2021 Vol.33(5)
Display Mode: |
2021, 33(5): 535-547.
doi: 10.21147/j.issn.1000-9604.2021.05.01
Abstract:
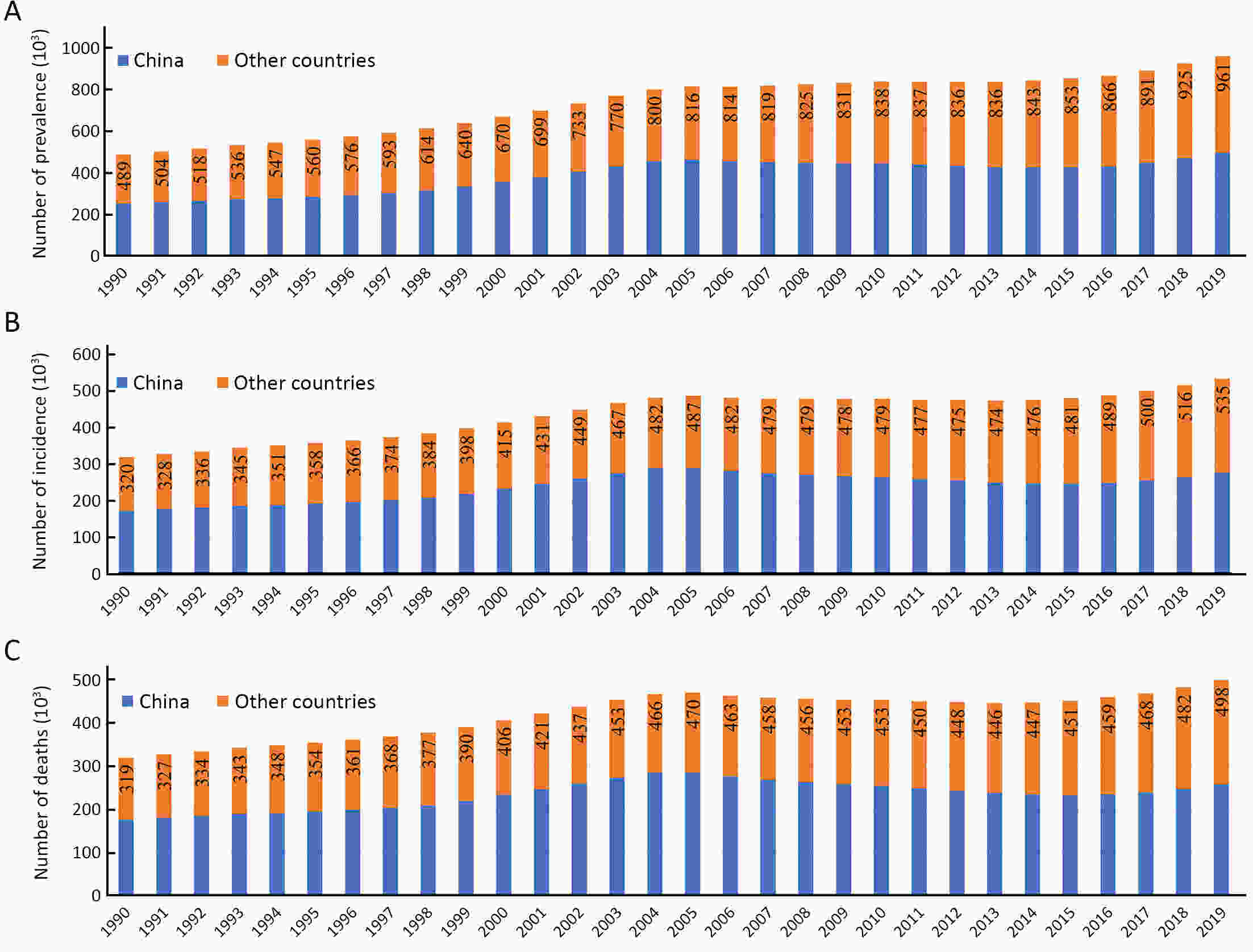
More than 600,000 people are diagnosed with esophageal cancer (EC) every year globally, and the five-year survival rate of EC is less than 20%. Two common histological subtypes of EC, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), have great geographical variations in incidence rates. About half of the world’s EC was diagnosed in China and a majority of which belong to ESCC. Globally, the overall incidence rate of EC is decreasing. In some high-risk Asian regions, such as China, the incidence rate of ESCC has generally declined, potentially due to economic growth and improvement of diet habits. In some European high-income countries and the United States, the decline is mainly attributed to the decrease in smoking and drinking. The risk factors of EC are not well understood, and the importance of environmental and genetic factors in the pathogenesis is also unclear. The incidence and mortality of advanced EC can be reduced through early diagnosis and screening. White light endoscopy is still the gold standard in the current screening technology. This article reviews the epidemiology, risk factors, and screening strategies of EC in recent years to help researchers determine the most effective management strategies to reduce the risk of EC.
