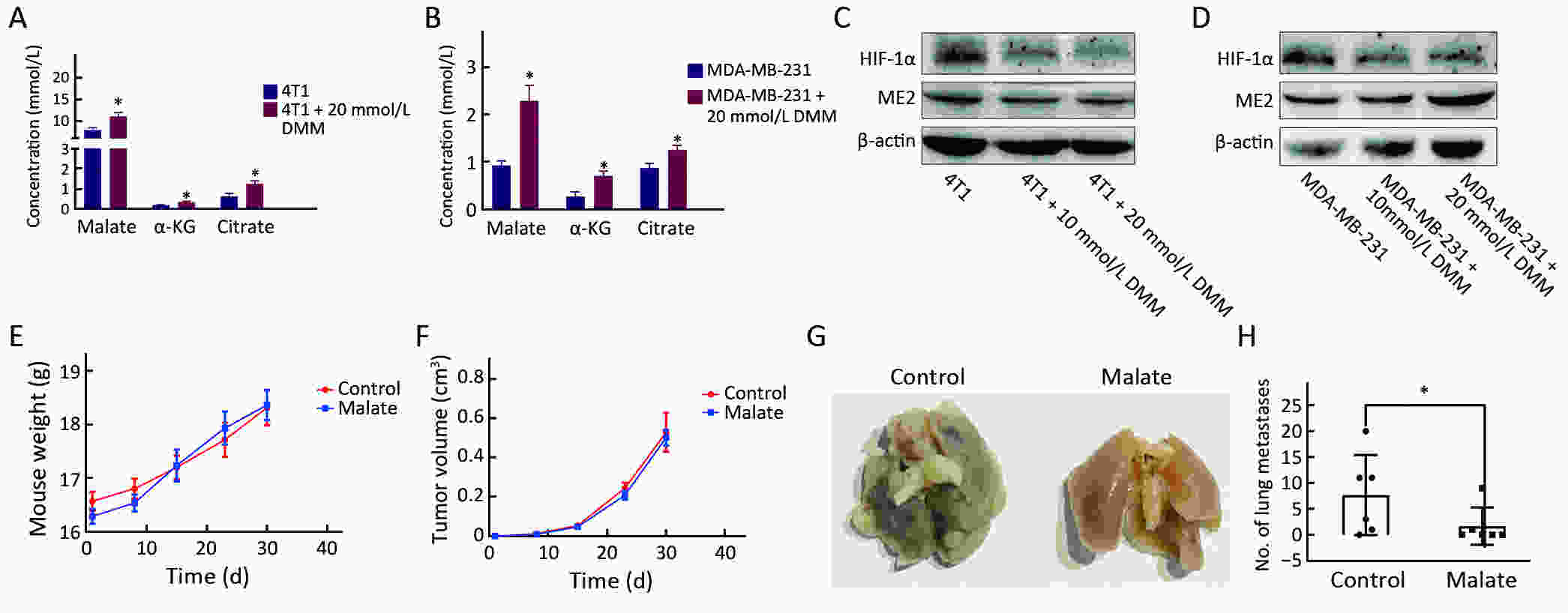
Objectiveα-ketoglutarate (α-KG) is the substrate to hydroxylate collagen and hypoxia-inducible factor-1α (HIF-1α), which are important for cancer metastasis. Previous studies have shown that the upregulation of collagen prolyl 4-hydroxylase in breast cancer cells stabilizes the expression of HIF-1α by depleting α-KG levels. We hypothesized that mitochondrial malic enzyme 2 (ME2) might also affect HIF-1α expression via modulating α-KG levels in breast cancer cells. MethodsWe evaluated ME2 protein expression in 100 breast cancer patients using immunohistochemistry and correlated with clinicopathological indicators. The effect of ME2 knockout on cancer metastasis was evaluated using an orthotopic breast cancer model. The effect of ME2 knockout or knockdown on the levels of α-KG and HIF-1α proteins in breast cancer cell lines was determined both in vitro and in vivo. ResultsME2 was found to be upregulated in the human breast cancerous tissues compared with the matched precancerous tissues (P<0.001). The elevated expression of ME2 was associated with a poor prognosis (P=0.019). ME2 upregulation was also related to lymph node metastasis (P=0.016), pathological staging (P=0.033), and vascular cancer embolus (P=0.014). Also, ME2 knockout significantly inhibited lung metastasis in vivo. In the tumors formed by ME2 knockout cells, the levels of α-KG were significantly increased and collagen hydroxylation level did not change significantly but HIF-1α protein expression was significantly decreased, compared to the control samples. In cell culture, cells with ME2 knockout or knockdown demonstrated significantly higher α-KG levels but significantly lower HIF-1α protein expression than control cells under hypoxia. Exogenous malate and α-KG exerted similar effect on HIF-1α in breast cancer cells to ME2 knockout or knockdown. Additionally, treatment with malate significantly decreased 4T1 breast cancer lung metastasis. ME2 expression was associated with HIF-1α levels in human breast cancer samples (P=0.008). ConclusionsOur results provide evidence that upregulation of ME2 is associated with a poor prognosis of breast cancer patients and propose a mechanistic understanding of a link between ME2 and breast cancer metastasis.
2021, 33(3): 323-330.
doi: 10.21147/j.issn.1000-9604.2021.03.04
Abstract:
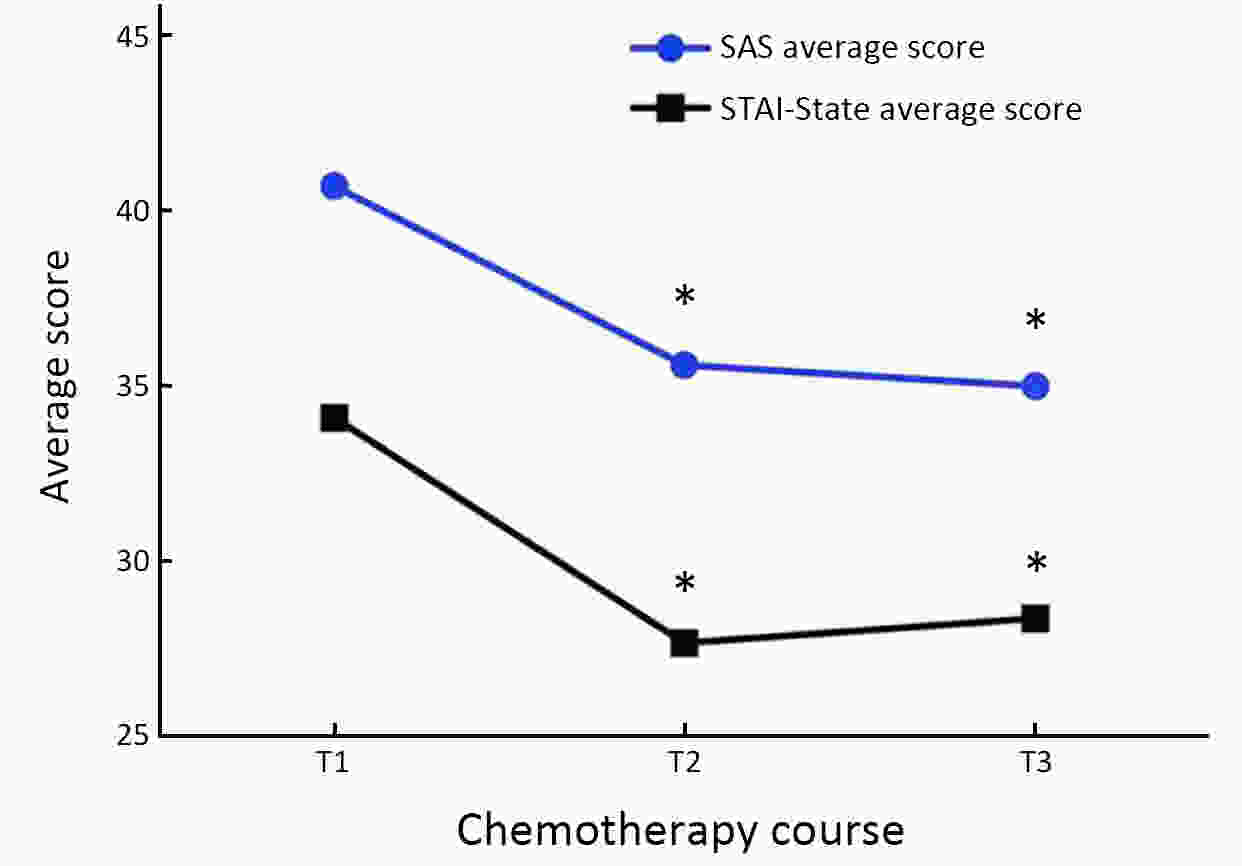
ObjectiveTo examine the trajectory of psychosomatic symptoms and to explore the impact of psychosomatic symptoms on setup error in patients undergoing breast cancer radiotherapy. MethodsA total of 102 patients with early breast cancer who received initial radiotherapy were consecutively recruited. The M.D. Anderson Symptom Inventory (MDASI) and three different anxiety scales, i.e., the Self-Rating Anxiety Scale (SAS), State-Trait Anxiety Inventory (STAI), and Anxiety Sensitivity Index (ASI), were used in this study. The radiotherapy setup errors were measured in millimetres by comparing the real-time isocratic verification film during radiotherapy with the digitally reconstructed radiograph (DRR). Patients completed the assessment at three time points: before the initial radiotherapy (T1), before the middle radiotherapy (T2), and before the last radiotherapy (T3). ResultsThe SAS and STAI-State scores of breast cancer patients at T1 were significantly higher than those at T2 and T3 (F=24.44, P<0.001; F=30.25, P<0.001). The core symptoms of MDASI were positively correlated with anxiety severity. The setup errors of patients with high SAS scores were greater than those of patients with low anxiety levels at T1 (Z=−2.01, P=0.044). We also found that higher SAS scores were associated with a higher risk of radiotherapy setup errors at T1 (B=0.458, P<0.05). ConclusionsThis study seeks to identify treatment-related psychosomatic symptoms and mitigate their impact on patients and treatment. Patients with early breast cancer experienced the highest level of anxiety before the initial radiotherapy, and then, anxiety levels declined. Patients with high somatic symptoms of anxiety may have a higher risk of radiotherapy setup errors.