More than 600,000 people are diagnosed with esophageal cancer (EC) every year globally, and the five-year survival rate of EC is less than 20%. Two common histological subtypes of EC, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), have great geographical variations in incidence rates. About half of the world’s EC was diagnosed in China and a majority of which belong to ESCC. Globally, the overall incidence rate of EC is decreasing. In some high-risk Asian regions, such as China, the incidence rate of ESCC has generally declined, potentially due to economic growth and improvement of diet habits. In some European high-income countries and the United States, the decline is mainly attributed to the decrease in smoking and drinking. The risk factors of EC are not well understood, and the importance of environmental and genetic factors in the pathogenesis is also unclear. The incidence and mortality of advanced EC can be reduced through early diagnosis and screening. White light endoscopy is still the gold standard in the current screening technology. This article reviews the epidemiology, risk factors, and screening strategies of EC in recent years to help researchers determine the most effective management strategies to reduce the risk of EC.
2021, 33(5): 548-562.
doi: 10.21147/j.issn.1000-9604.2021.05.02
Abstract:
Lung cancer is the leading cause of cancer-related mortality globally, accounting for 1.8 million deaths in 2020. While the vast majority are caused by tobacco smoking, 15%−25% of all lung cancer cases occur in lifelong never-smokers. The International Agency for Research on Cancer (IARC) has classified multiple agents with sufficient evidence for lung carcinogenesis in humans, which include tobacco smoking, as well as several environmental exposures such as radon, second-hand tobacco smoke, outdoor air pollution, household combustion of coal and several occupational hazards. However, the IARC evaluation had not been stratified based on smoking status, and notably lung cancer in never-smokers (LCINS) has different epidemiological, clinicopathologic and molecular characteristics from lung cancer in ever-smokers. Among several risk factors proposed for the development of LCINS, environmental factors have the most available evidence for their association with LCINS and their roles cannot be overemphasized. Additionally, while initial genetic studies largely focused on lung cancer as a whole, recent studies have also identified genetic risk factors for LCINS. This article presents an overview of several environmental factors associated with LCINS, and some of the emerging evidence for genetic factors associated with LCINS. An increased understanding of the risk factors associated with LCINS not only helps to evaluate a never-smoker’s personal risk for lung cancer, but also has important public health implications for the prevention and early detection of the disease. Conclusive evidence on causal associations could inform longer-term policy reform in a range of areas including occupational health and safety, urban design, energy use and particle emissions, and the importance of considering the impacts of second-hand smoke in tobacco control policy.
Lung cancer is the leading cause of cancer-related mortality globally, accounting for 1.8 million deaths in 2020. While the vast majority are caused by tobacco smoking, 15%−25% of all lung cancer cases occur in lifelong never-smokers. The International Agency for Research on Cancer (IARC) has classified multiple agents with sufficient evidence for lung carcinogenesis in humans, which include tobacco smoking, as well as several environmental exposures such as radon, second-hand tobacco smoke, outdoor air pollution, household combustion of coal and several occupational hazards. However, the IARC evaluation had not been stratified based on smoking status, and notably lung cancer in never-smokers (LCINS) has different epidemiological, clinicopathologic and molecular characteristics from lung cancer in ever-smokers. Among several risk factors proposed for the development of LCINS, environmental factors have the most available evidence for their association with LCINS and their roles cannot be overemphasized. Additionally, while initial genetic studies largely focused on lung cancer as a whole, recent studies have also identified genetic risk factors for LCINS. This article presents an overview of several environmental factors associated with LCINS, and some of the emerging evidence for genetic factors associated with LCINS. An increased understanding of the risk factors associated with LCINS not only helps to evaluate a never-smoker’s personal risk for lung cancer, but also has important public health implications for the prevention and early detection of the disease. Conclusive evidence on causal associations could inform longer-term policy reform in a range of areas including occupational health and safety, urban design, energy use and particle emissions, and the importance of considering the impacts of second-hand smoke in tobacco control policy.
2021, 33(5): 563-573.
doi: 10.21147/j.issn.1000-9604.2021.05.03
Abstract:
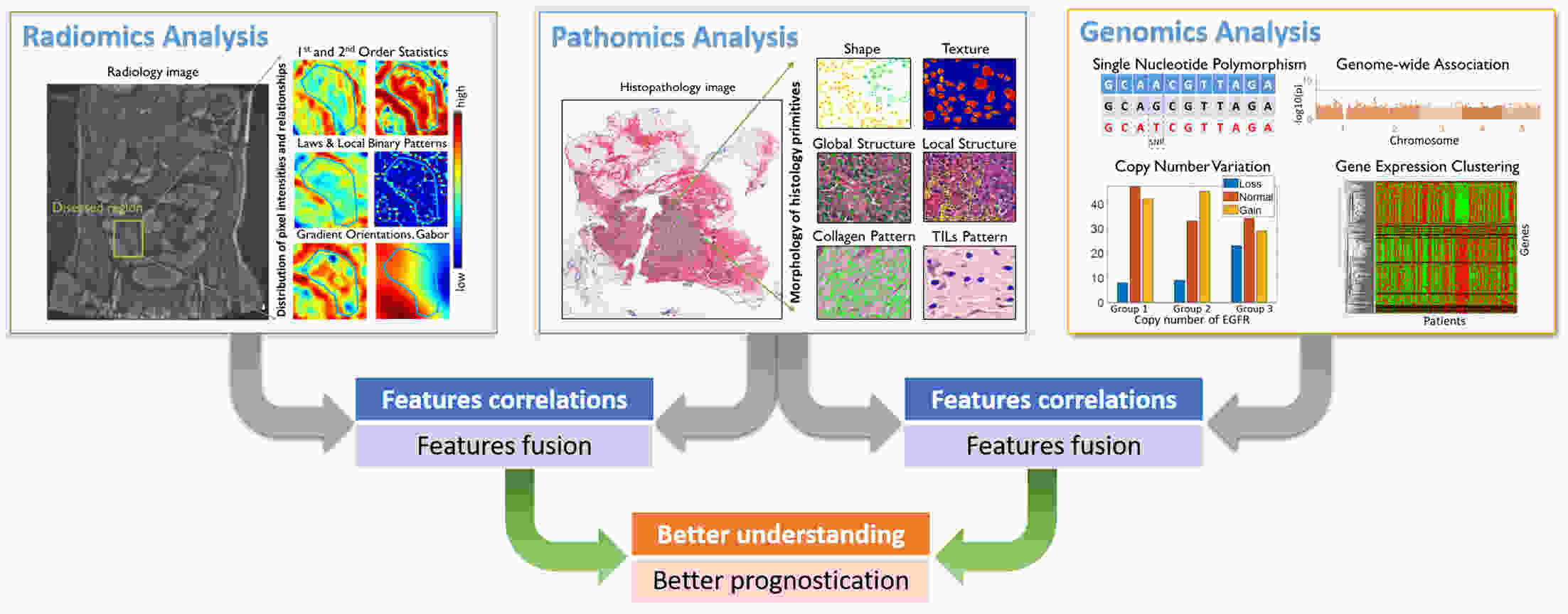
In the last decade, the focus of computational pathology research community has shifted from replicating the pathological examination for diagnosis done by pathologists to unlocking and discovering “sub-visual” prognostic image cues from the histopathological image. While we are getting more knowledge and experience in digital pathology, the emerging goal is to integrate other-omics or modalities that will contribute for building a better prognostic assay. In this paper, we provide a brief review of representative works that focus on integrating pathomics with radiomics and genomics for cancer prognosis. It includes: correlation of pathomics and genomics; fusion of pathomics and genomics; fusion of pathomics and radiomics. We also present challenges, potential opportunities, and avenues for future work.
In the last decade, the focus of computational pathology research community has shifted from replicating the pathological examination for diagnosis done by pathologists to unlocking and discovering “sub-visual” prognostic image cues from the histopathological image. While we are getting more knowledge and experience in digital pathology, the emerging goal is to integrate other-omics or modalities that will contribute for building a better prognostic assay. In this paper, we provide a brief review of representative works that focus on integrating pathomics with radiomics and genomics for cancer prognosis. It includes: correlation of pathomics and genomics; fusion of pathomics and genomics; fusion of pathomics and radiomics. We also present challenges, potential opportunities, and avenues for future work.
2021, 33(5): 574-582.