2021, 33(3): 331-342.
doi: 10.21147/j.issn.1000-9604.2021.03.05
Abstract:
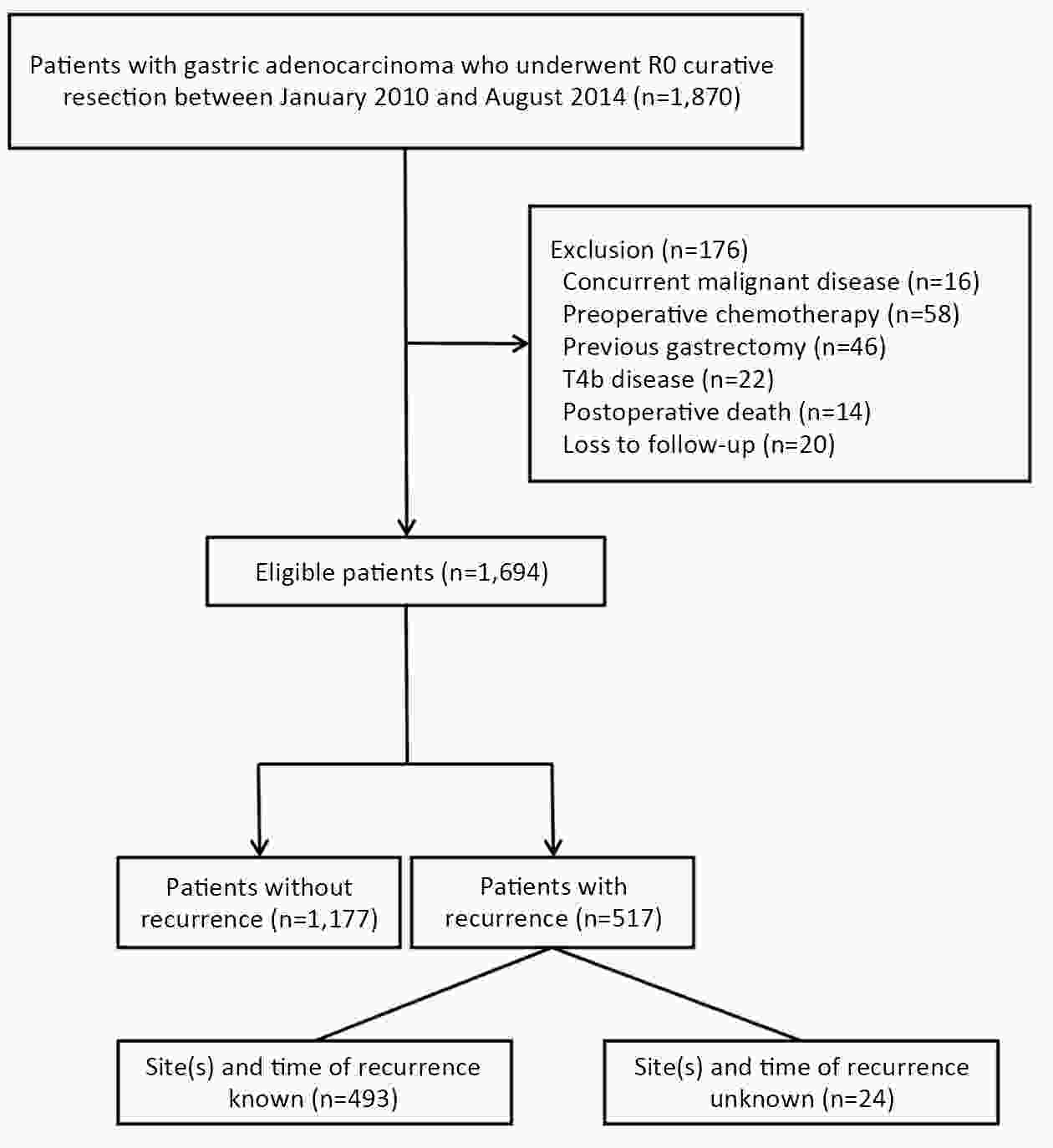
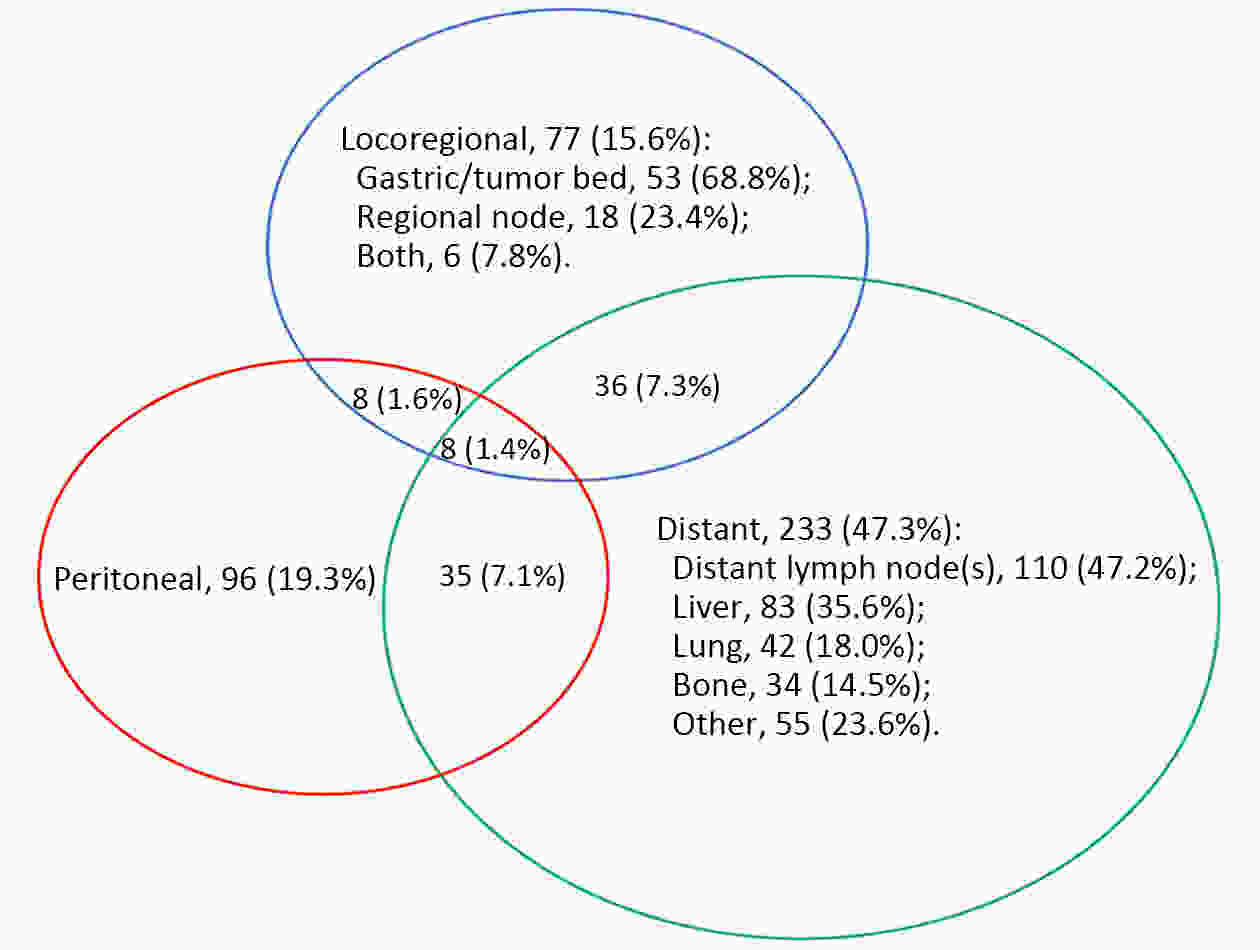
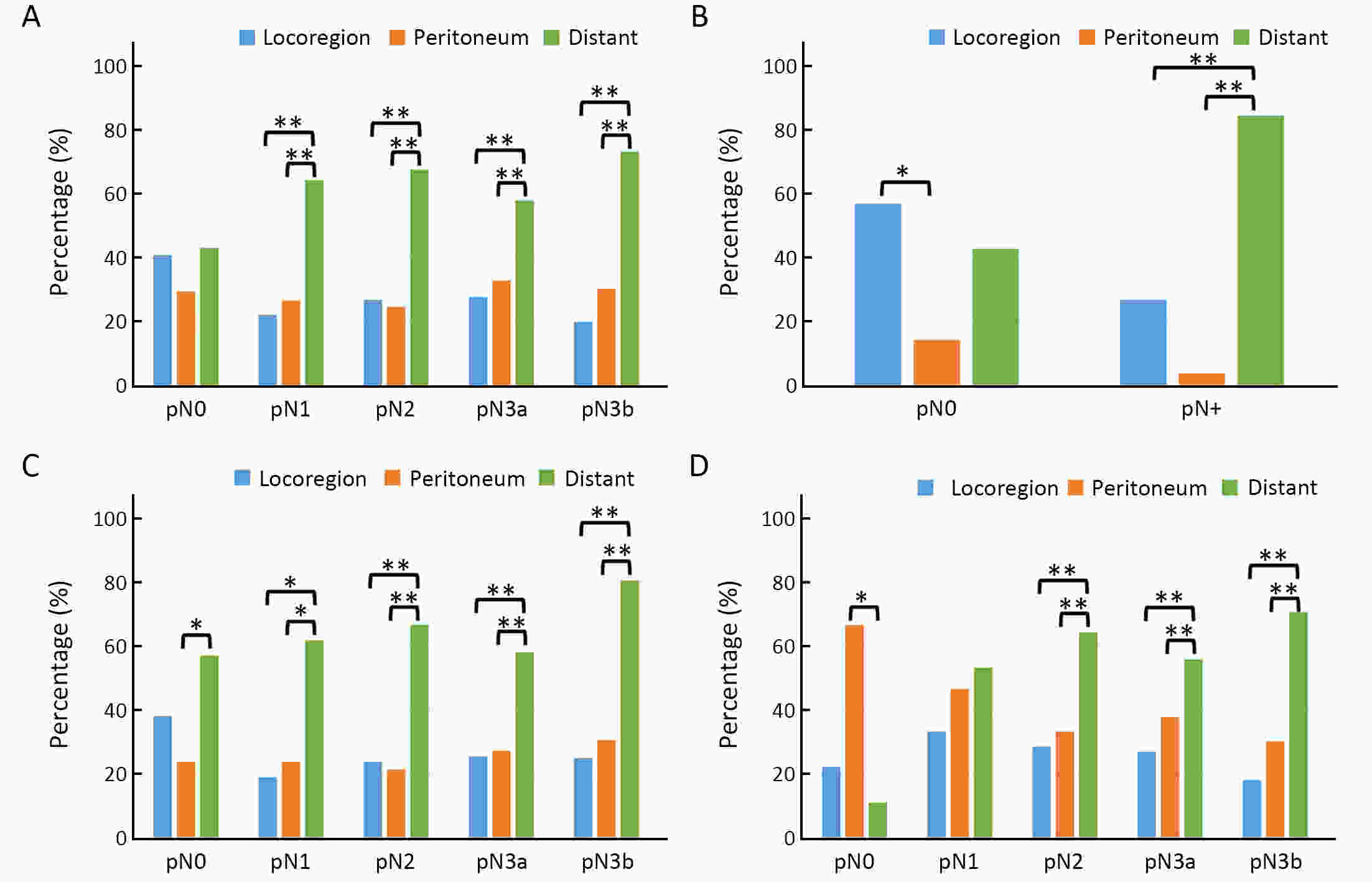
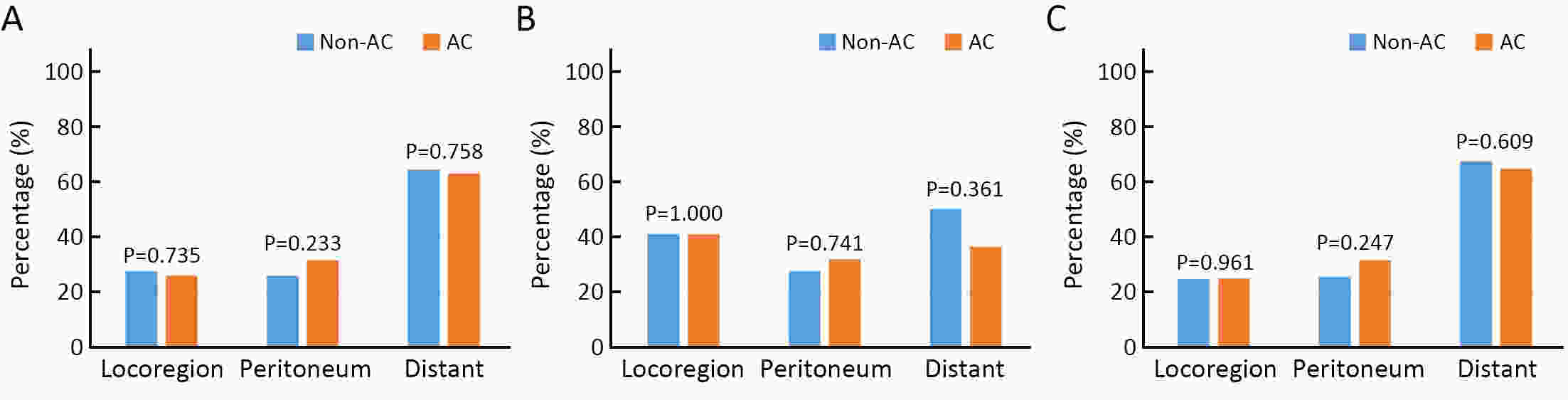
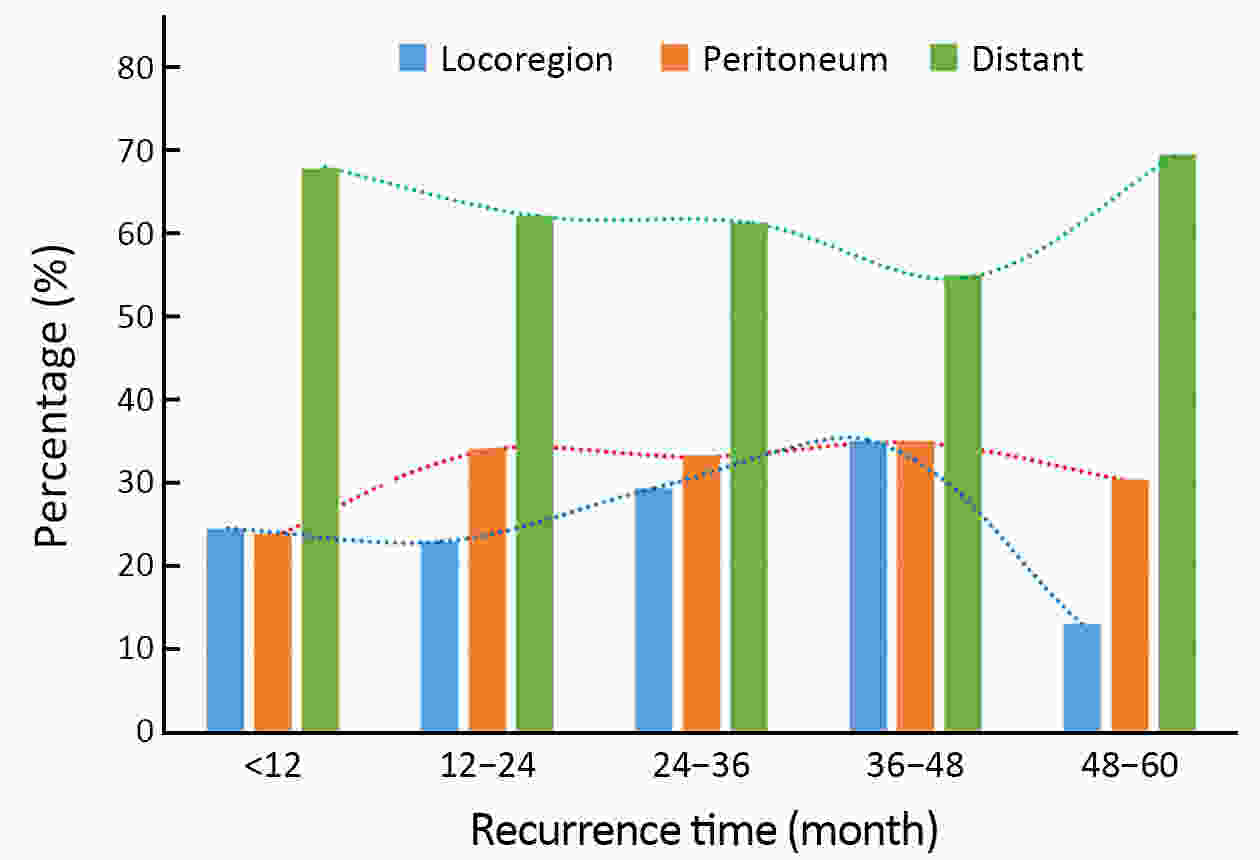
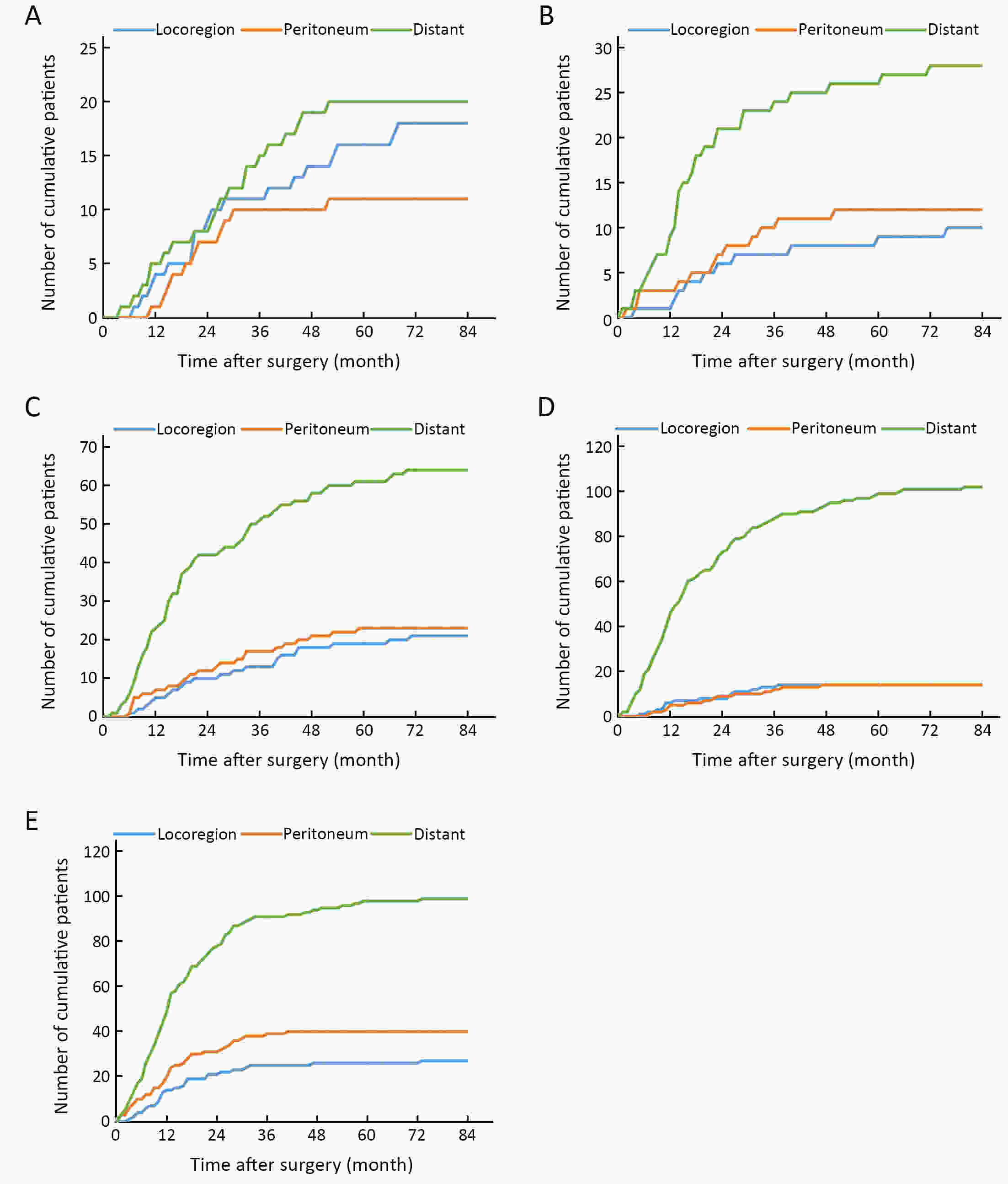
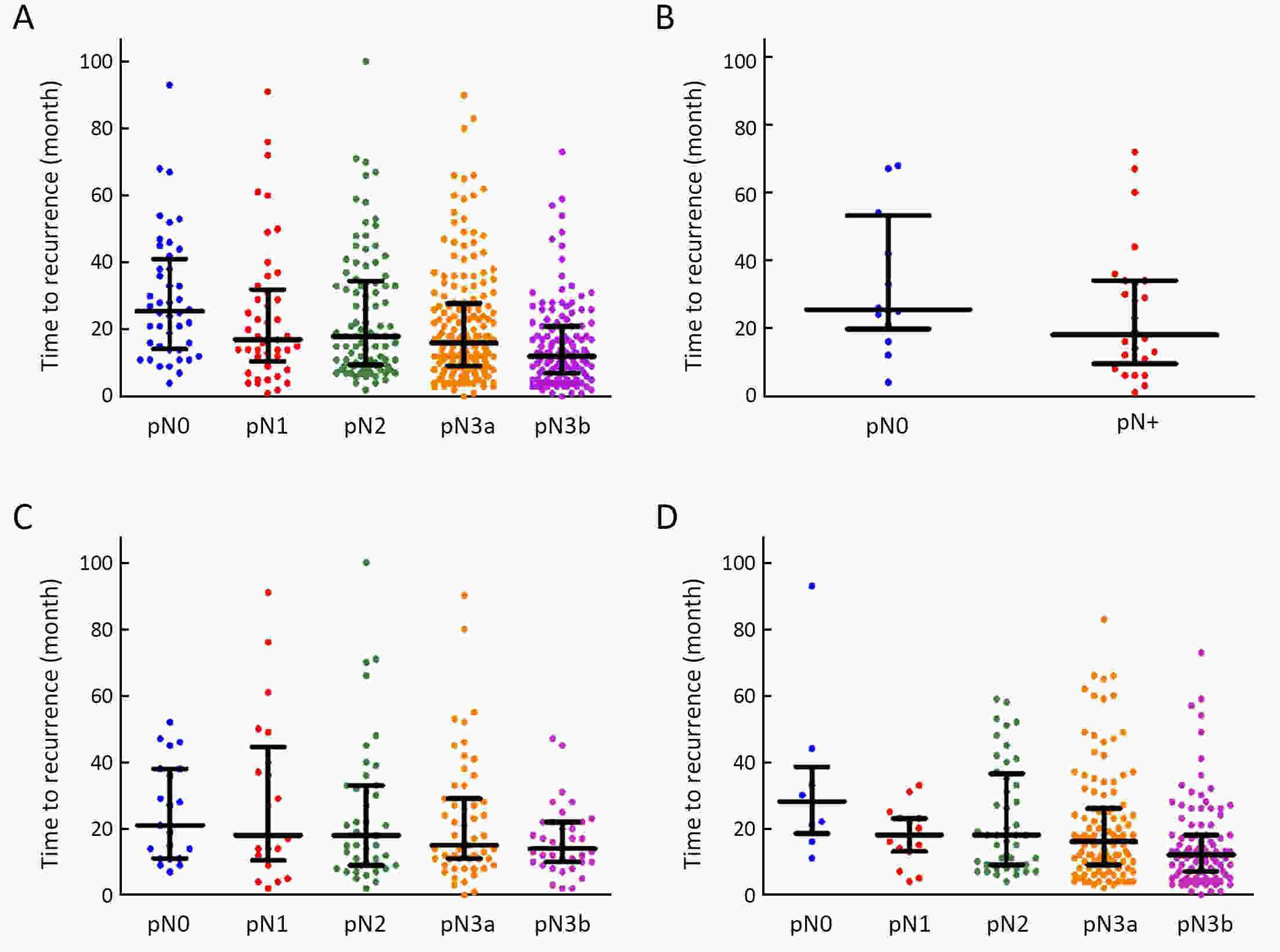
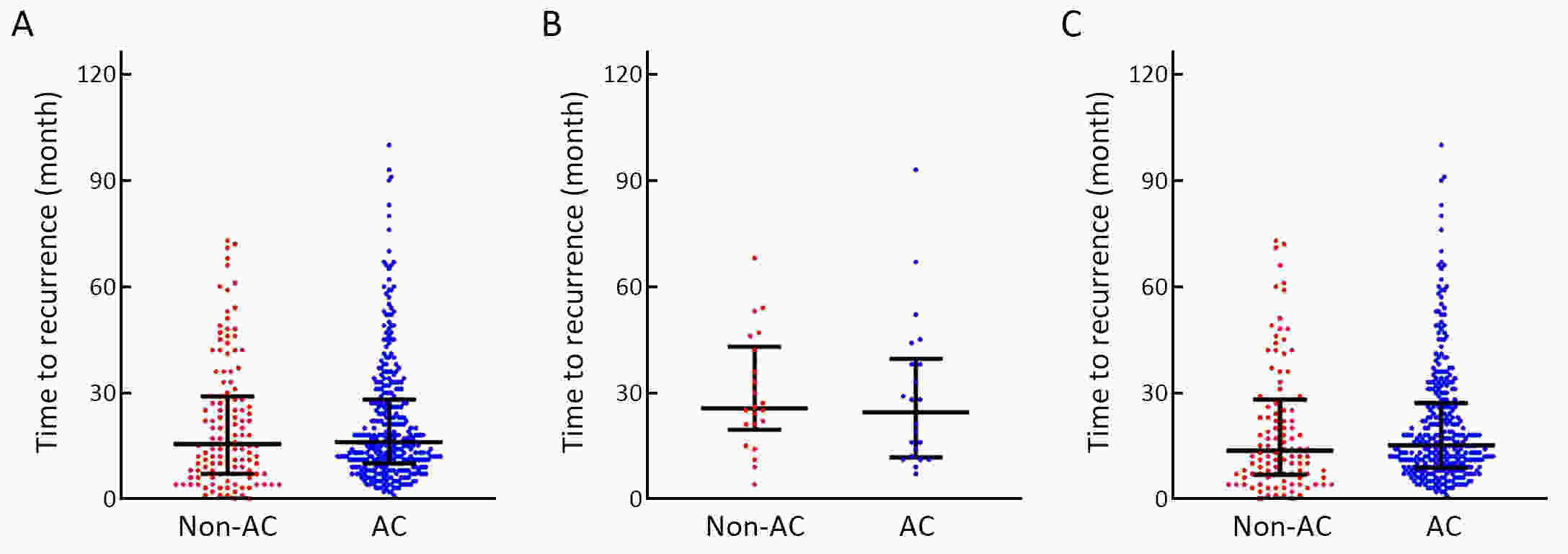
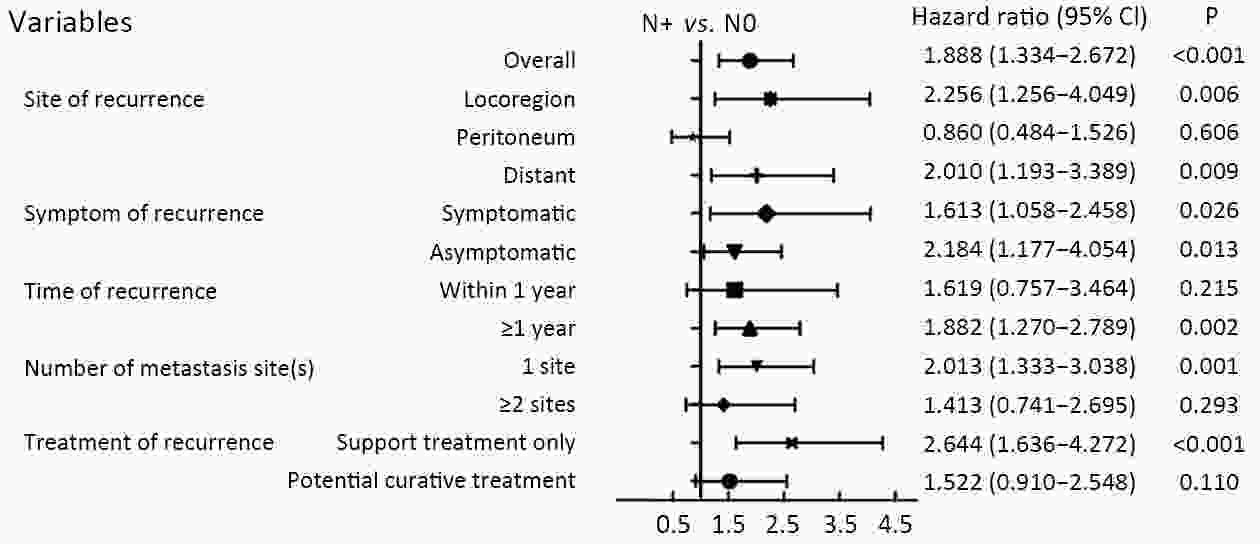
ObjectiveTo examine the association between lymph node status and recurrence patterns in completely resected gastric adenocarcinoma. MethodsWe retrospectively assessed 1,694 patients who underwent curative gastrectomy from January 2010 to August 2014. Patients stratified according to lymph node status and recurrence patterns among different subgroups were compared. ResultsOf all, 517 (30.5%) patients developed recurrent disease, and complete data of recurrence could be obtained in 493 (95.4%) patients. For pN0 patients, the patterns of recurrence were different according to pT stage: locoregional recurrence was most common in patients with pT1−2 disease (57.1%), distant recurrence was most common in patients with pT3 disease (57.1%), and peritoneal recurrence was most common in patients with pT4a disease (66.7%). For pN+ patients, distant metastasis was most common pattern irrespective of pT stage. The site-specific trend of recurrence showed that locoregional recurrence increased within 5 years in patients with pN0−2 disease but plateaued 3 years after surgery in patients with pN3 disease. Time to recurrence was significantly longer for the pN0 patients compared with the pN+ patients (median: 25 vs. 16 months, P=0.001). Moreover, post-recurrence survival was significantly better for the pN0 patients than for the pN+ patients (median: 12 vs. 6 months, P<0.001), especially in patients with non-peritoneal recurrence, late recurrence, single recurrence, and receipt of potential curative treatment. ConclusionsAmong clinicopathologic factors, lymph node status is the most important factor associated with recurrence patterns after curative gastrectomy. Lymph node status may be used as an adjunct in clinical decision-making about postoperative therapeutic and follow-up strategies.
2021, 33(3): 343-351.
doi: 10.21147/j.issn.1000-9604.2021.03.06
Abstract:
ObjectiveThis prospective cohort study explored factors related to postoperative pain in gastric cancer patients. MethodsA total of 236 patients who underwent gastrectomy were enrolled. All patients enrolled in the study completed the Hospital Anxiety and Depression Scale (HADS) questionnaire and Life Orientation Test-Revised (LOT-R) questionnaire on the day before surgery. Heat pain threshold (HPT), cold pain threshold (CPT) and pressure pain threshold (PPT) were measured for all patients one day prior to surgery and demographic details were collected. All patients were connected to a patient-controlled intravenous analgesia (PCIA) pump at the end of the surgery. The occurrence of postoperative pain was used as a dependent variable, and multivariate logistic regression analyses were conducted to screen for factors affecting postoperative pain. ResultsIn total, 83 patients (35.2%) had postoperative pain. Body mass index (BMI) ≥28 kg/m2 [odds ratio (OR): 2.67; 95% confidence interval (95% CI): 1.07−6.67], total gastrectomy (OR: 2.64; 95% CI: 1.42−4.91), preoperative anxiety score ≥8 (OR: 2.37; 95% CI: 1.12−5.02), heat pain threshold ≤4.9 s (OR: 2.14; 95% CI: 1.06−4.32), pressure pain threshold ≤4 g (OR: 2.05; 95% CI: 1.05−4.03), and female gender (OR: 1.99; 95% CI: 1.04−3.83) were risk factors for postoperative pain. ConclusionsObesity, wide range of gastrectomy, high preoperative anxiety, low HPT and PPT, and female gender are associated with increased risk for postoperative pain.