doi: 10.21147/j.issn.1000-9604.2021.05.04
Abstract:
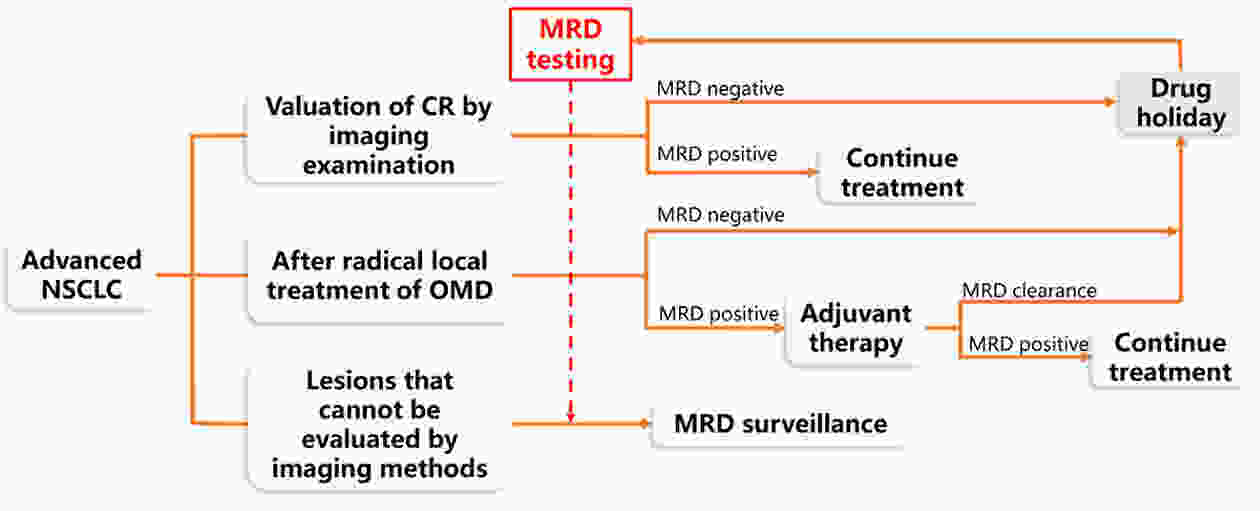
Curative therapy was not previously available for patients with advanced non-small cell lung cancer (NSCLC); thus, the concept of minimal/measurable (or molecular) residual disease (MRD) was not applicable to these patients. However, advances in targeted and immunotherapy have revolutionized the treatment landscape for patients with advanced NSCLC, with emerging evidence of long-term survival and even the hope of complete remission (CR) by imaging examination. The latest research shows that patients with oligometastatic lung cancer can benefit from local treatment. After removing the lesions, the choice of follow-up therapy and monitoring of the lesions could remain uncertain. MRD plays a role in identifying early-stage NSCLC patients with high risks of recurrence and determining adjuvant therapy after radical treatment. In recent years, evidence has been accumulating regarding the use of circulating cell-free tumor DNA (ctDNA) to assess MRD in solid tumors. This study discussed the possible applications of ctDNA-based MRD monitoring in advanced NSCLC and described the current challenges and unresolved problems in the application of MRD in advanced NSCLC.
Curative therapy was not previously available for patients with advanced non-small cell lung cancer (NSCLC); thus, the concept of minimal/measurable (or molecular) residual disease (MRD) was not applicable to these patients. However, advances in targeted and immunotherapy have revolutionized the treatment landscape for patients with advanced NSCLC, with emerging evidence of long-term survival and even the hope of complete remission (CR) by imaging examination. The latest research shows that patients with oligometastatic lung cancer can benefit from local treatment. After removing the lesions, the choice of follow-up therapy and monitoring of the lesions could remain uncertain. MRD plays a role in identifying early-stage NSCLC patients with high risks of recurrence and determining adjuvant therapy after radical treatment. In recent years, evidence has been accumulating regarding the use of circulating cell-free tumor DNA (ctDNA) to assess MRD in solid tumors. This study discussed the possible applications of ctDNA-based MRD monitoring in advanced NSCLC and described the current challenges and unresolved problems in the application of MRD in advanced NSCLC.
2021, 33(5): 583-591.
doi: 10.21147/j.issn.1000-9604.2021.05.05
Abstract:
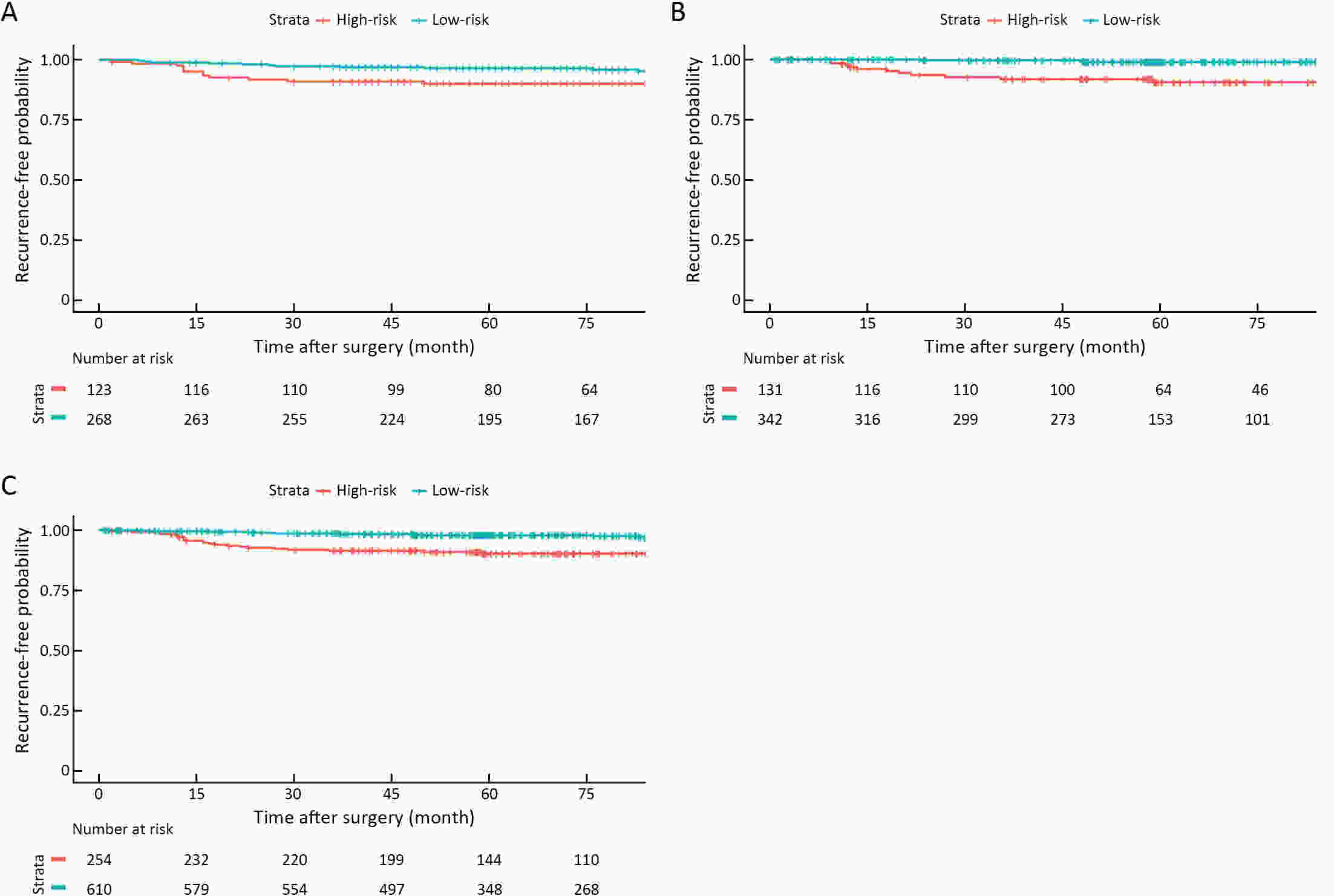
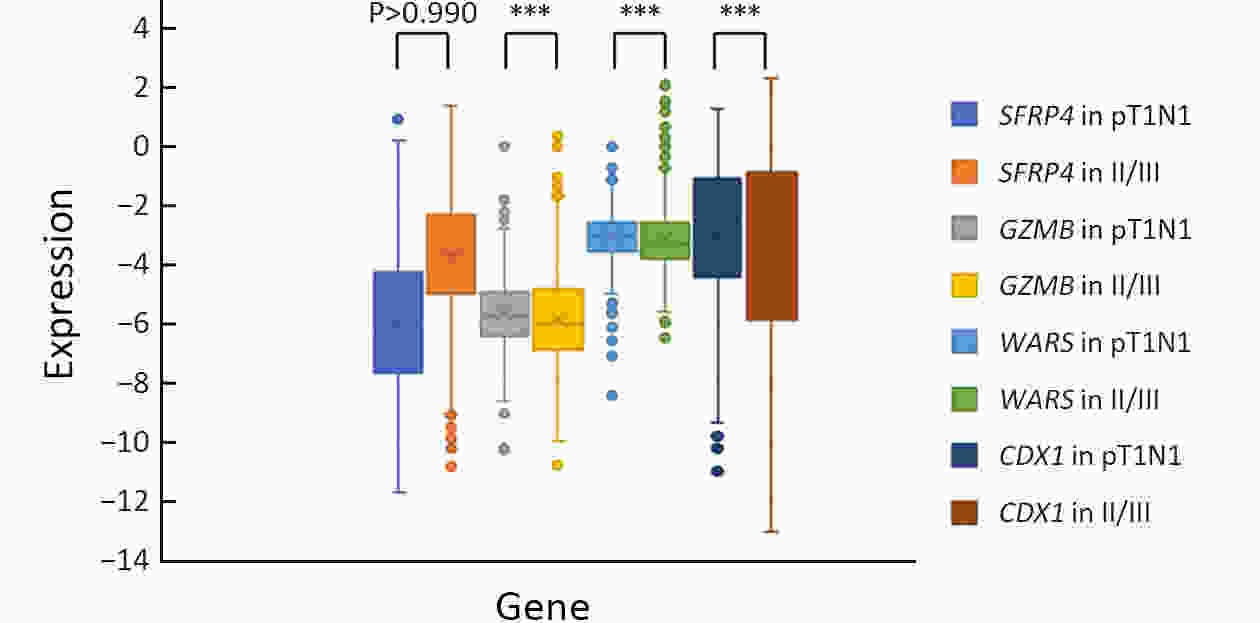
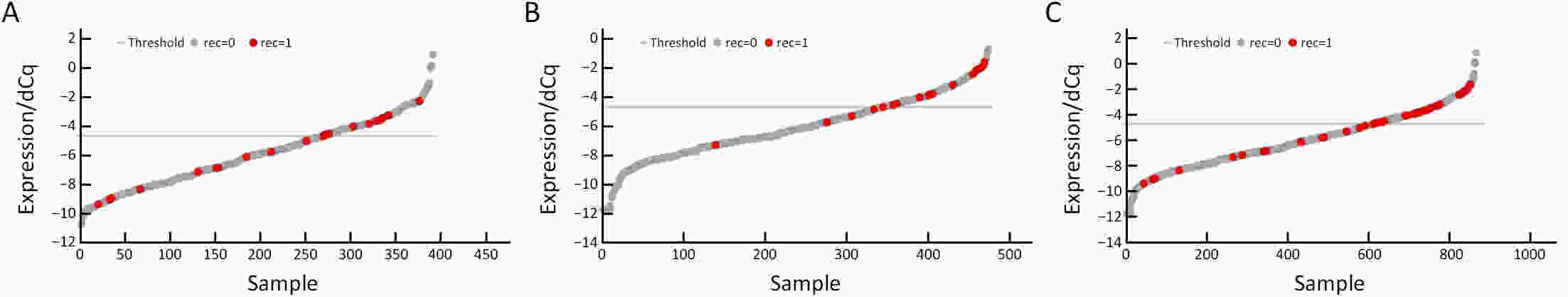
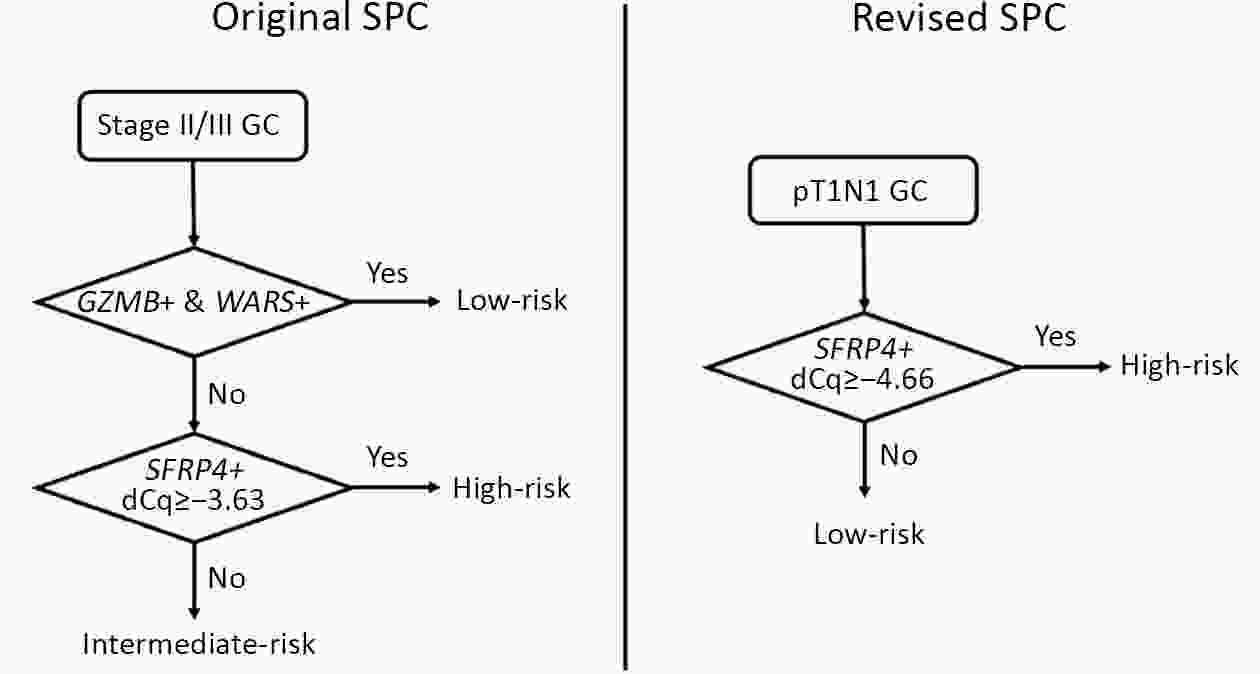
ObjectiveBenefits of adjuvant treatment in pT1N1 gastric cancer (GC) remain controversial. Additionally, an effective biomarker for early GC is the need of the hour. The prognostic and predictive roles of single patient classifier (SPC) were validated in stage II/III GC. In this study, we aimed to elucidate the role of SPC as a biomarker for pT1N1 