2021, 33(3): 352-363.
doi: 10.21147/j.issn.1000-9604.2021.03.07
Abstract:
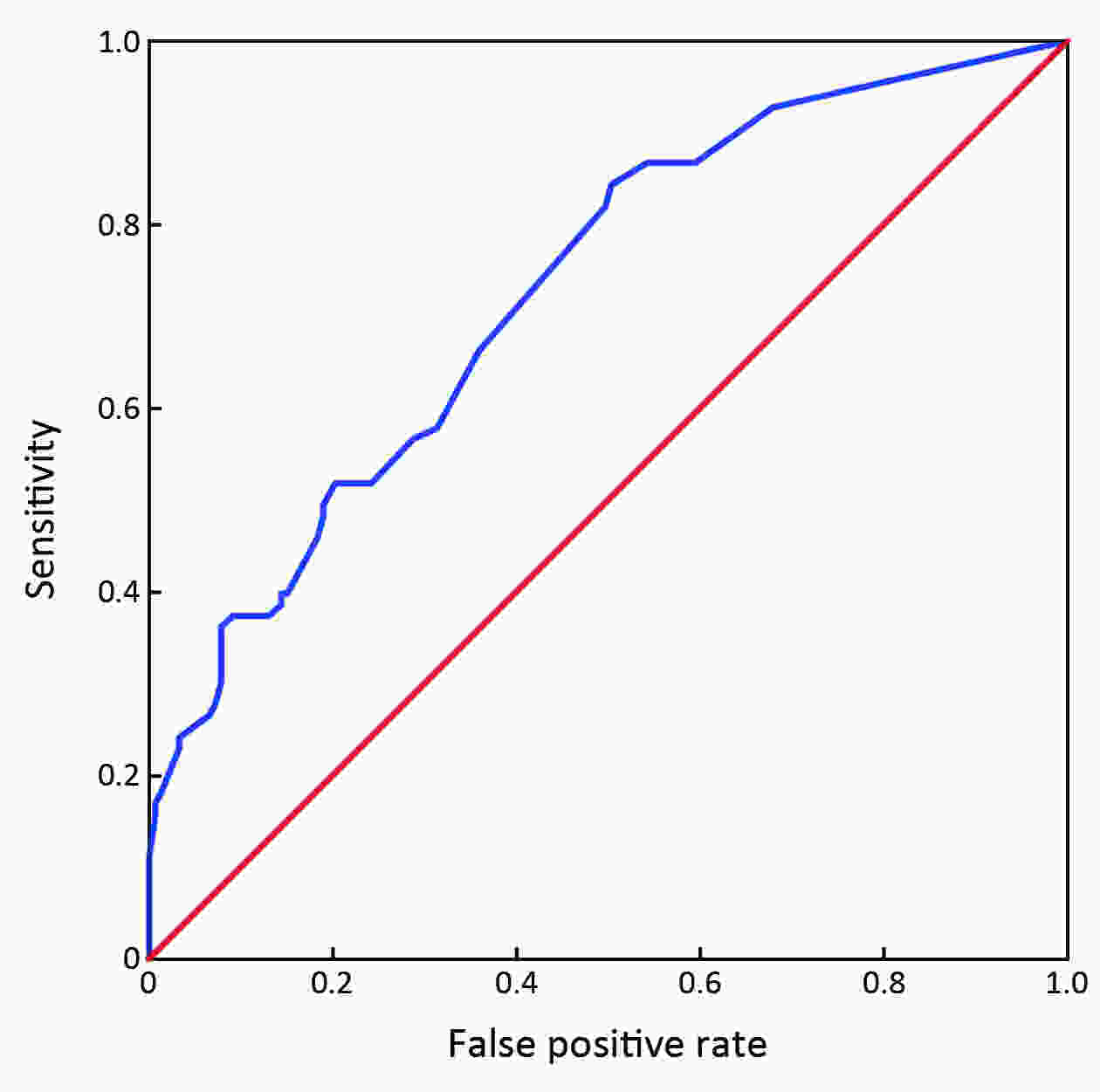
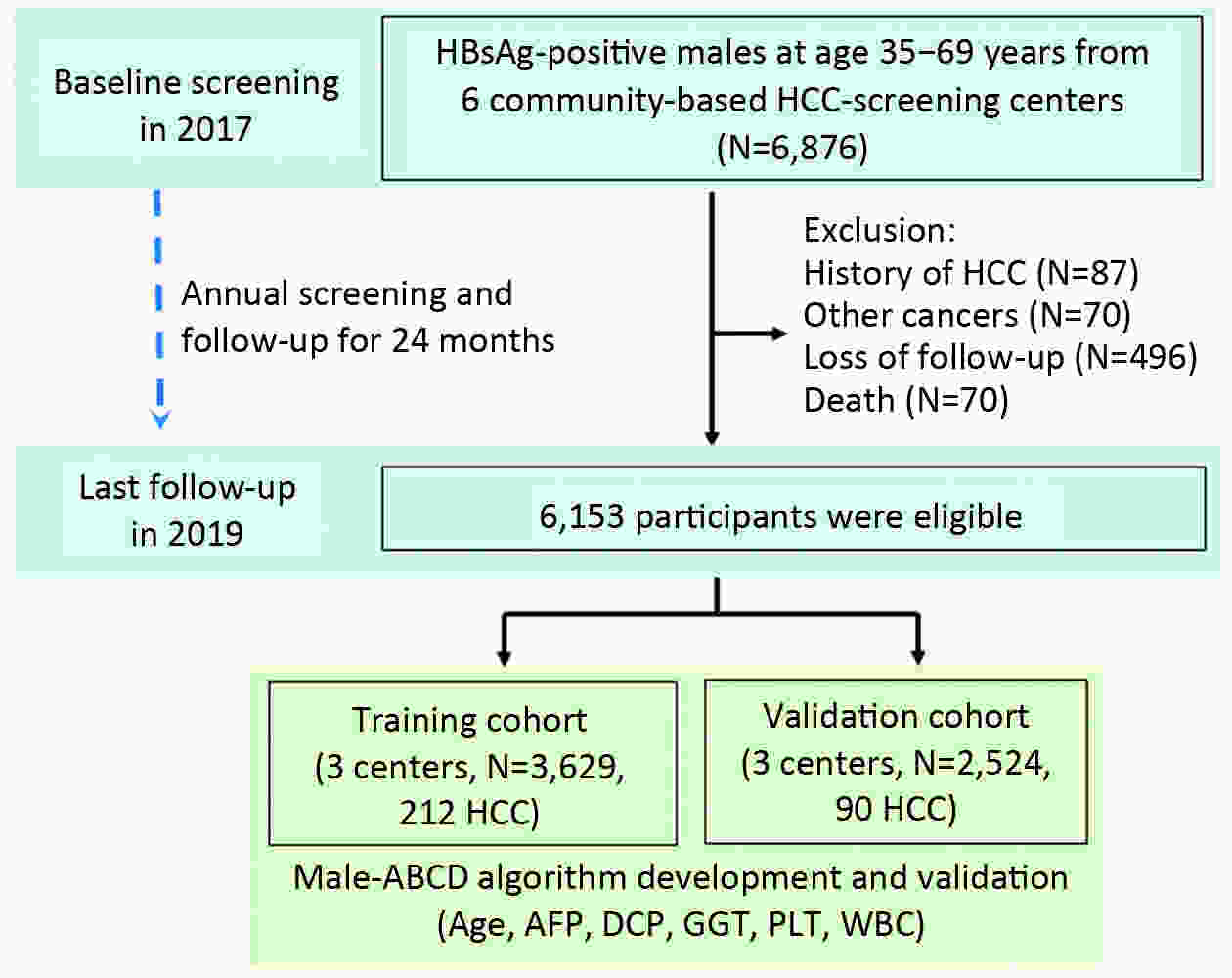
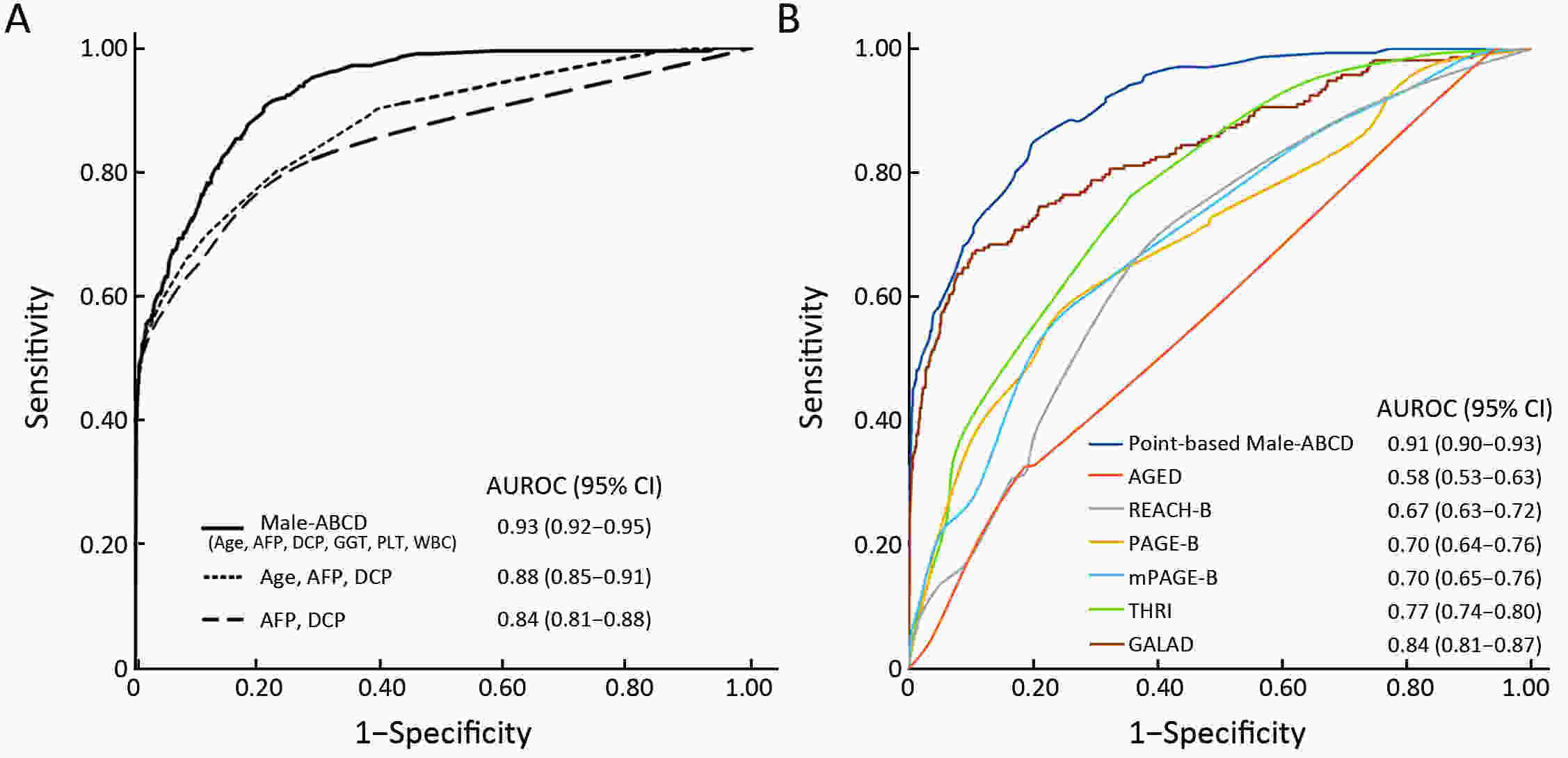
ObjectiveHepatocellular carcinoma (HCC) development among hepatitis B surface antigen (HBsAg) carriers shows gender disparity, influenced by underlying liver diseases that display variations in laboratory tests. We aimed to construct a risk-stratified HCC prediction model for HBsAg-positive male adults. MethodsHBsAg-positive males of 35−69 years old (N=6,153) were included from a multi-center population-based liver cancer screening study. Randomly, three centers were set as training, the other three centers as validation. Within 2 years since initiation, we administrated at least two rounds of HCC screening using B-ultrasonography and α-fetoprotein (AFP). We used logistic regression models to determine potential risk factors, built and examined the operating characteristics of a point-based algorithm for HCC risk prediction. ResultsWith 2 years of follow-up, 302 HCC cases were diagnosed. A male-ABCD algorithm was constructed including participant’s age, blood levels of GGT (γ-glutamyl-transpeptidase), counts of platelets, white cells, concentration of DCP (des-γ-carboxy-prothrombin) and AFP, with scores ranging from 0 to 18.3. The area under receiver operating characteristic was 0.91 (0.90−0.93), larger than existing models. At 1.5 points of risk score, 26.10% of the participants in training cohort and 14.94% in validation cohort were recognized at low risk, with sensitivity of identifying HCC remained 100%. At 2.5 points, 46.51% of the participants in training cohort and 33.68% in validation cohort were recognized at low risk with 99.06% and 97.78% of sensitivity, respectively. At 4.5 points, only 20.86% of participants in training cohort and 23.73% in validation cohort were recognized at high risk, with positive prediction value of 22.85% and 12.35%, respectively. ConclusionsMale-ABCD algorithm identified individual’s risk for HCC occurrence within short term for their HCC precision surveillance.
2021, 33(3): 364-378.
doi: 10.21147/j.issn.1000-9604.2021.03.08
Abstract:
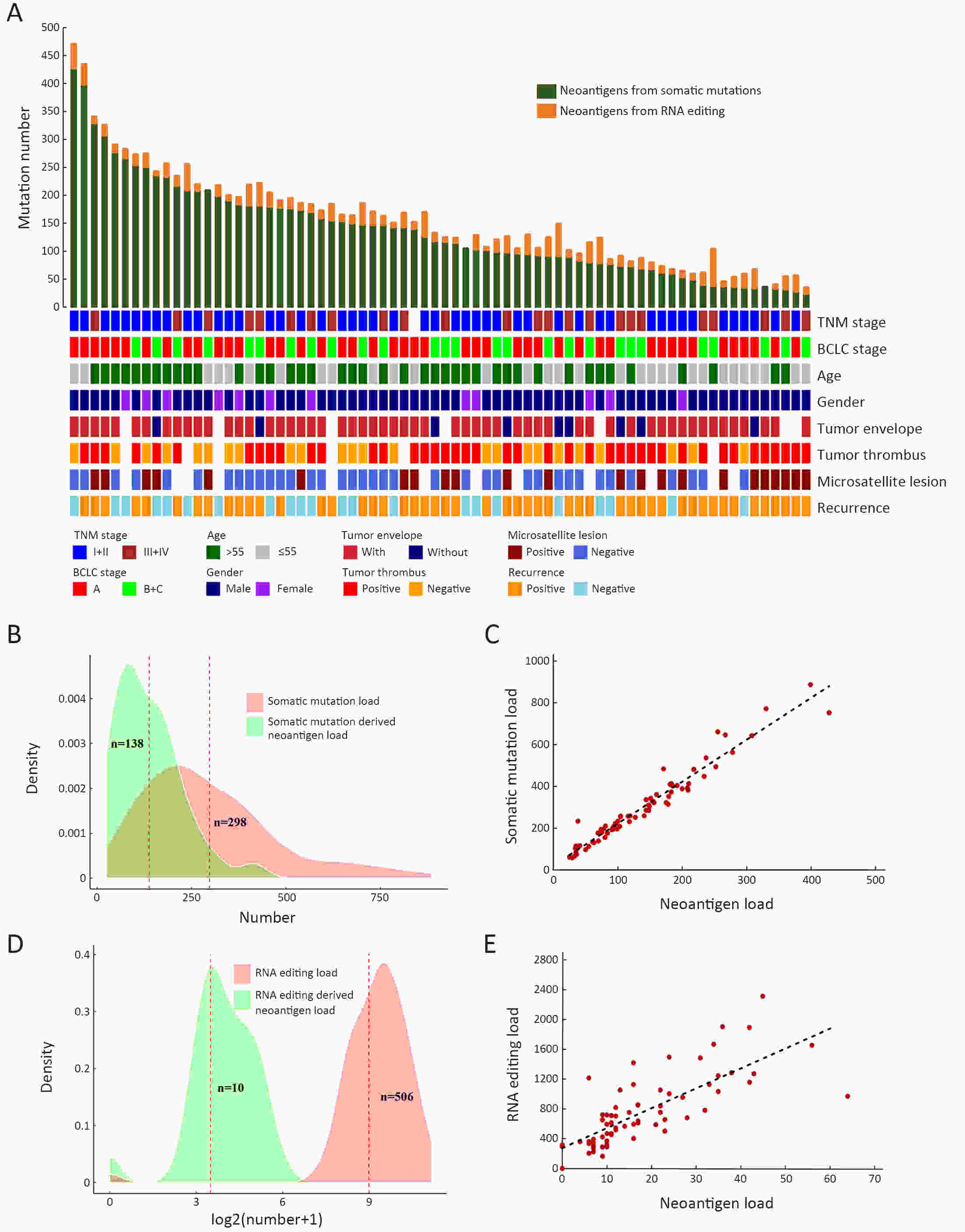
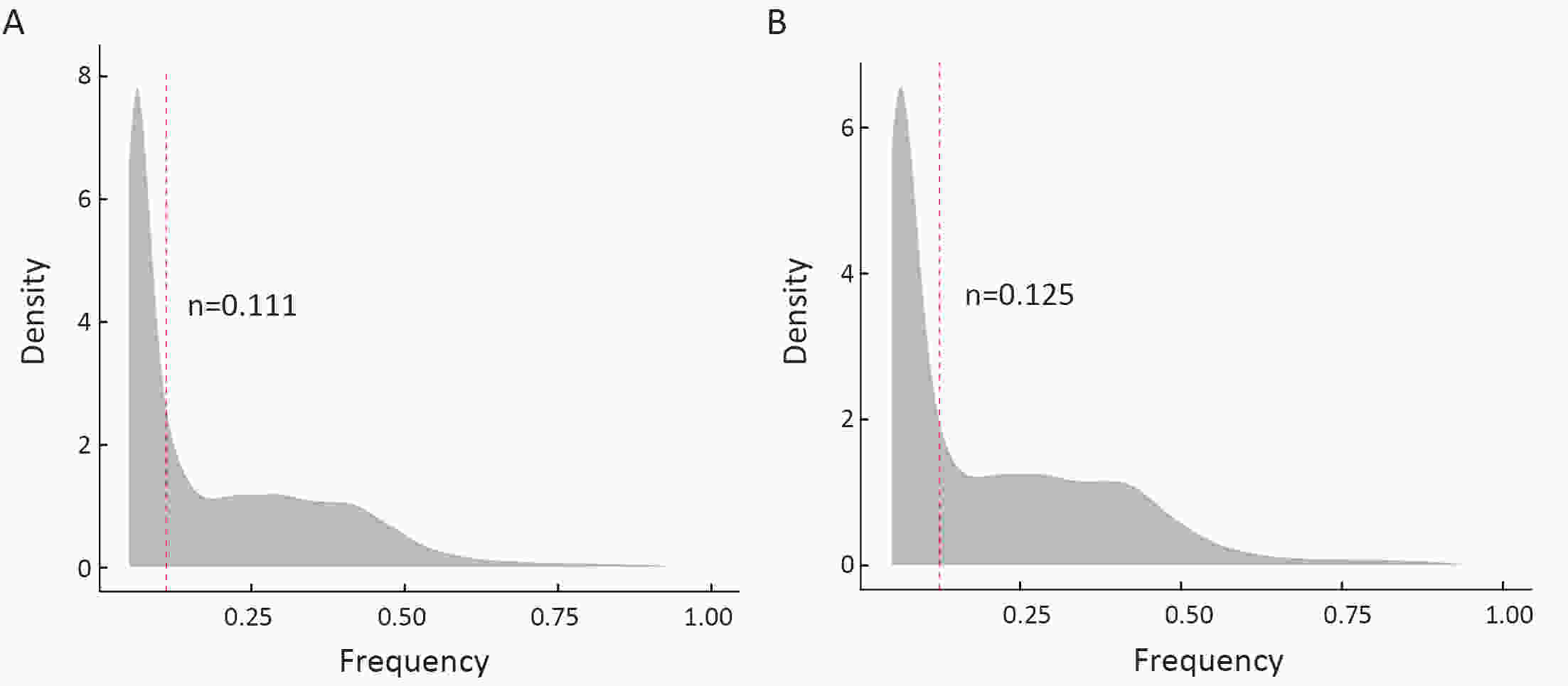
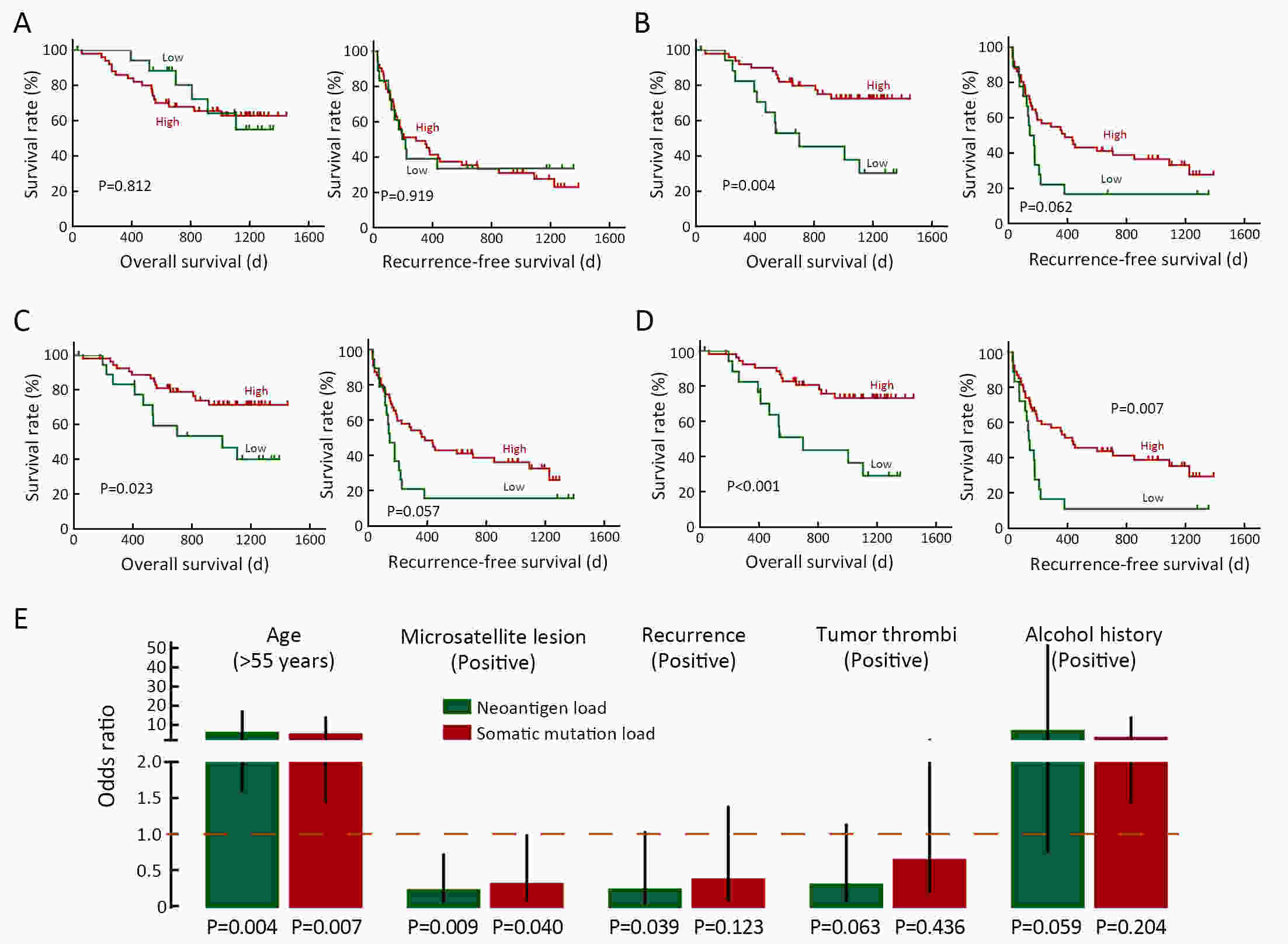
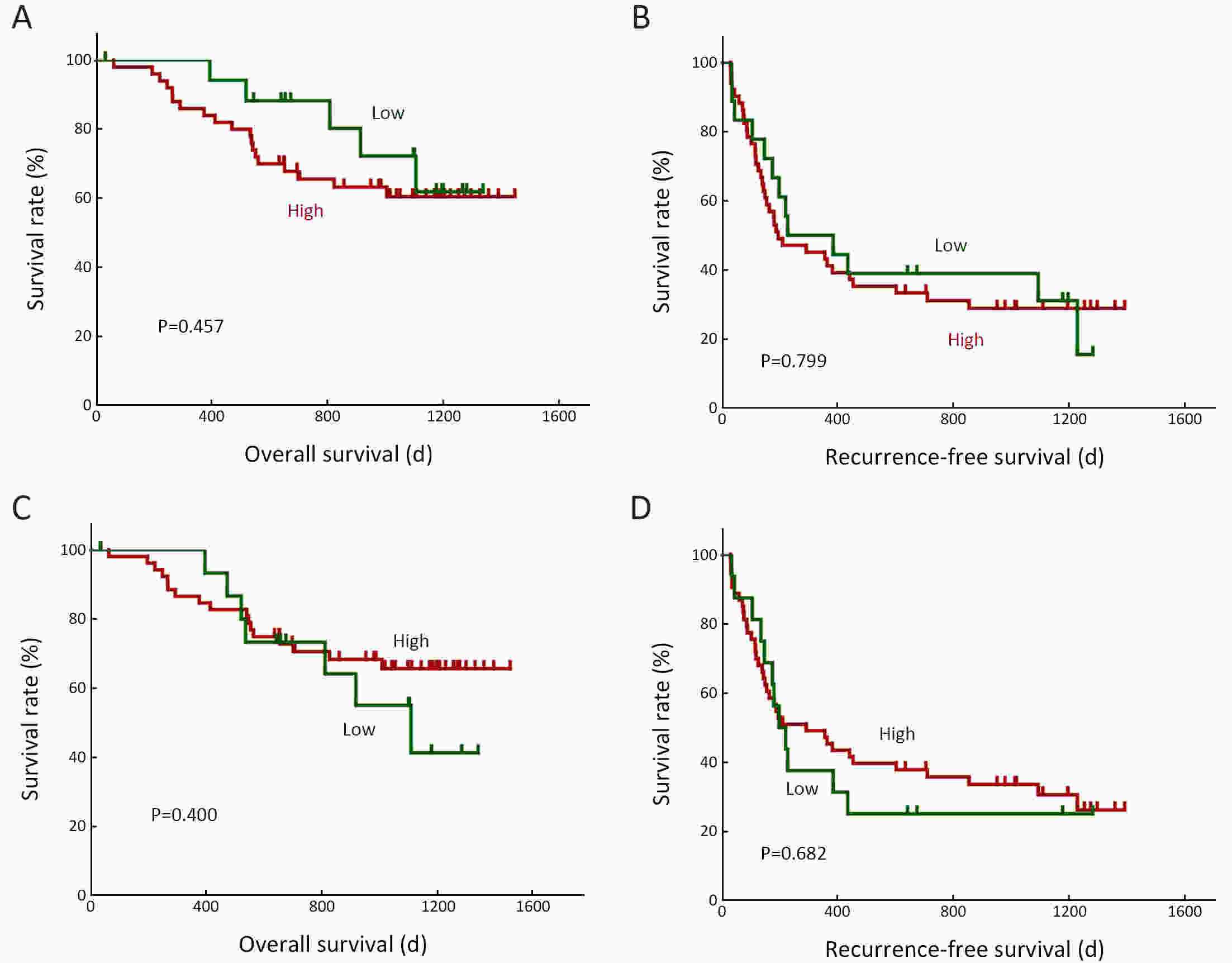
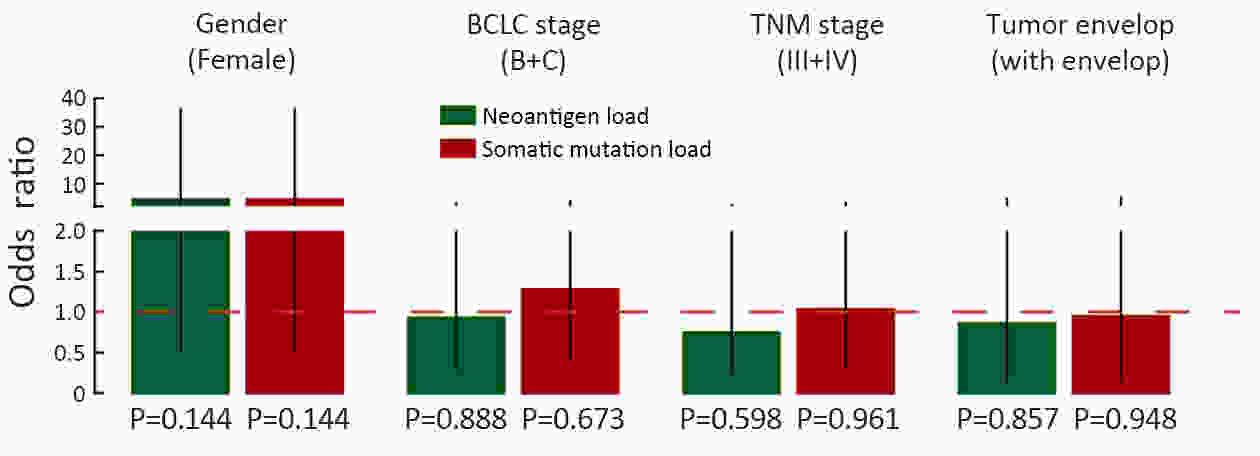
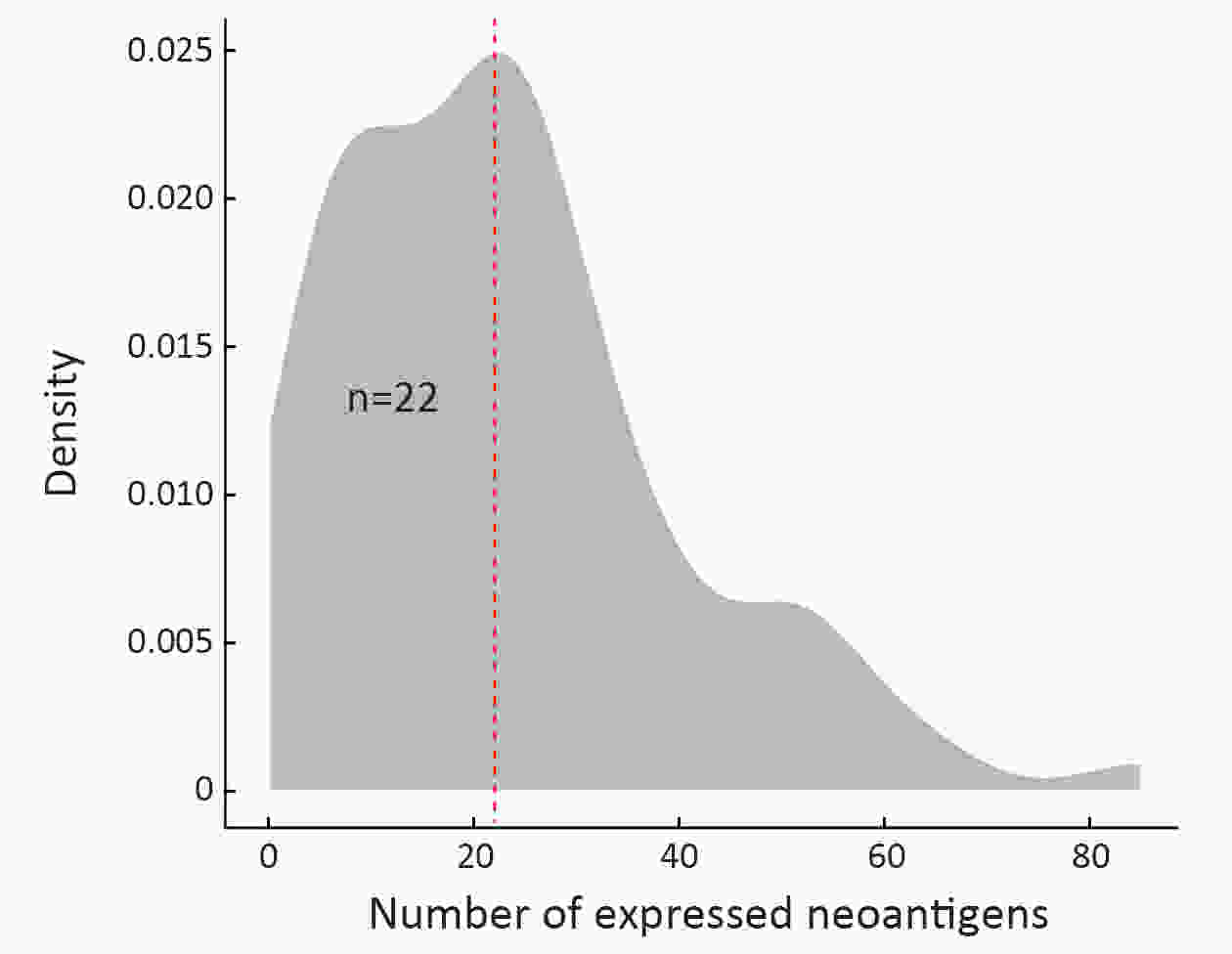
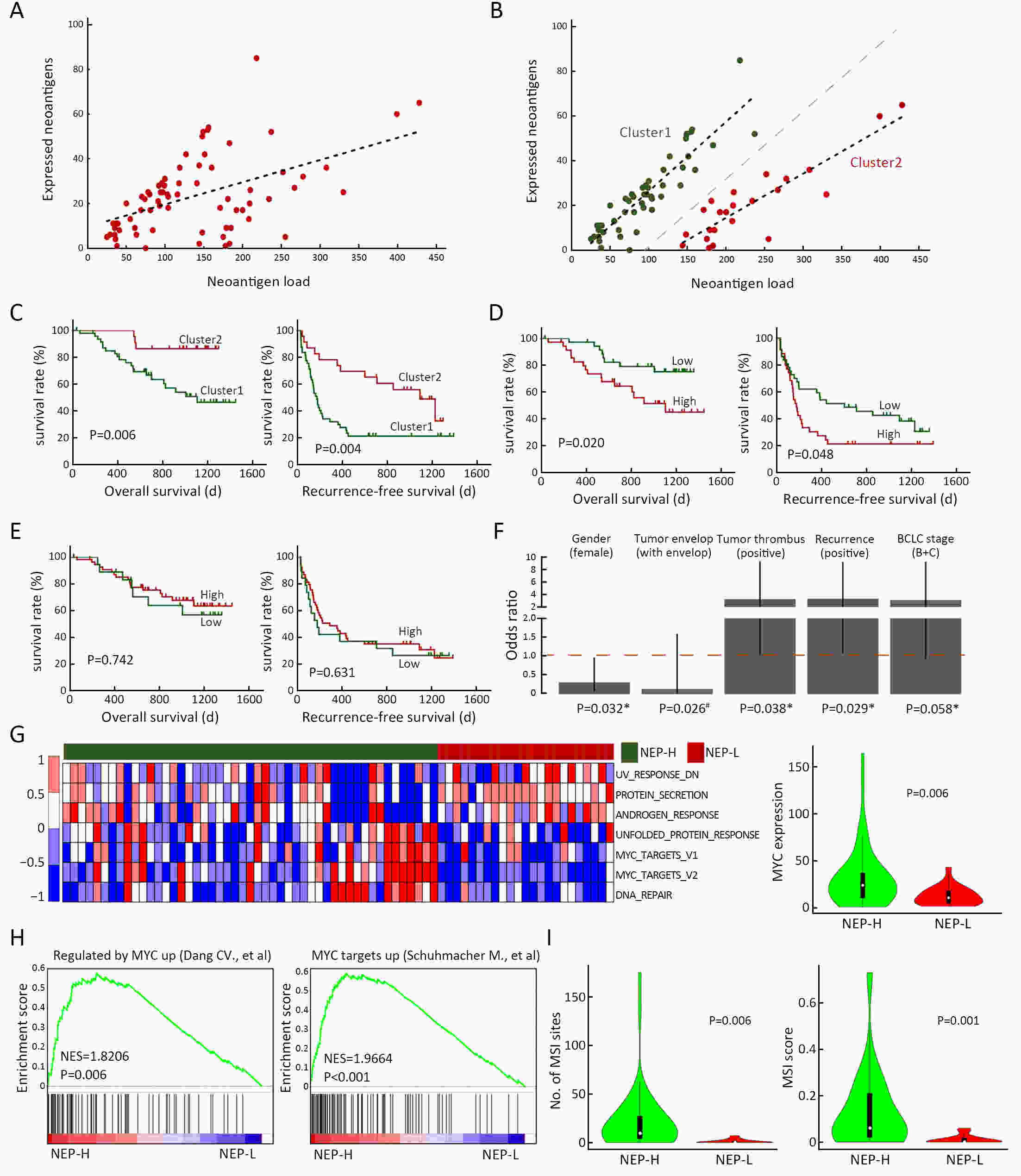
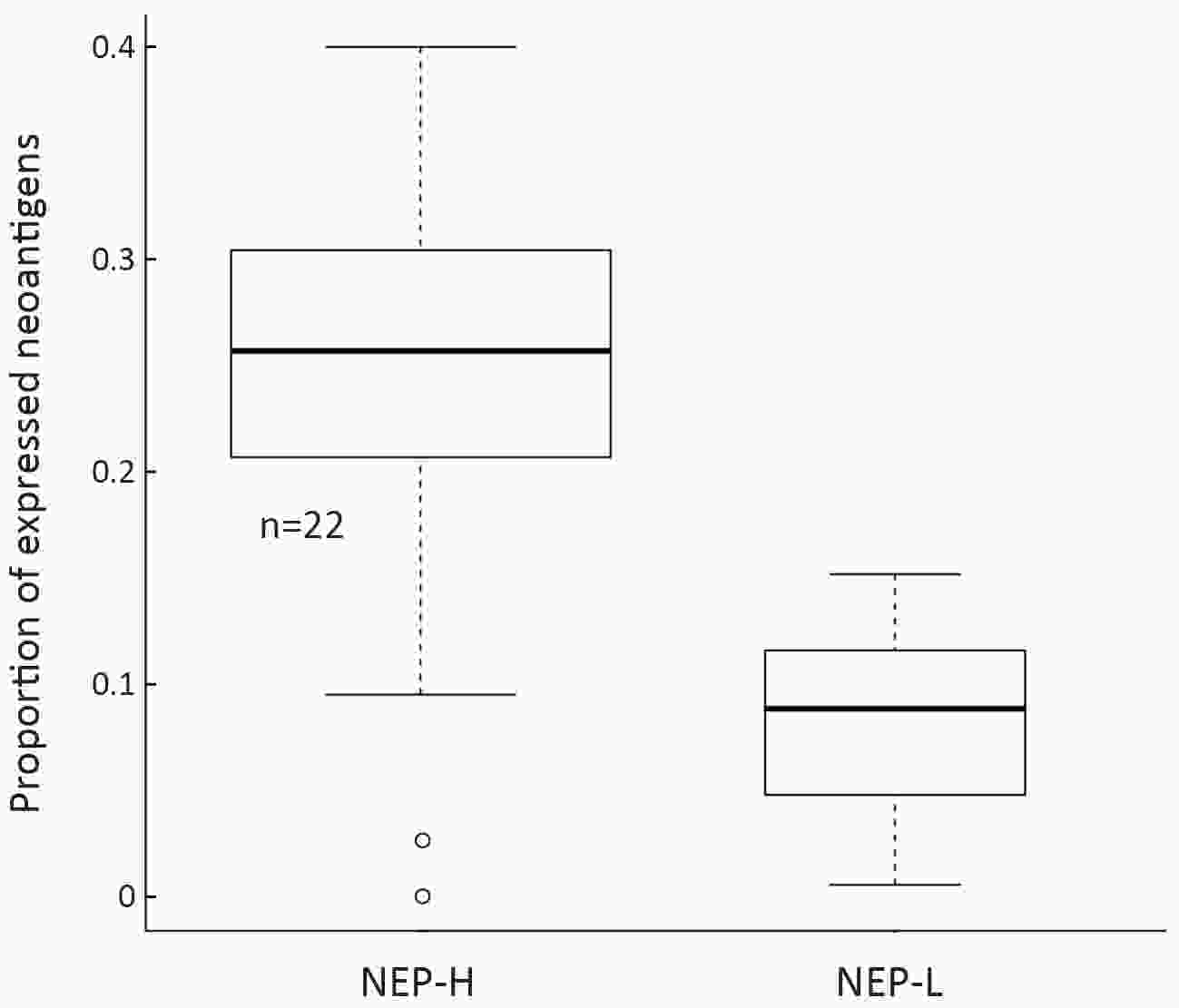
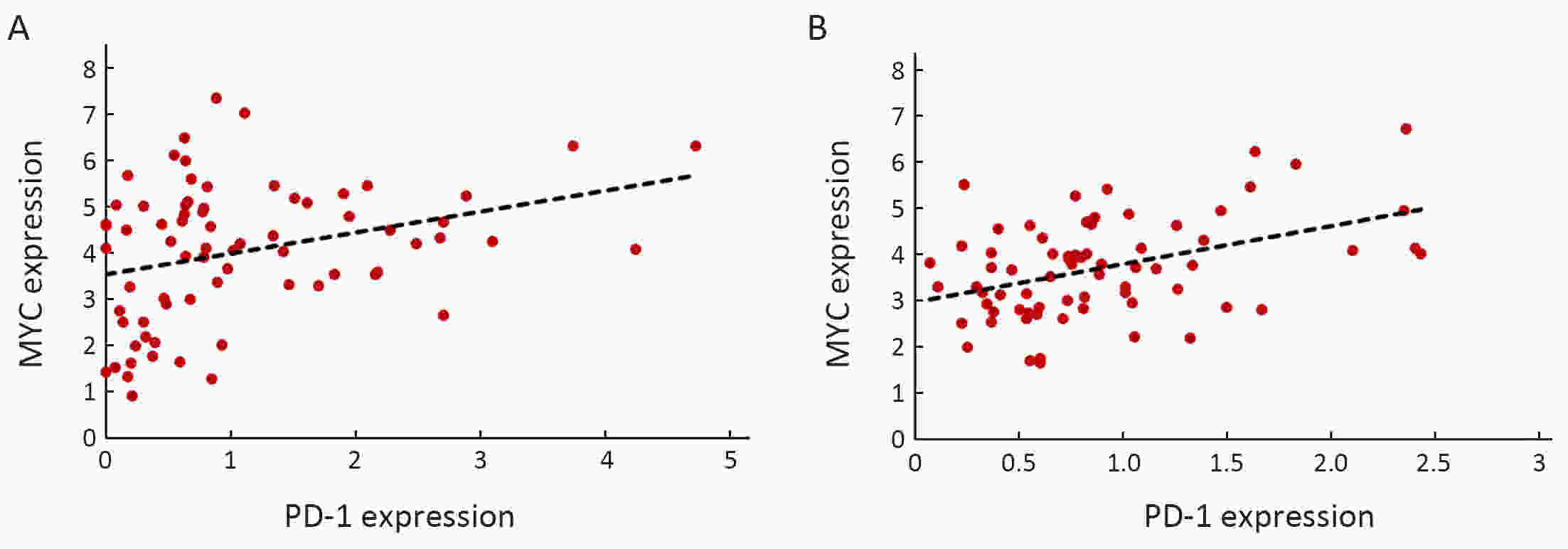
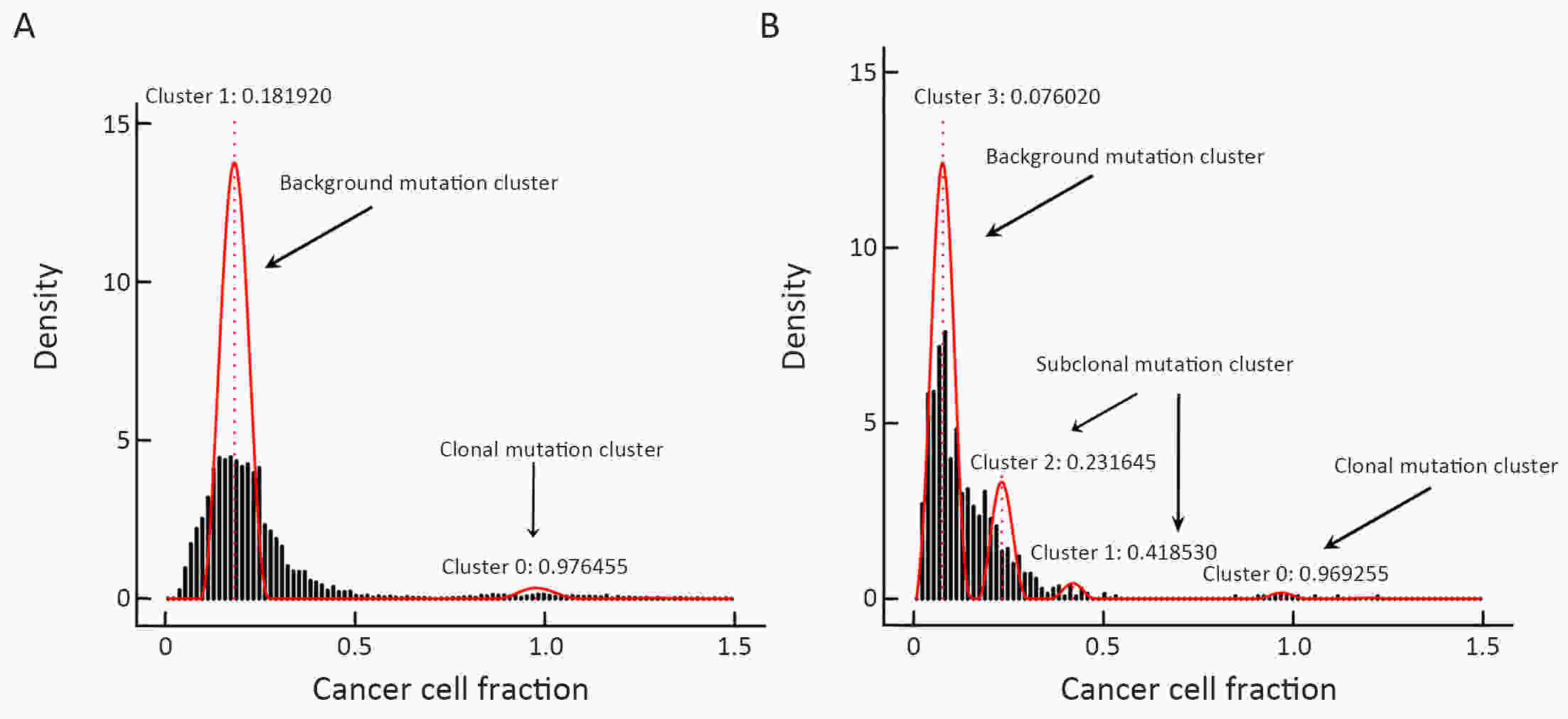
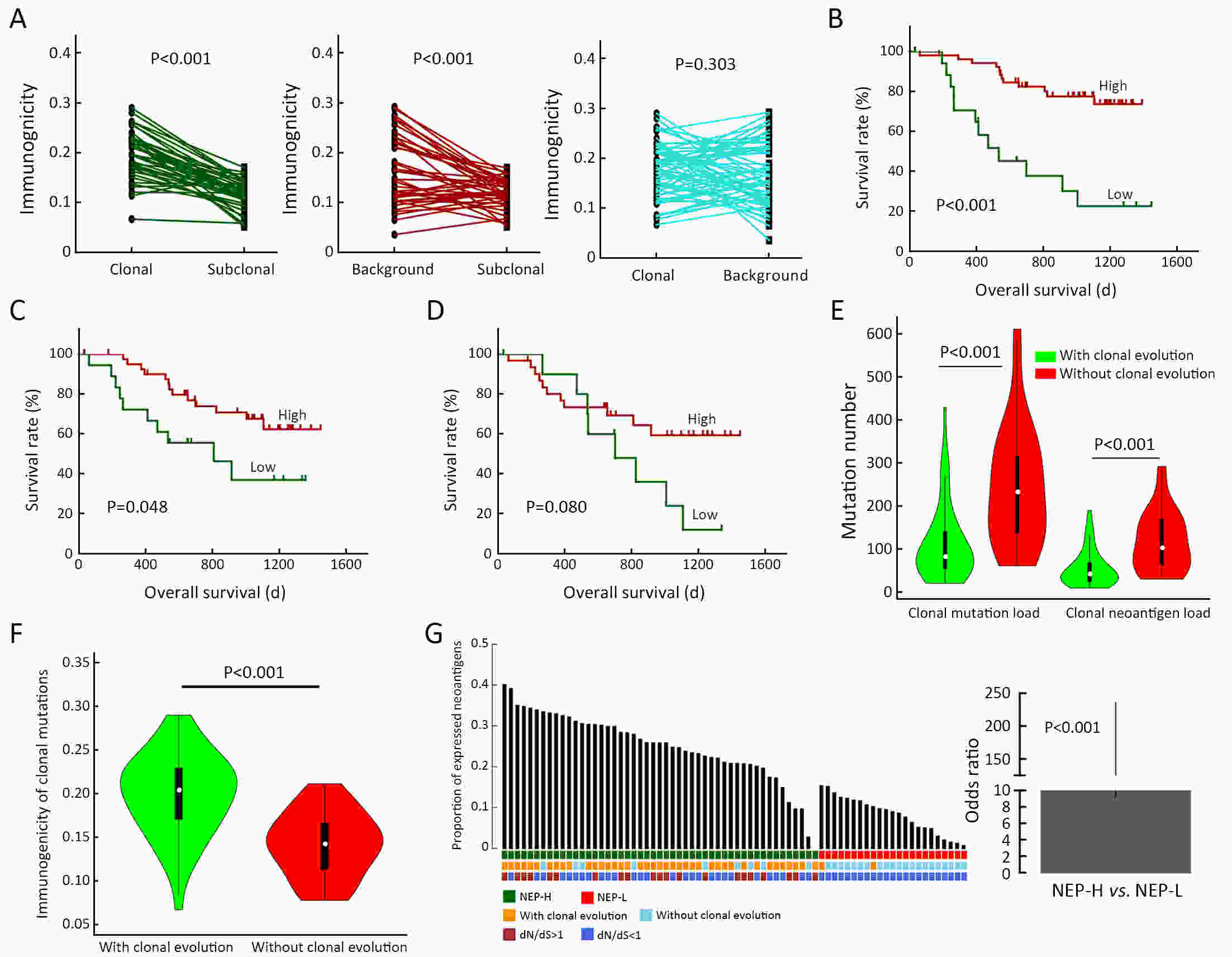
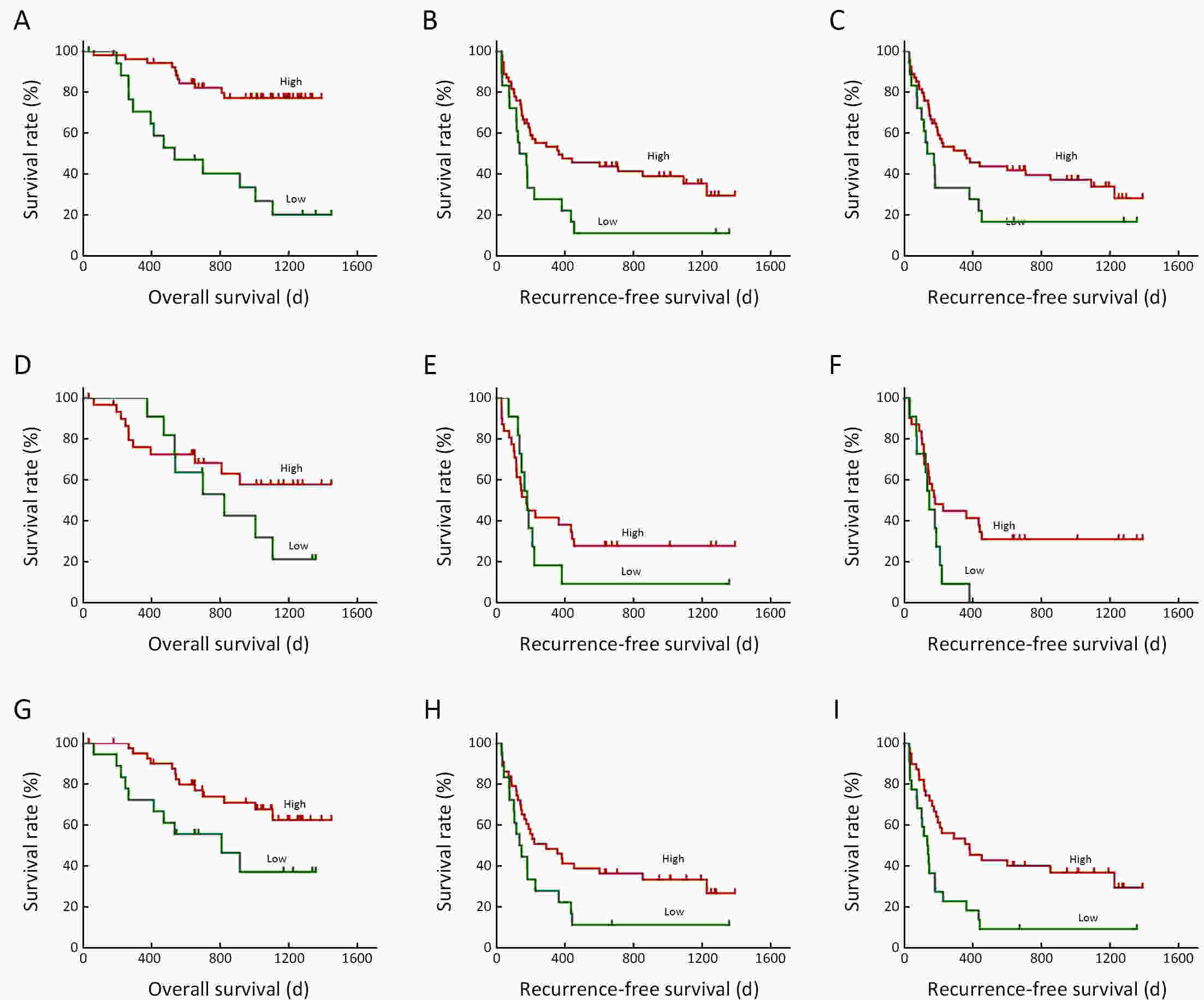
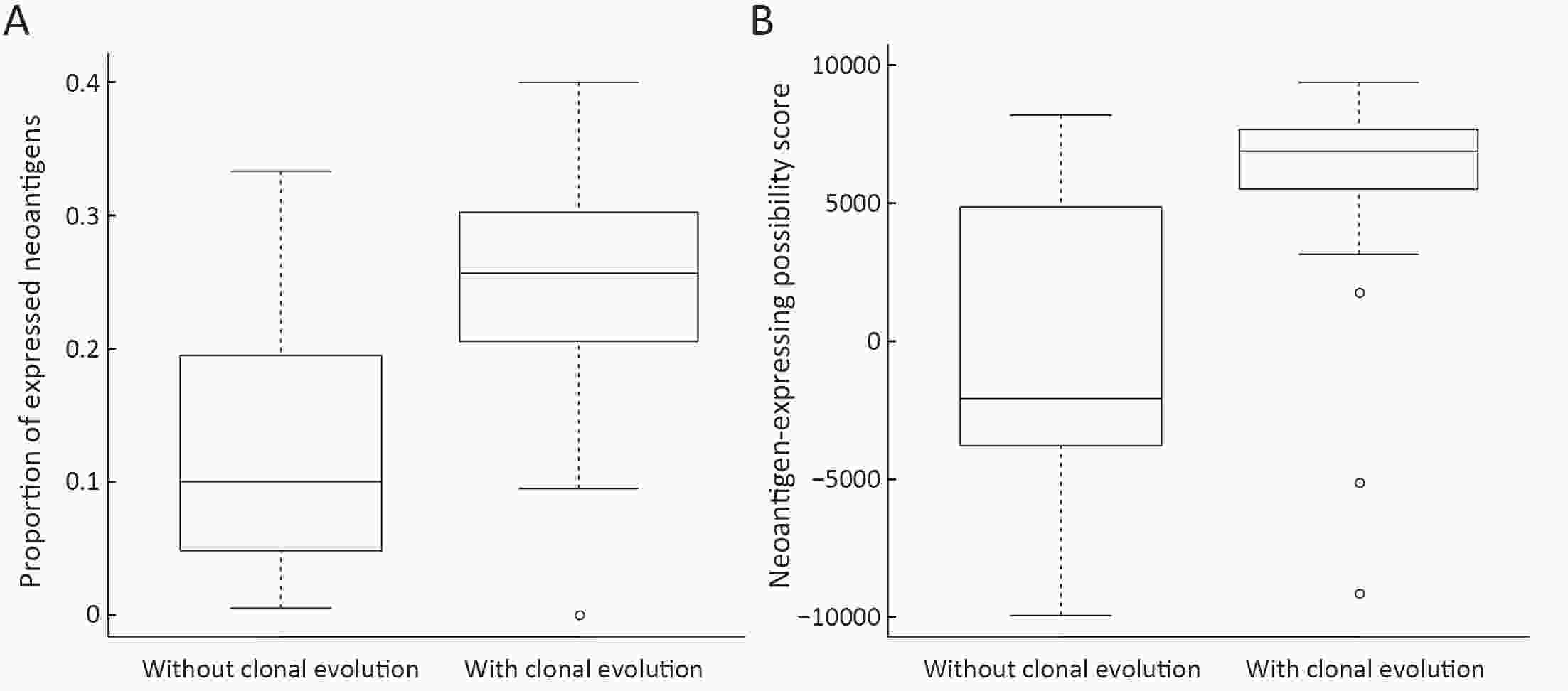
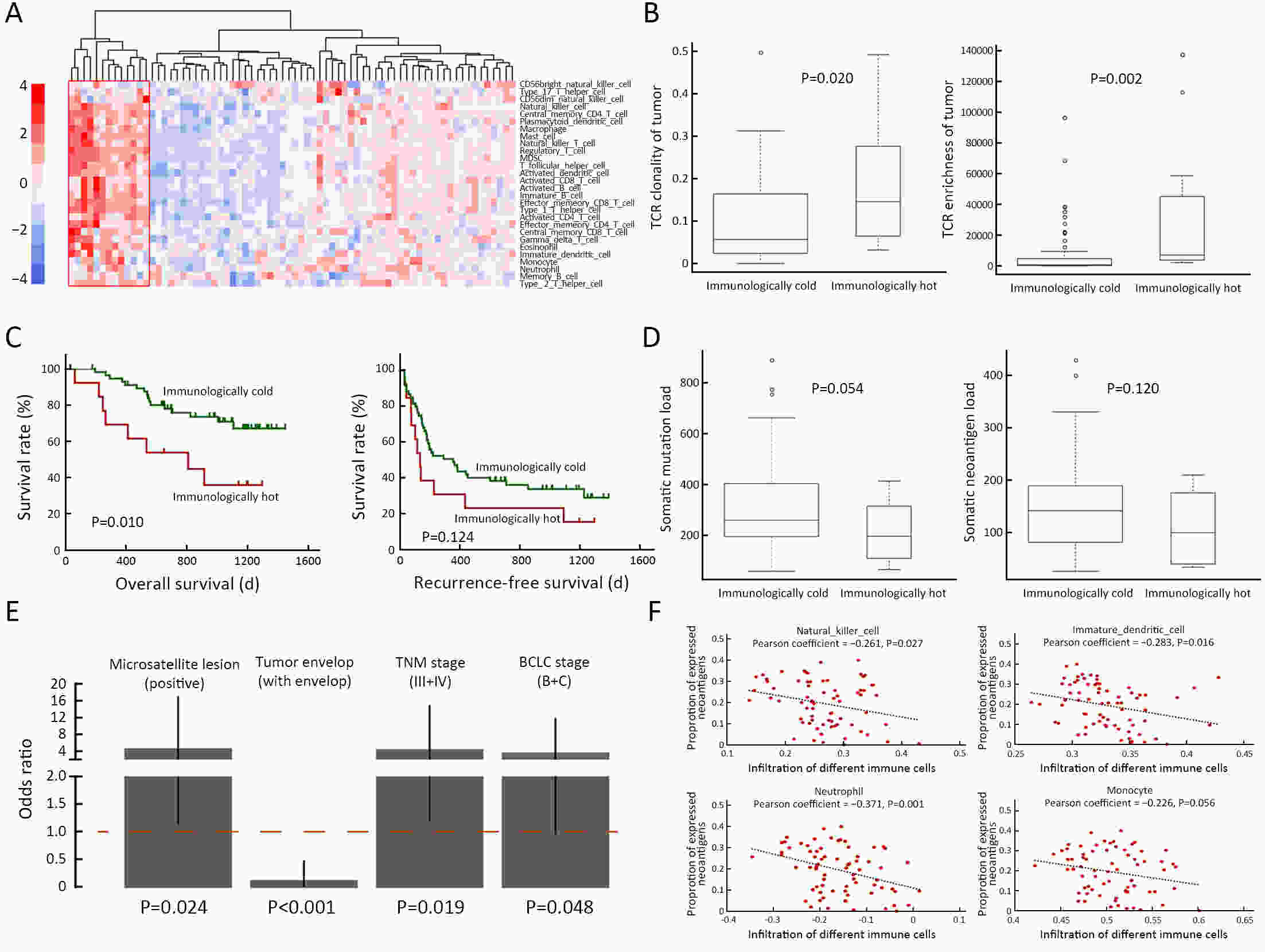
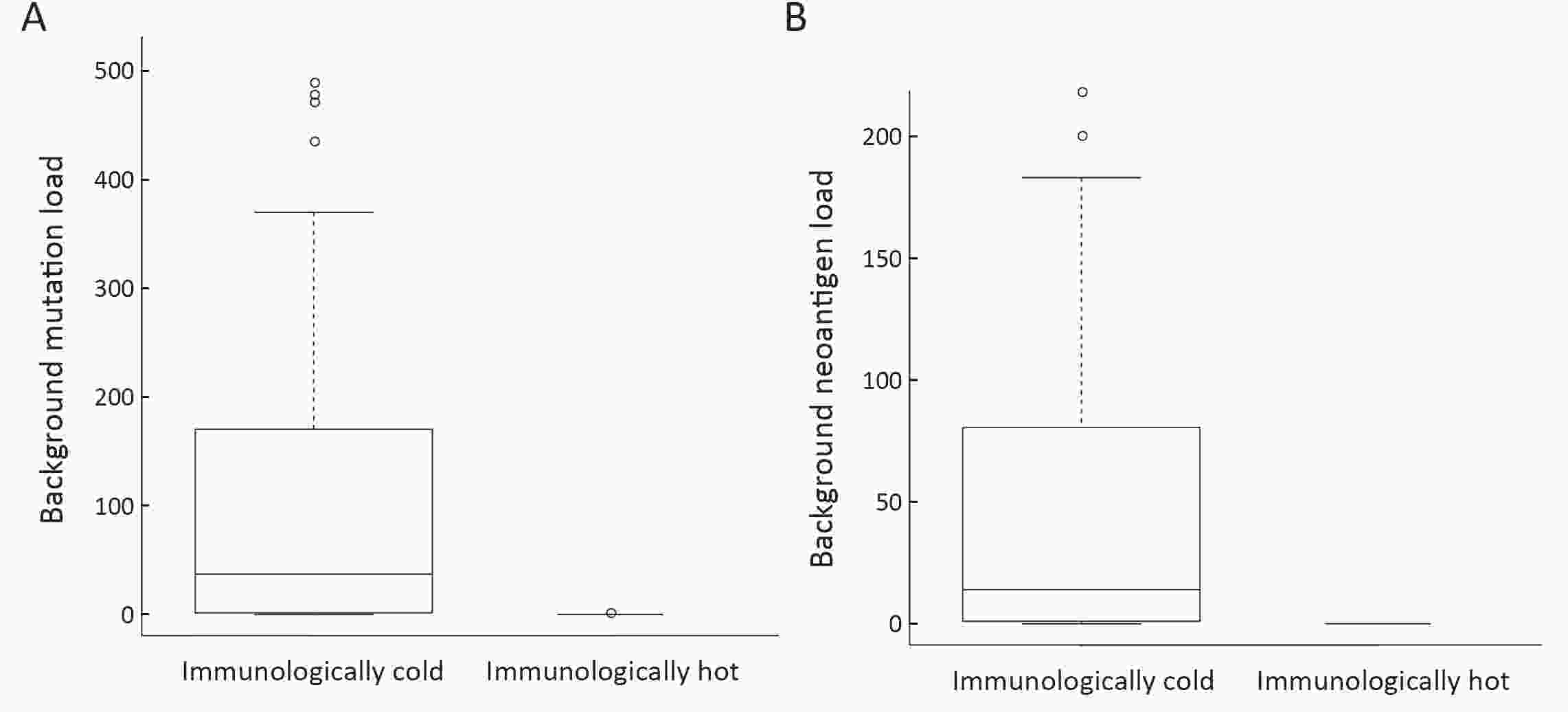
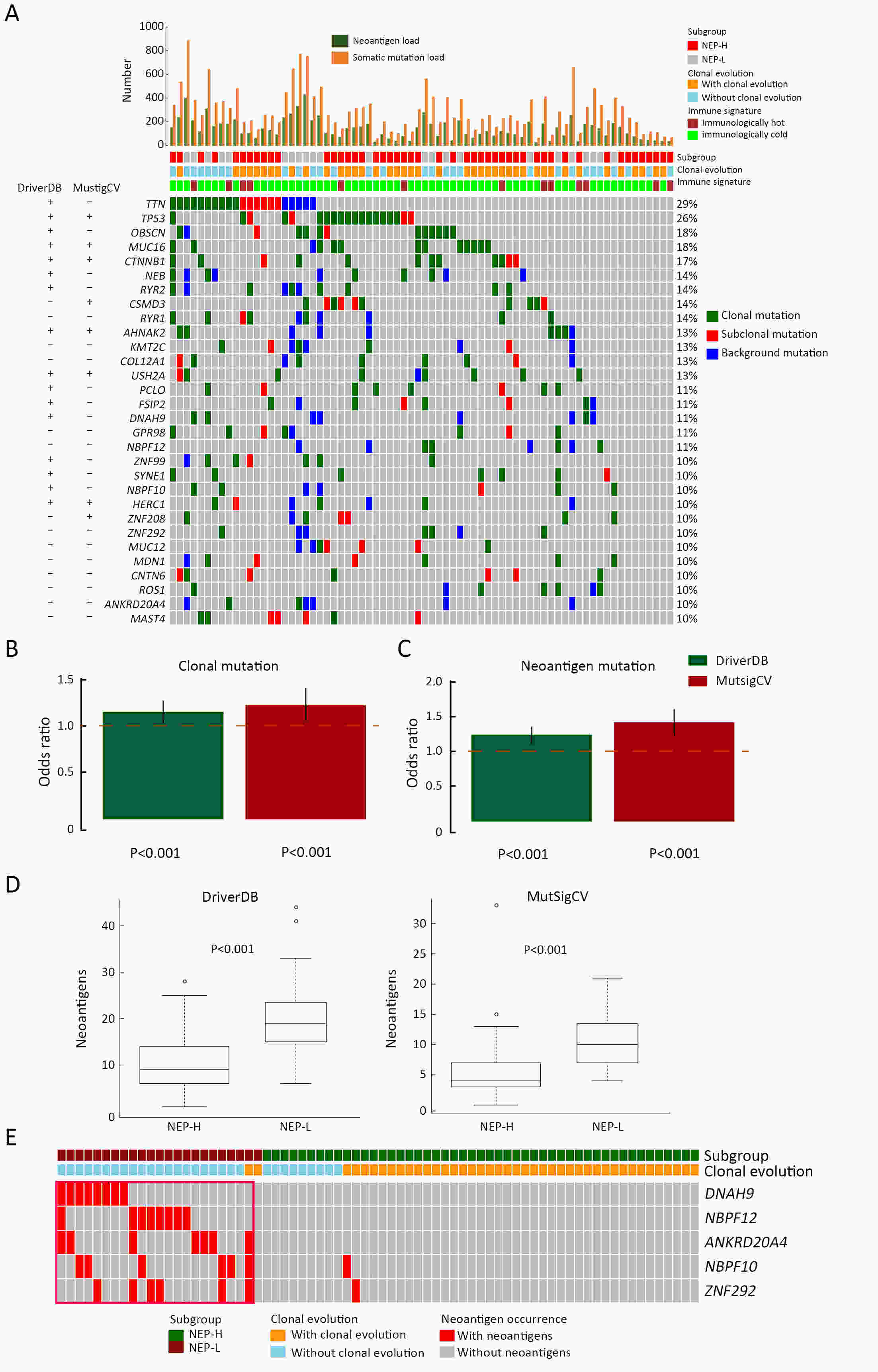
ObjectiveNeoantigens derived from tumor-specific genomic alterations have demonstrated great potential for immunotherapeutic interventions in cancers. However, the comprehensive profile of hepatocellular carcinoma (HCC) neoantigens and their complex interplay with immune microenvironment and tumor evolution have not been fully addressed. MethodsHere we integrated whole exome sequencing data, transcriptome sequencing data and clinical information of 72 primary HCC patients to characterize the HCC neoantigen profile, and systematically explored its interactions with tumor clonal evolution, driver mutations and immune microenvironments. ResultsWe observed that higher somatic mutation/neoantigen load was associated with better clinical outcomes and HCC patients could be further divided into two subgroups with distinct prognosis based on their neoantigen expression patterns. HCC subgroup with neoantigen expression probability high (NEP-H) showed more aggressive pathologic features including increased incidence of tumor thrombus (P=0.038), higher recurrence rate (P=0.029), more inclined to lack tumor capsule (P=0.026) and with more microsatellite instability sites (P=0.006). In addition, NEP-H subgroup was also characterized by higher chance to be involved in tumor clonal evolution [odds ratio (OR)=46.7, P<0.001]. Gene set enrichment analysis revealed that upregulation of MYC and its targets could suppress immune responses, leading to elevated neoantigen expression proportion in tumor cells. Furthermore, we discovered an immune escape mechanism that tumors could become more inconspicuous by evolving subclones with less immunogenicity. We observed that smaller clonal mutation clusters with higher immunogenicity in tumor were more likely to involve in clonal evolution. Based on identified neoantigen profiles, we also discovered series of neoantigenic hotspot genes, which could serve as potential actionable targets in future. ConclusionsOur results revealed the landscape of HCC neoantigens and discovered two clinically relevant subgroups with distinct neoantigen expression patterns, suggesting the neoantigen expression should be fully considered in future immunotherapeutic interventions.
2021, 33(3): 379-390.
doi: 10.21147/j.issn.1000-9604.2021.03.09
Abstract:
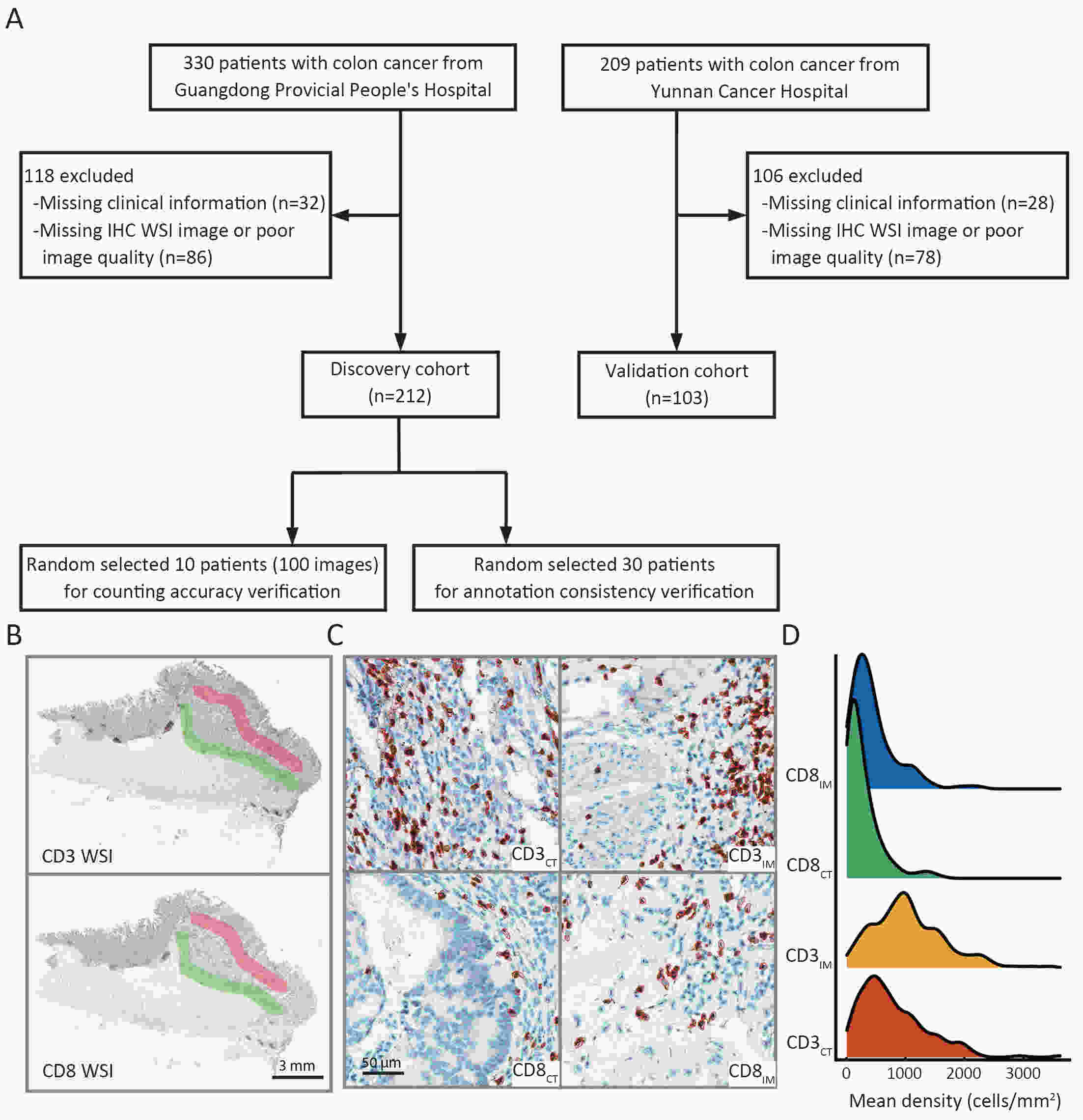
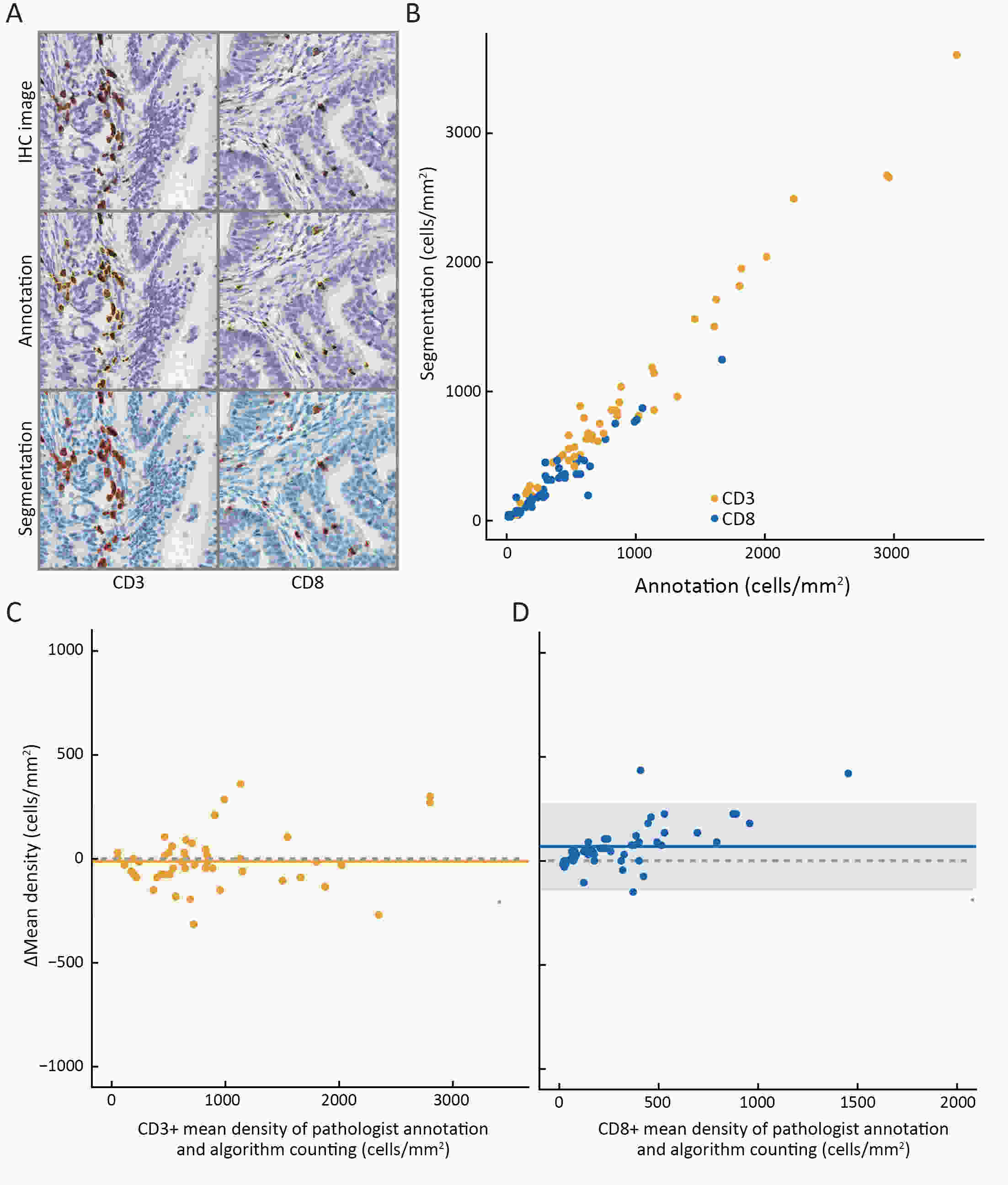
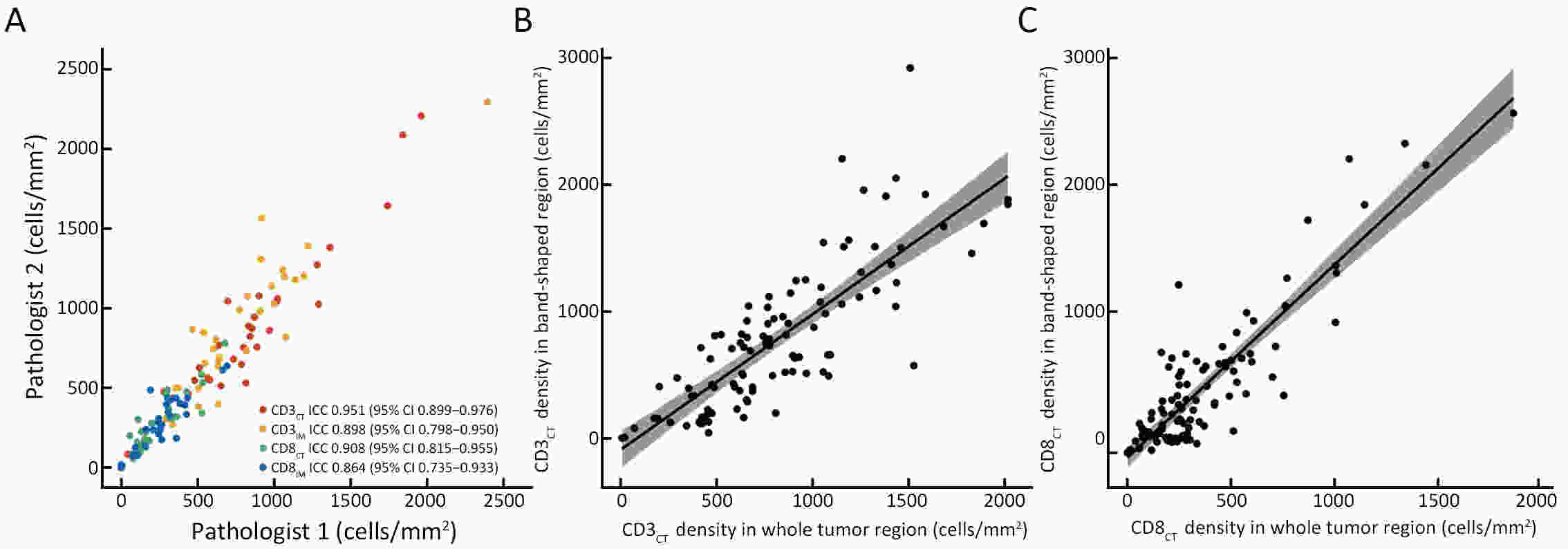
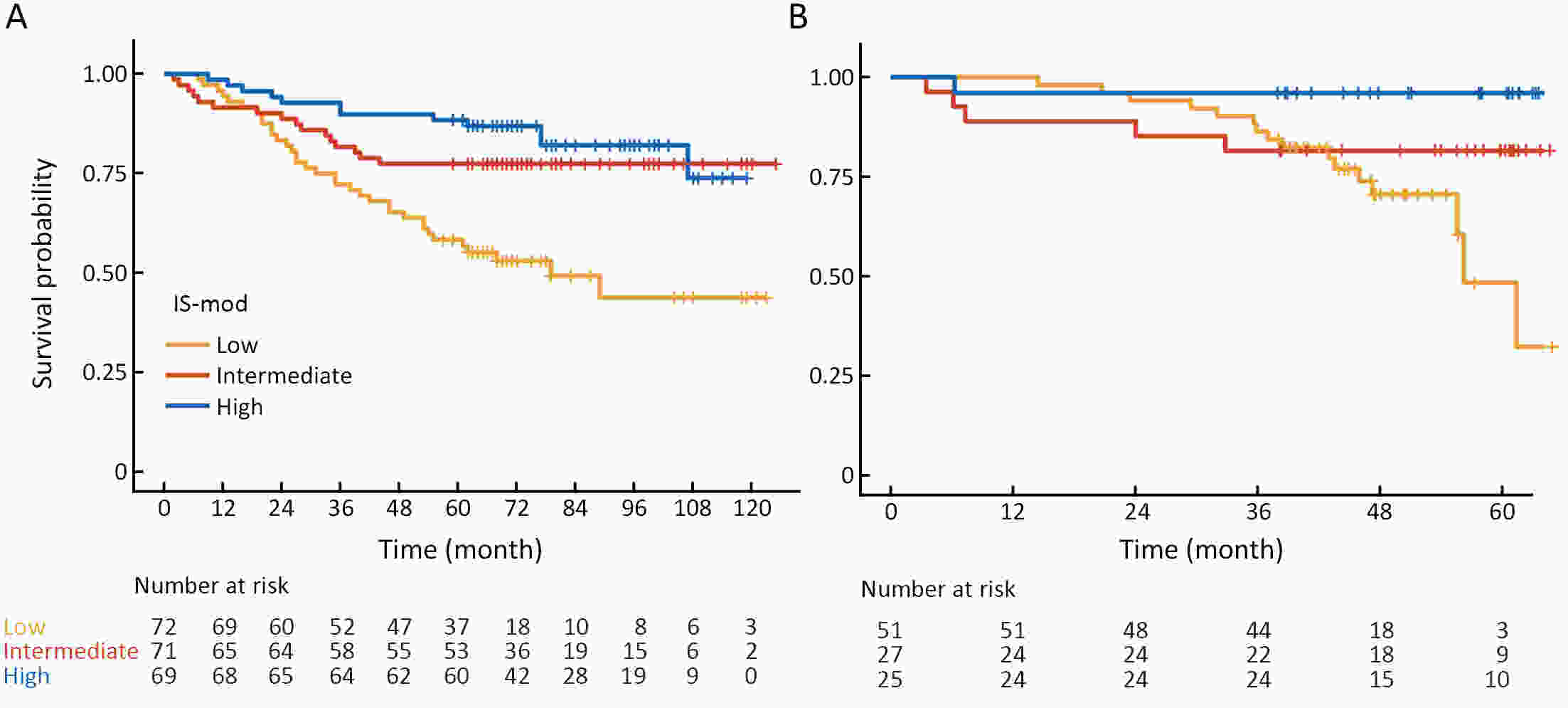
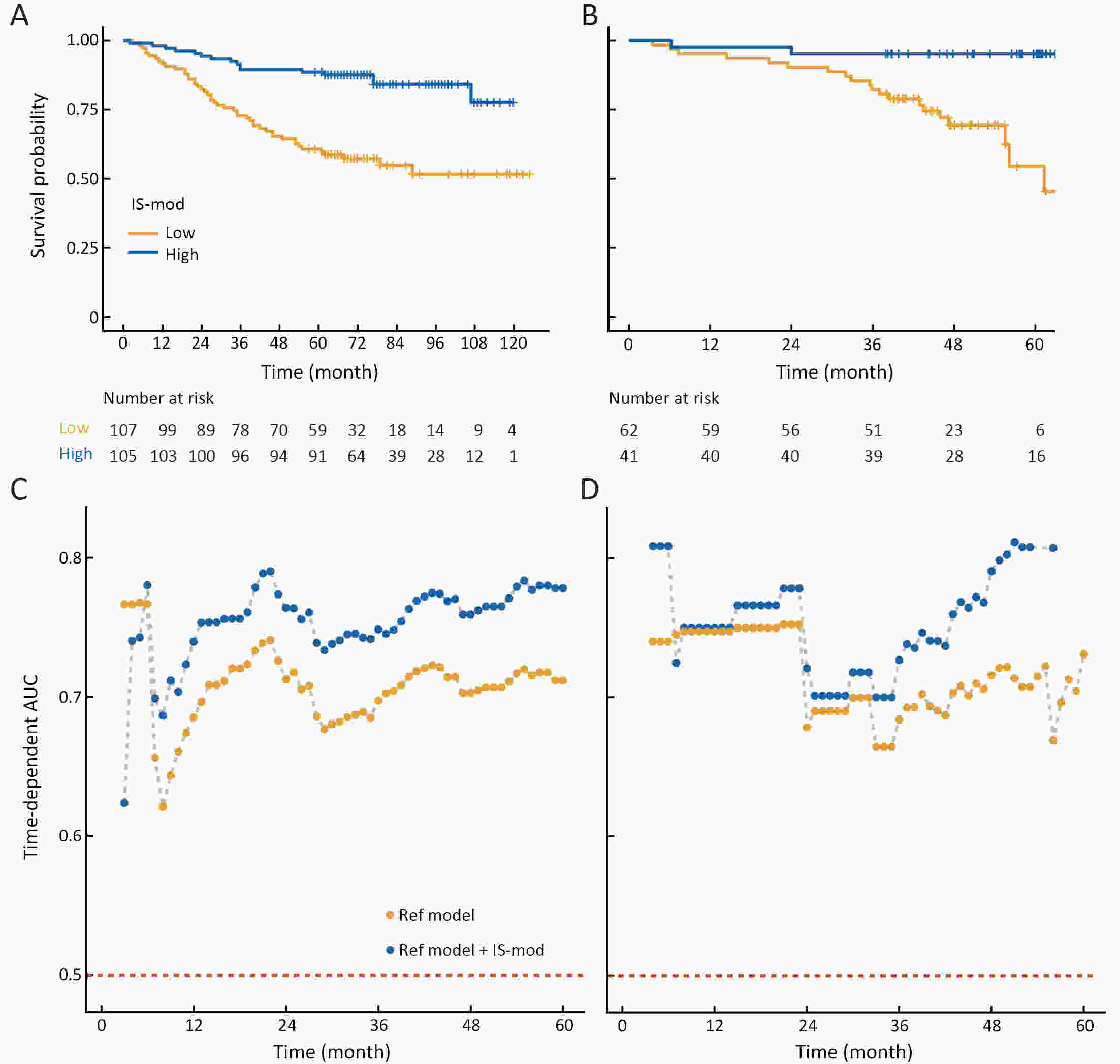
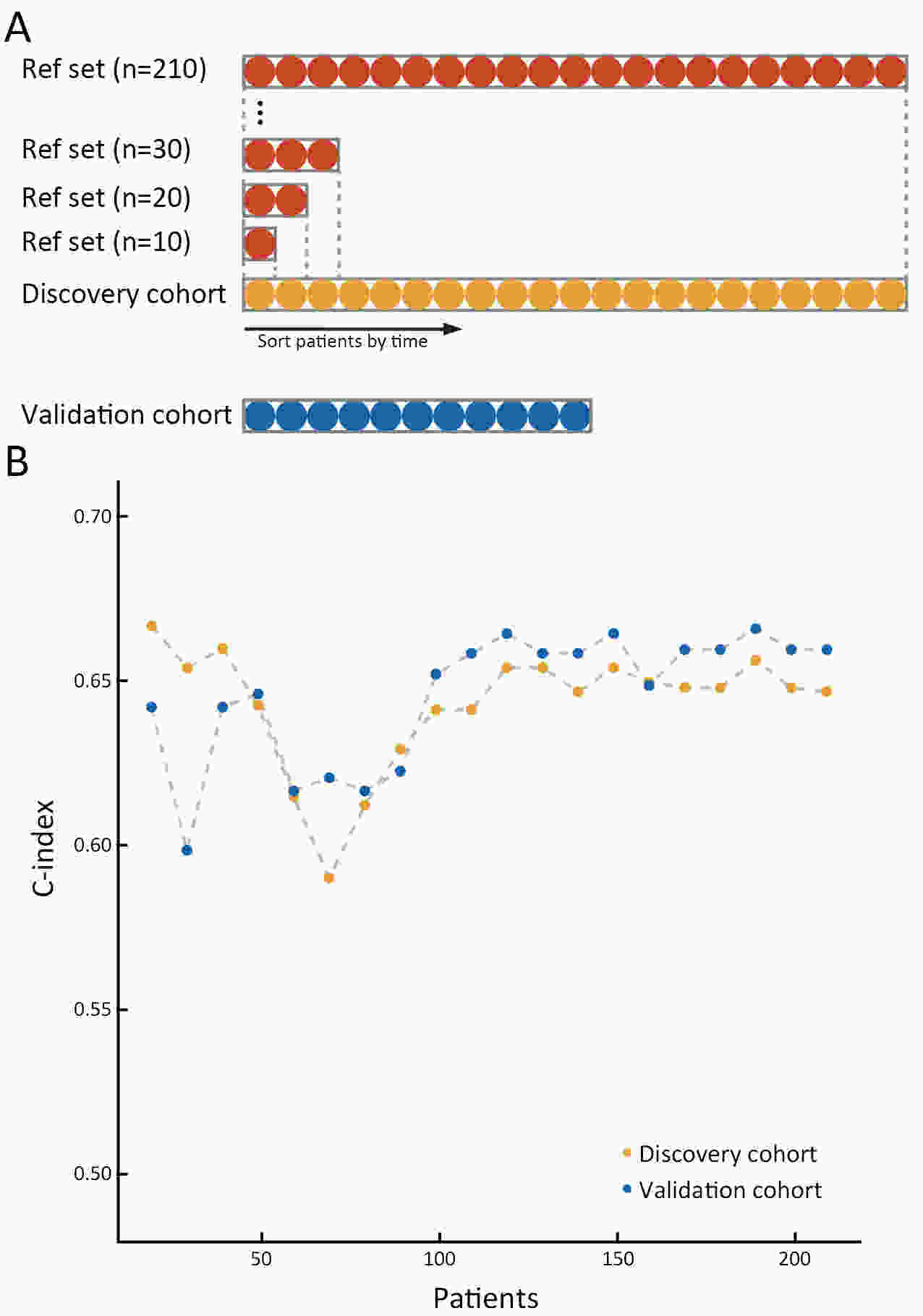
ObjectiveThe Immunoscore method has proved fruitful for predicting prognosis in patients with colon cancer. However, there is still room for improvement in this scoring method to achieve further advances in its clinical translation. This study aimed to develop and validate a modified Immunoscore (IS-mod) system for predicting overall survival (OS) in patients with stage I−III colon cancer. MethodsThe IS-mod was proposed by counting CD3+ and CD8+ immune cells in regions of the tumor core and its invasive margin by drawing two lines of interest. A discovery cohort (N=212) and validation cohort (N=103) from two centers were used to evaluate the prognostic value of the IS-mod. ResultsIn the discovery cohort, 5-year survival rates were 88.6% in the high IS-mod group and 60.7% in the low IS-mod group. Multivariate analysis confirmed that the IS-mod was an independent prognostic factor for OS [adjusted hazard ratio (HR)=0.36, 95% confidence interval (95% CI): 0.20−0.63]. With less annotation and computation cost, the IS-mod achieved performance comparable to that of the Immunoscore-like (IS-like) system (C-index, 0.676 vs. 0.661, P=0.231). The 2-category IS-mod using 47.5% as the threshold had a better prognostic value than that using a fixed threshold of 25% (C-index, 0.653 vs. 0.573, P=0.004). Similar results were confirmed in the validation cohort. ConclusionsOur method simplifies the annotation and accelerates the calculation of Immunoscore method, thus making it easier for clinical implementation. The IS-mod achieved comparable prognostic performance when compared to the IS-like system in both cohorts. Besides, we further found that even with a small reference set (N≥120), the IS-mod still demonstrated a stable prognostic value. This finding may inspire other institutions to develop a local reference set of an IS-mod system for more accurate risk stratification of colon cancer.
2021, 33(3): 391-404.
doi: 10.21147/j.issn.1000-9604.2021.03.10
Abstract:
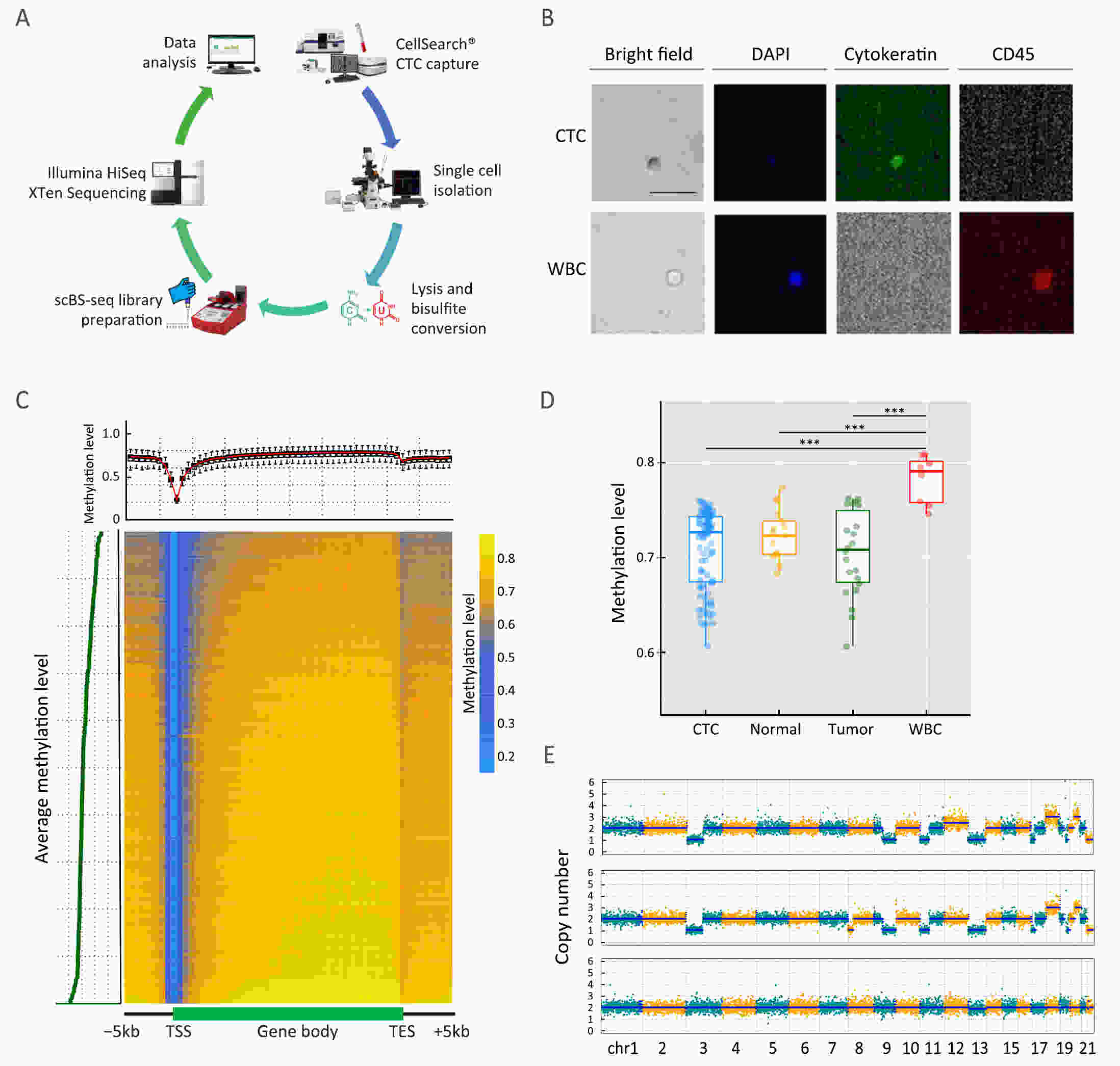
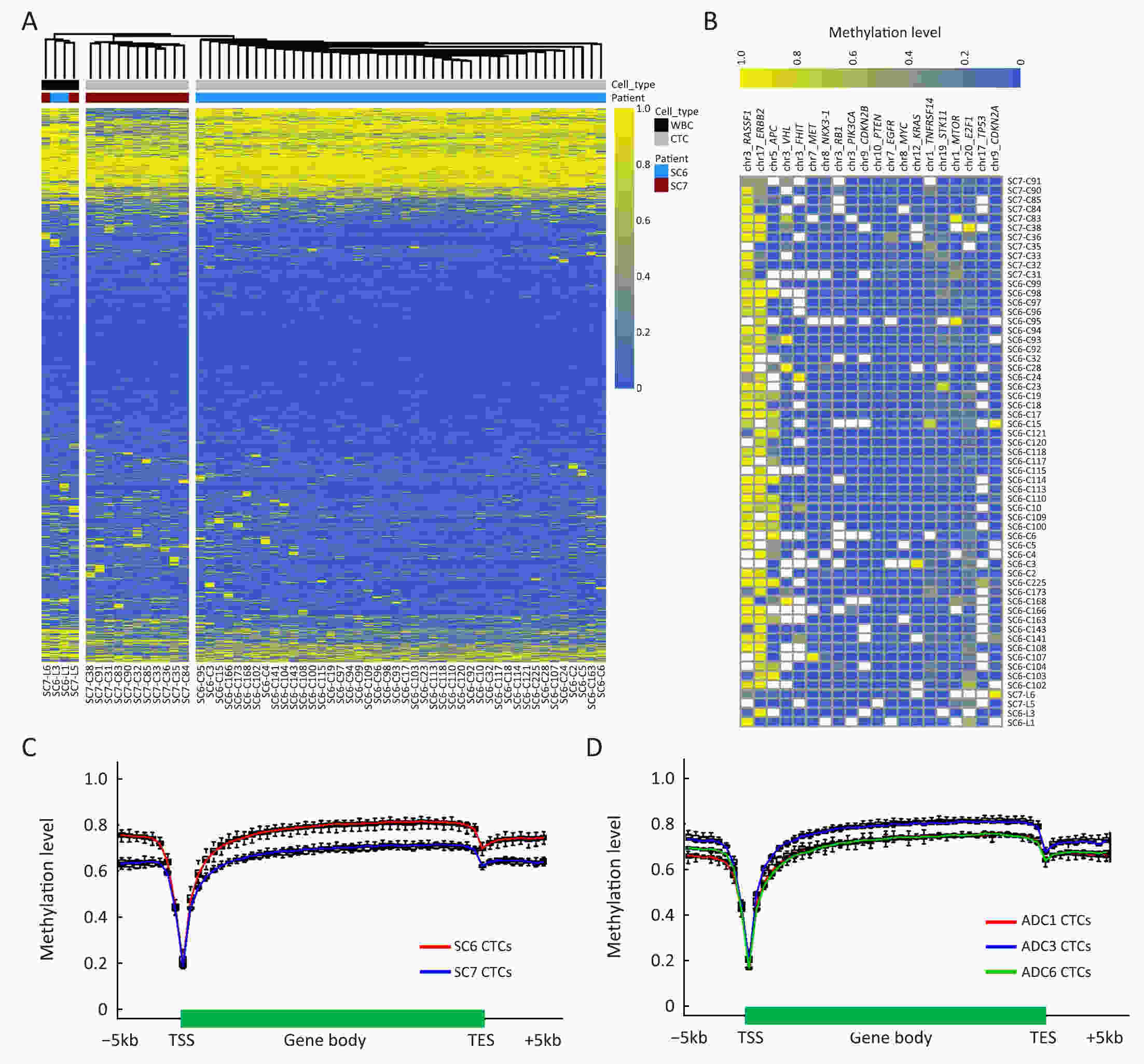
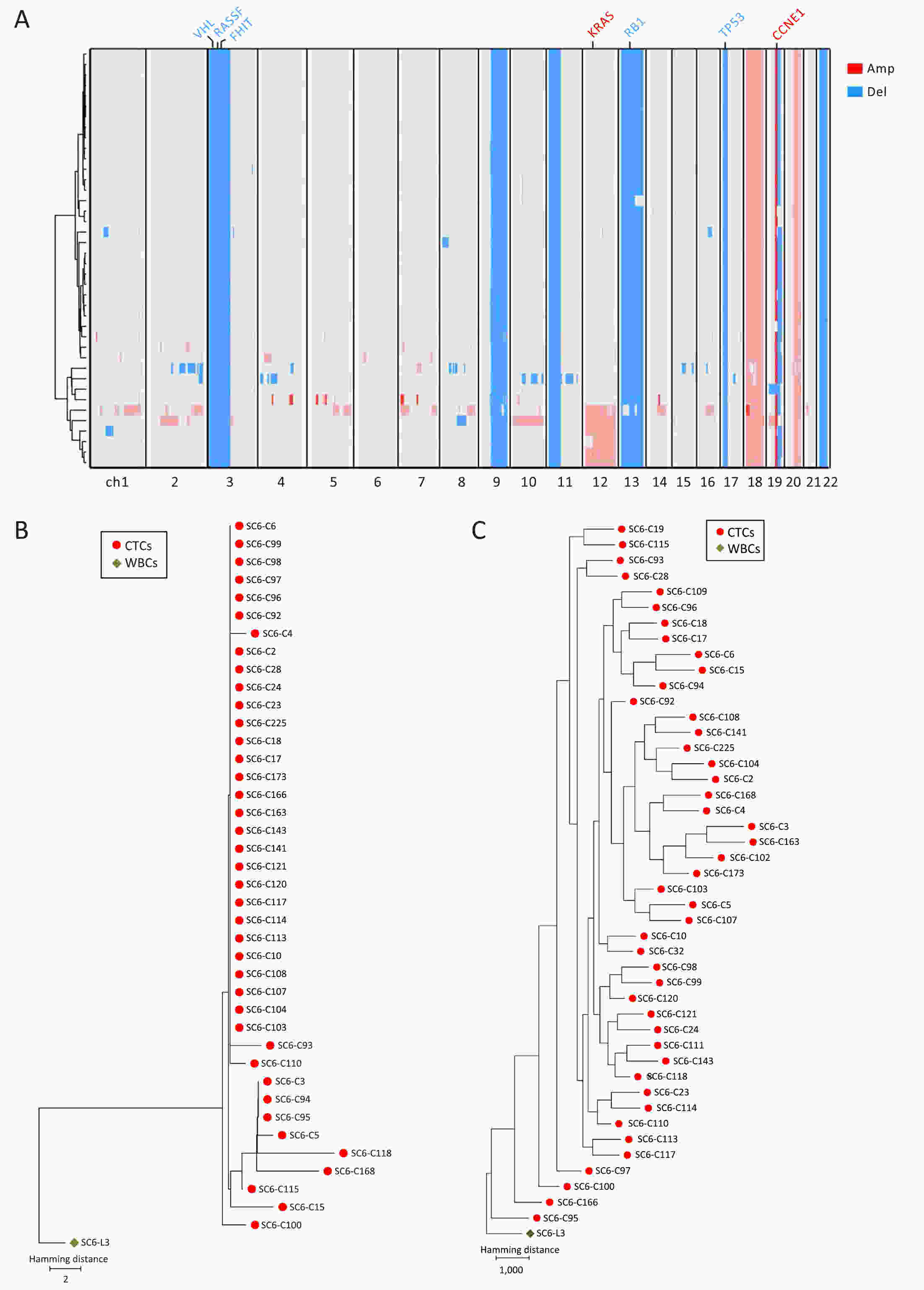
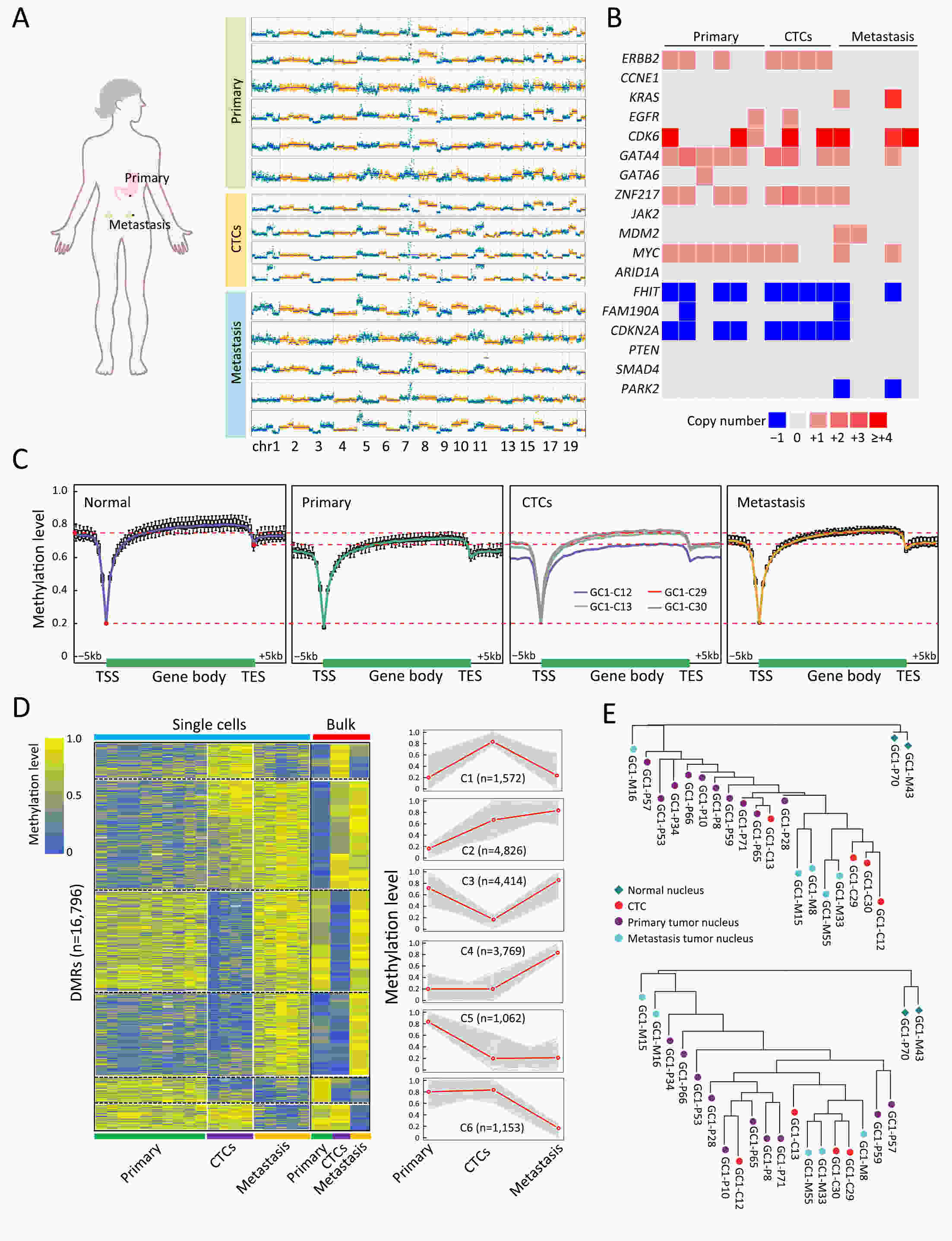
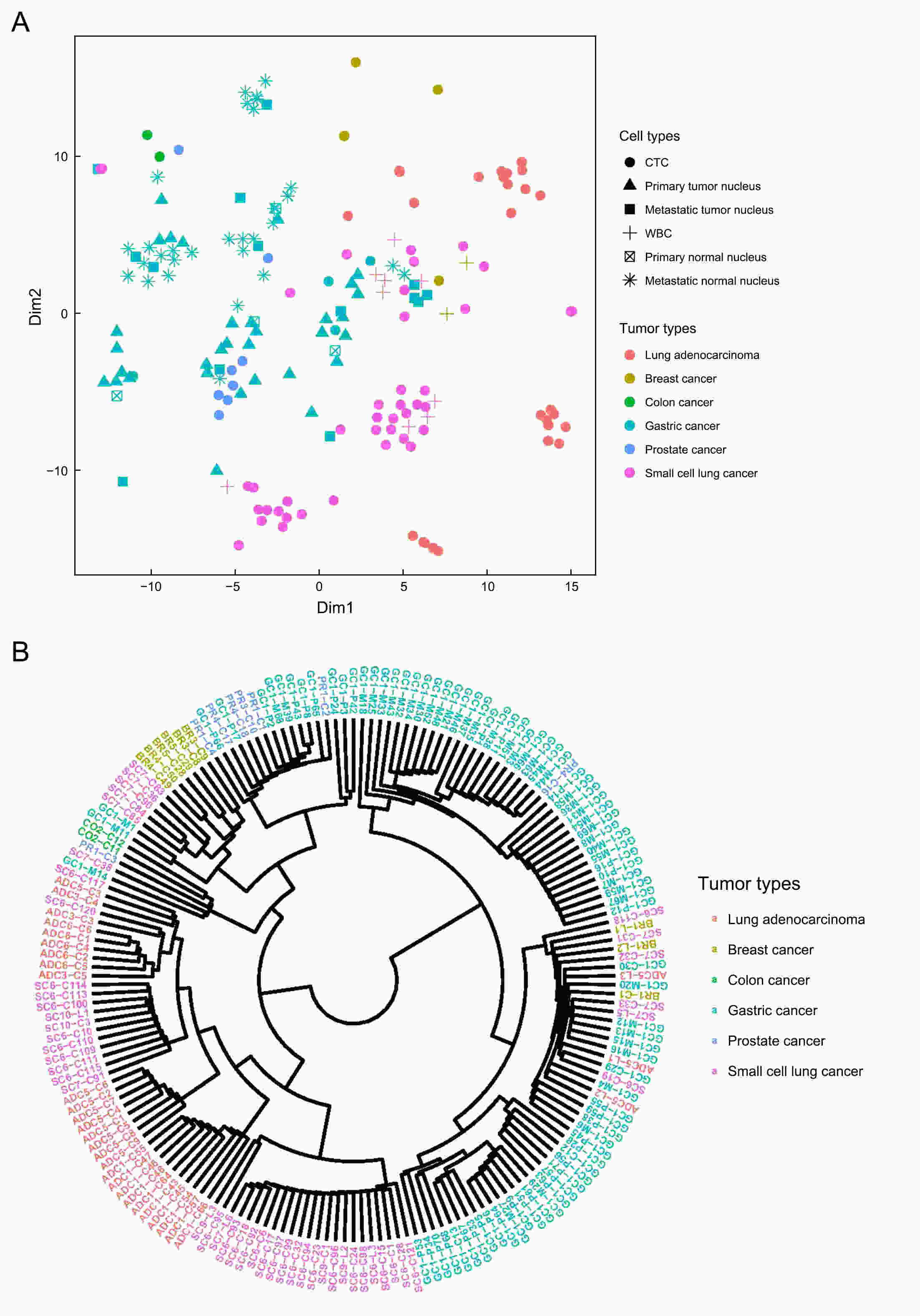
ObjectivePrevious investigations of circulating tumor cells (CTCs) have mainly focused on their genomic or transcriptomic features, leaving their epigenetic landscape relatively uncharacterized. Here, we investigated the genome-wide DNA methylome of CTCs with a view to understanding the epigenetic regulatory mechanisms underlying cancer metastasis. MethodsWe evaluated single-cell DNA methylome and copy number alteration (CNA) in 196 single cells, including 107 CTCs collected from 17 cancer patients covering six different cancer types. Our single-cell bisulfite sequencing (scBS-seq) covered on average 11.78% of all CpG dinucleotides and accurately deduced the CNA patterns at 500 kb resolution. ResultsWe report distinct subclonal structures and different evolutionary histories of CTCs inferred from CNA and DNA methylation profiles. Furthermore, we demonstrate potential tumor origin classification based on the tissue-specific DNA methylation profiles of CTCs. ConclusionsOur work provides a comprehensive survey of genome-wide DNA methylome in single CTCs and reveals 5-methylcytosine (5-mC) heterogeneity in CTCs, addressing the potential epigenetic regulatory mechanisms underlying cancer metastasis and facilitating the future clinical application of CTCs.
2021, 33(3): 405-416.
doi: 10.21147/j.issn.1000-9604.2021.03.11
Abstract:
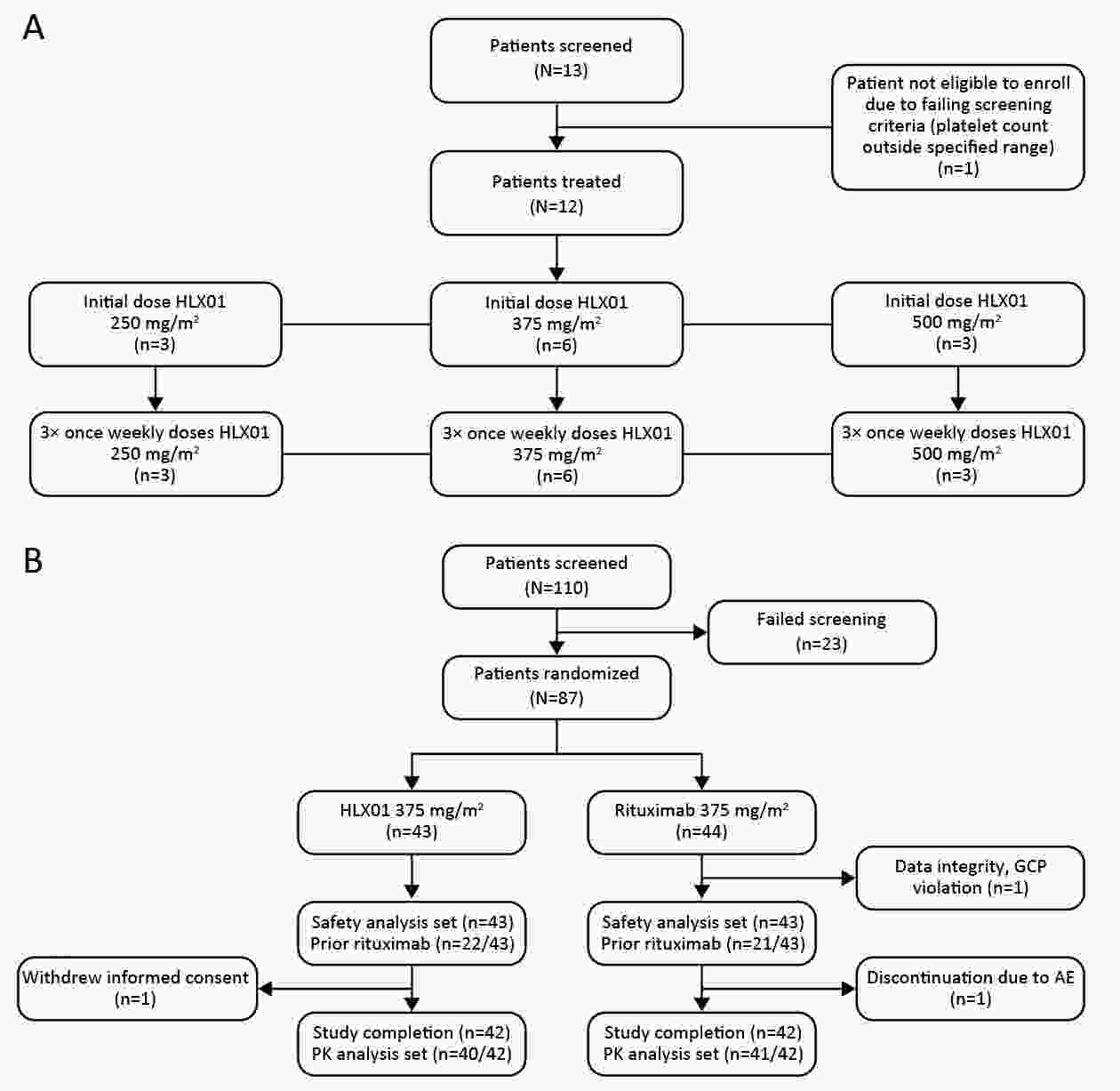
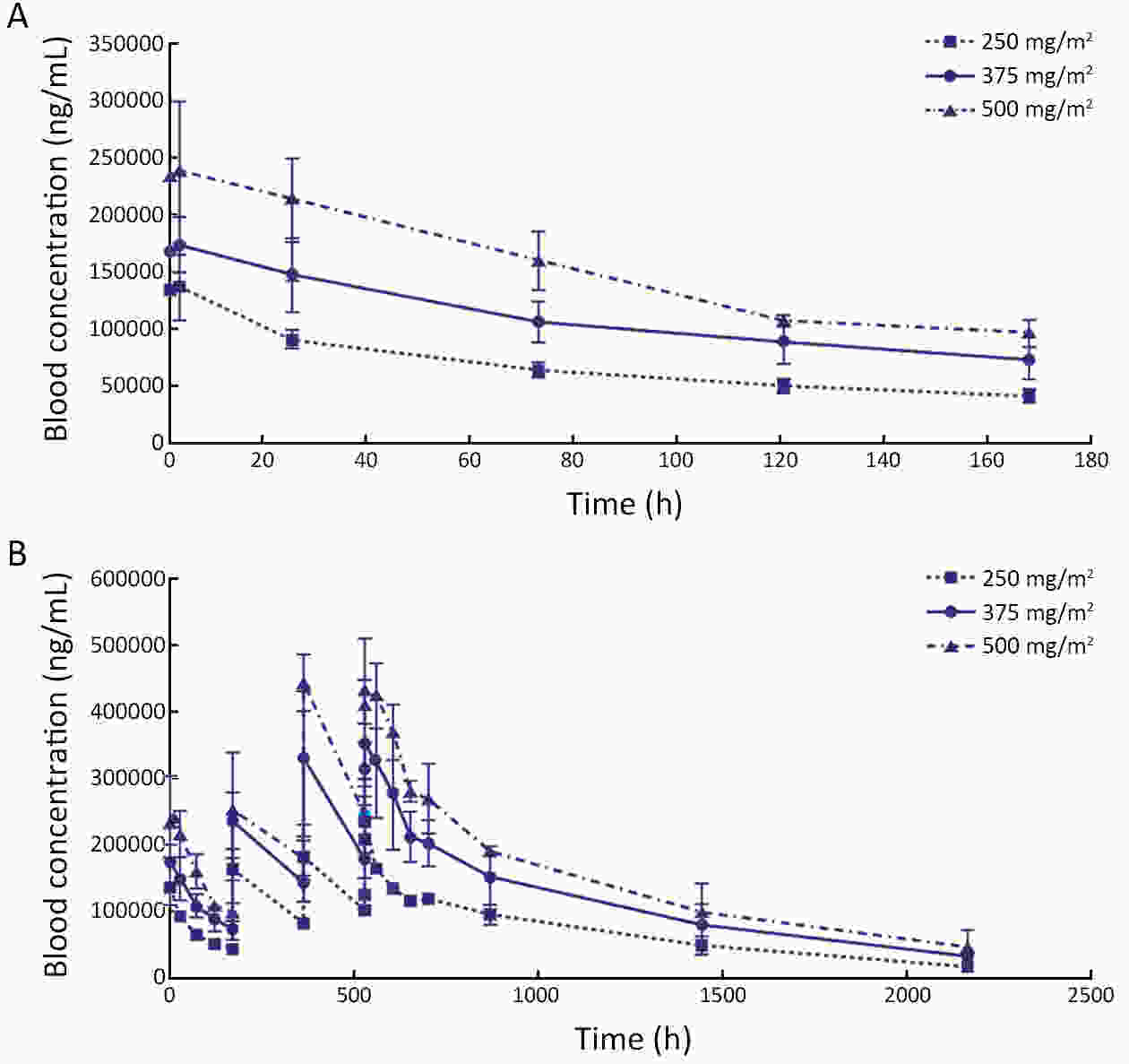
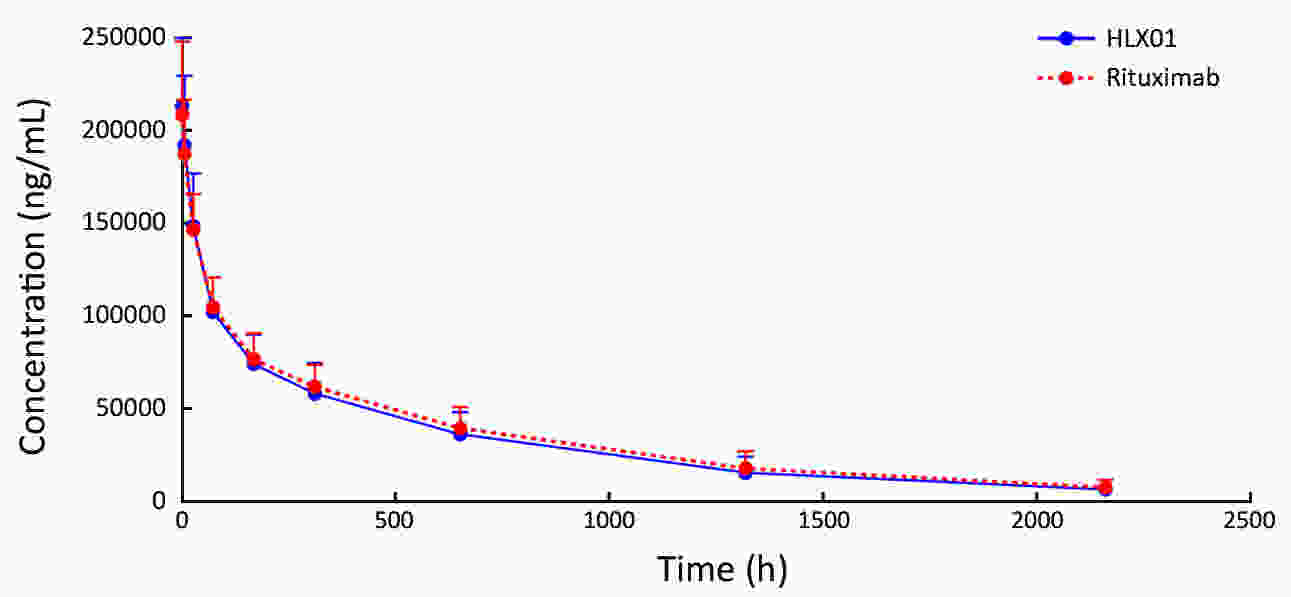
ObjectiveThis study aimed to compare the pharmacokinetic, pharmacodynamic and safety profiles of HLX01 (a rituximab biosimilar) and reference rituximab sourced from China (MabThera®; rituximab-CN). MethodsHere we report the results of two phase 1 studies. In the phase 1a, open-label, dose-escalation study (NCT03218072, CTR20140400), eligible patients received 250, 375 and 500 mg/m2 HLX01 sequentially at 7-day intervals, after confirming no dose-limiting toxicity (DLT). In the phase 1b, double-blind study (NCT02584920, CTR20140764), eligible patients were given a single dose of 375 mg/m2 HLX01 or rituximab-CN. The primary endpoints included safety and tolerability parameters for the phase 1a and the area under the plasma concentration-time curve from time zero to day 91 (AUC0−91 d) for the phase 1b study. Equivalence was concluded if 90% confidence interval (90% CI) for the geometric least squares mean ratio (GLSMR) fell in the pre-specified equivalence criteria (80%−125%). ResultsBetween June 20, 2014 and January 5, 2015, 12 patients were enrolled in the phase 1a study. The pharmacokinetics of HLX01 showed dose proportionality and accumulation to steady state. HLX01 was well tolerated, with no serious adverse events (AEs), discontinuations or DLTs. Between November 8, 2014 and August 13, 2015, 87 eligible patients were enrolled in the phase 1b study, including 43 who received HLX01 and 44 who were treated with rituximab-CN. The equivalence endpoint was met with GLSMR for AUC0−91 d being 89.6% (90% CI: 80.4%−99.8%). AEs, anti-drug antibodies, and CD19+ and CD20+ B lymphocyte counts were similar between the HLX01 and rituximab-CN treatment groups. ConclusionsTreatment with HLX01 was safe and well tolerated in Chinese patients with B-cell lymphoma. HLX01 and rituximab-CN have similar pharmacokinetic, pharmacodynamic and safety profiles.
2021, 33(3): 417-432.
doi: 10.21147/j.issn.1000-9604.2021.03.12
Abstract:
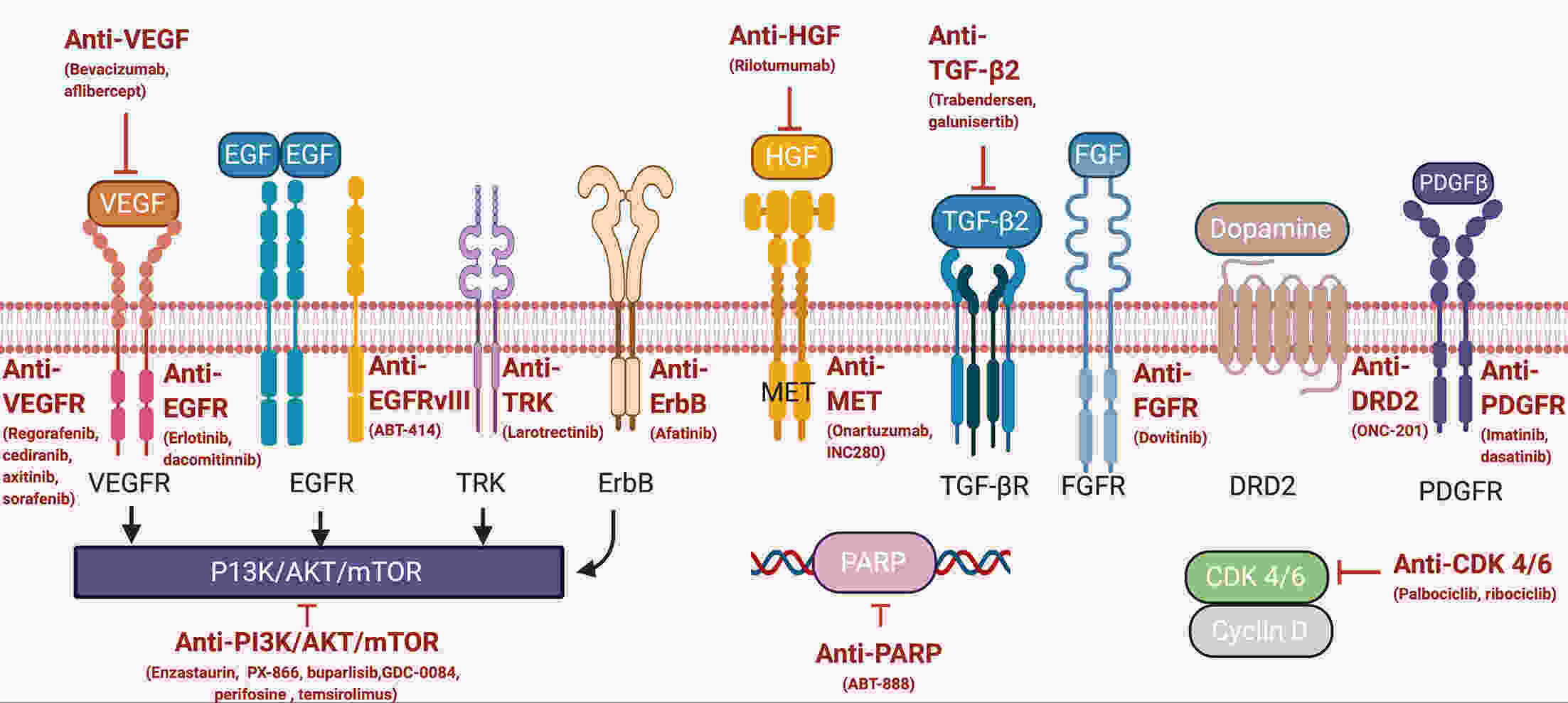
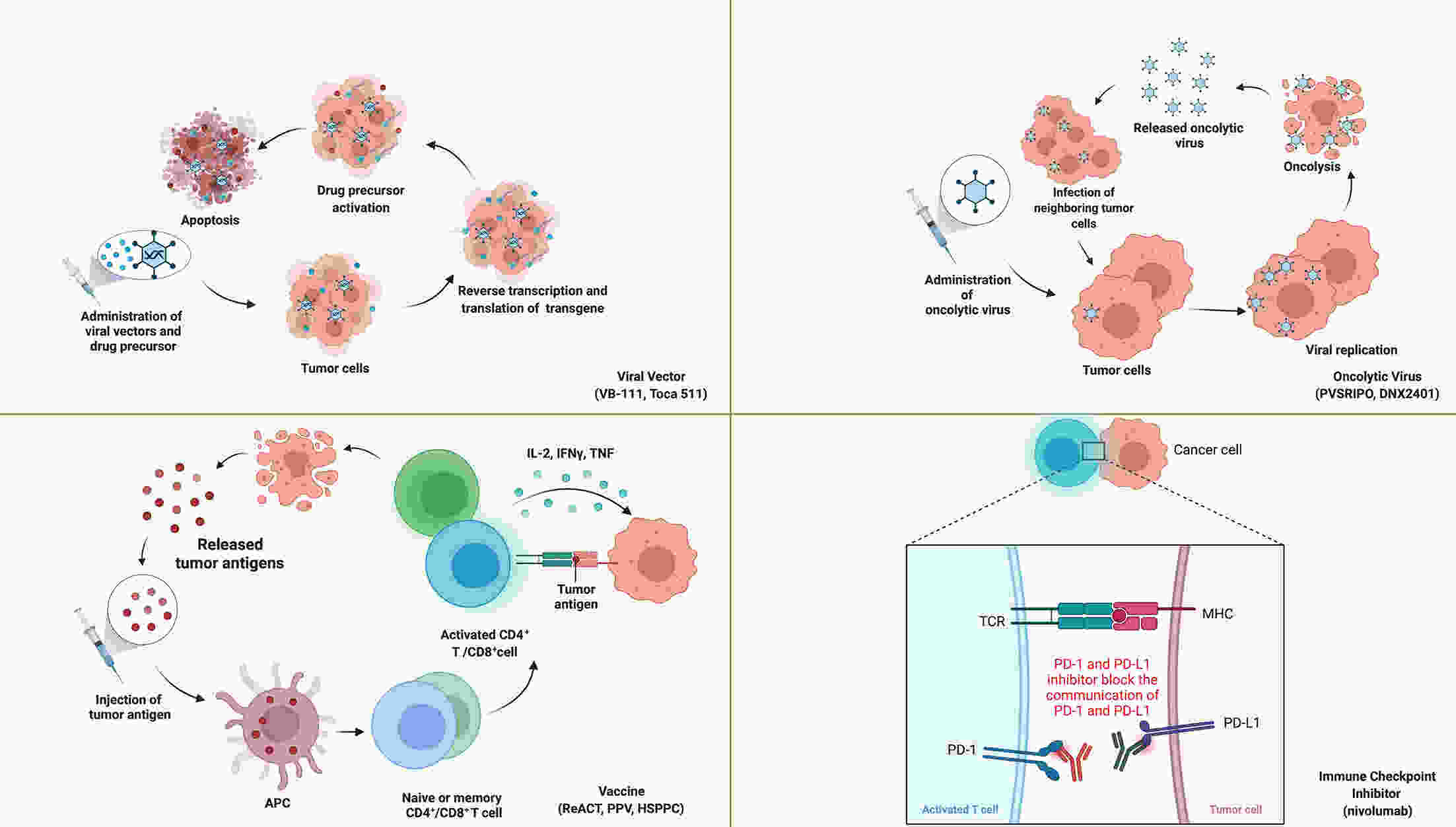
Recurrence is a major concern for adult patients with glioblastomas (GBMs), and the prognosis remains poor. Although several therapies have been assessed, most of them have not achieved satisfactory results. Therefore, there is currently no standard treatment for adult recurrent GBM (rGBM). Here, we review the results of clinical trials for the systematic therapy of rGBM. Regorafenib, rindopepimut and neoadjuvant programmed death 1 (PD-1) inhibitors are promising agents for rGBM, while regorafenib is effective in both O6-methylguanine DNA methyltransferase (MGMT) promoter methylated and unmethylated patients. Temozolomide rechallenge and alkylating agents combined with bevacizumab can be useful for patients with MGMT methylation, and patients with isocitrate dehydrogenase (IDH) mutations or second recurrence can benefit from vocimagene amiretrorepvec (Toca 511). Some phase I trials on targeted therapy and immunotherapy have shown positive results, and results from further studies are expected. In addition to the analysis of existing clinical trial results, forthcoming trials should be well designed, and patients are encouraged to participate in appropriate clinical trials.
Recurrence is a major concern for adult patients with glioblastomas (GBMs), and the prognosis remains poor. Although several therapies have been assessed, most of them have not achieved satisfactory results. Therefore, there is currently no standard treatment for adult recurrent GBM (rGBM). Here, we review the results of clinical trials for the systematic therapy of rGBM. Regorafenib, rindopepimut and neoadjuvant programmed death 1 (PD-1) inhibitors are promising agents for rGBM, while regorafenib is effective in both O6-methylguanine DNA methyltransferase (MGMT) promoter methylated and unmethylated patients. Temozolomide rechallenge and alkylating agents combined with bevacizumab can be useful for patients with MGMT methylation, and patients with isocitrate dehydrogenase (IDH) mutations or second recurrence can benefit from vocimagene amiretrorepvec (Toca 511). Some phase I trials on targeted therapy and immunotherapy have shown positive results, and results from further studies are expected. In addition to the analysis of existing clinical trial results, forthcoming trials should be well designed, and patients are encouraged to participate in appropriate clinical trials.

 Abstract
Abstract FullText HTML
FullText HTML PDF 544KB
PDF 544KB