GC. MethodsThe present retrospective biomarker study (NCT03485105) enrolled patients treated for pT1N1 GC between 1996 and 2012 from two large hospitals (the Y cohort and S cohort). For SPC, mRNA expression of four classifier genes (GZMB, WARS, SFRP4 and CDX1) were evaluated by real-time reverse transcription-polymerase chain reaction assay. The SPC was revised targeting pT1 stages and the prognosis was stratified as high- and low-risk group by the expression of SFRP4, a representative epithelial-mesenchymal transition marker. ResultsSPC was evaluated in 875 patients (n=391 and 484 in the Y and S cohorts, respectively). Among 864 patients whose SPC result was available, 41 (4.7%) patients experience GC recurrence. According to revised SPC, 254 (29.4%) patients were classified as high risk [123 (31.5%) and 131 (27.1%) in the Y and S cohorts, respectively]. The high risk was related to frequent recurrence in both Y and S cohort (log-rank P=0.023, P<0.001, respectively), while there was no difference by GZMB and WARS expression. Multivariable analyses of the overall-cohort confirmed the high risk of revised SPC as a significant prognostic factor [hazard ratio (HR): 4.402 (2.293−8.449), P<0.001] of GC. A significant difference was not detected by SPC in the prognosis of patients in the presence and absence of adjuvant treatment (log-rank P=0.670). ConclusionsThe present study revealed the revised SPC as a prognostic biomarker of pT1N1 GC and suggested the use of the revised SPC for early-stage GC as like stage II/III.
2021, 33(5): 592-605.
doi: 10.21147/j.issn.1000-9604.2021.05.06
Abstract:
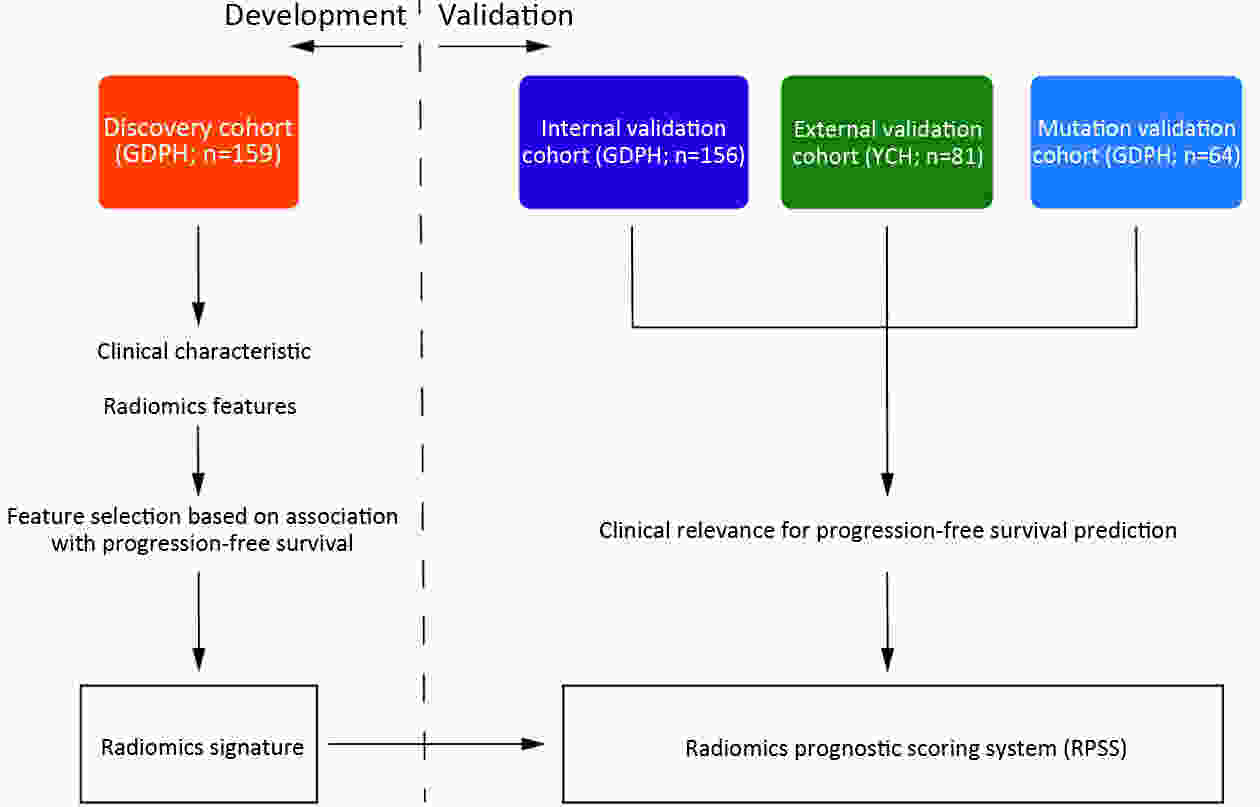
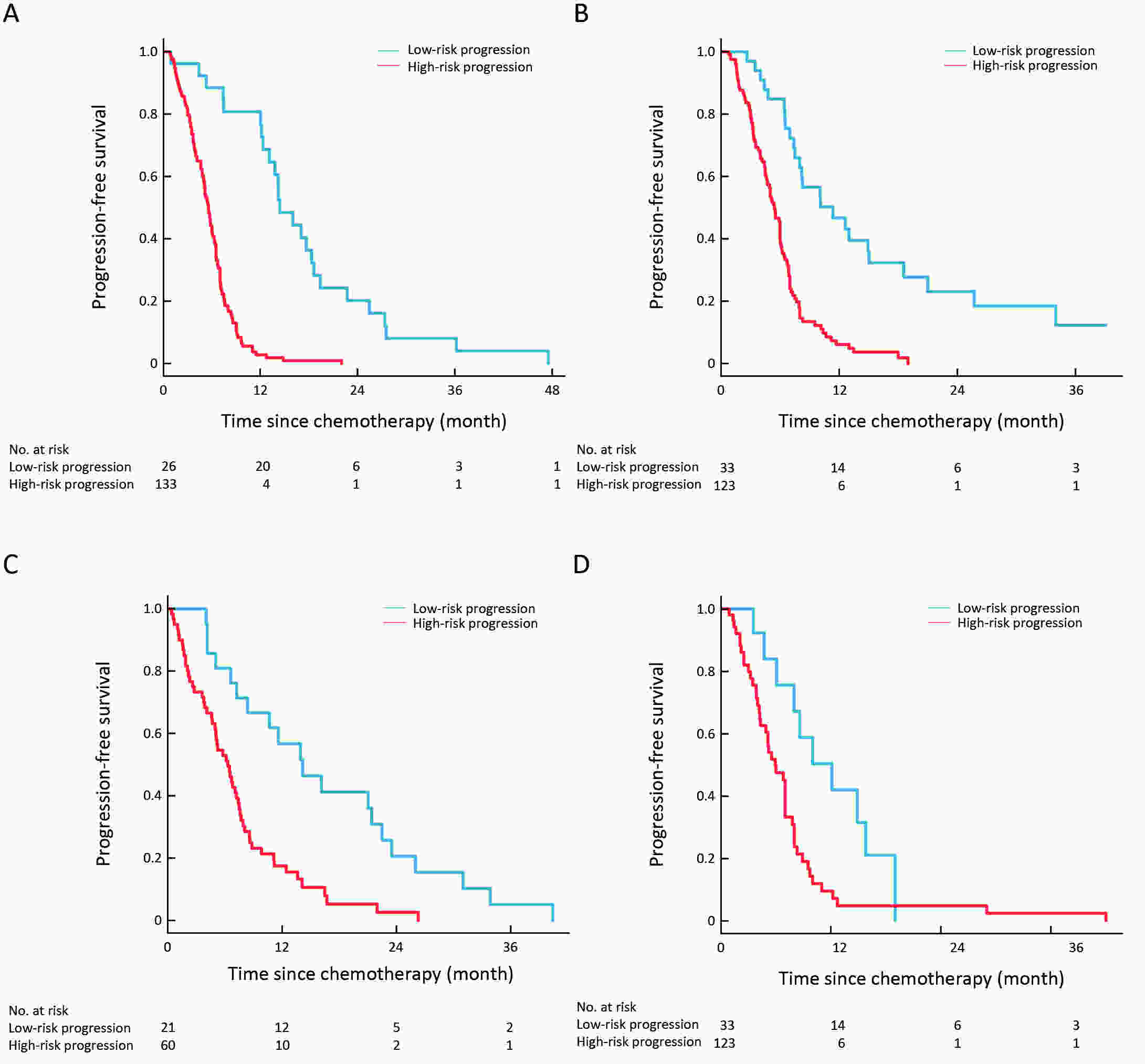
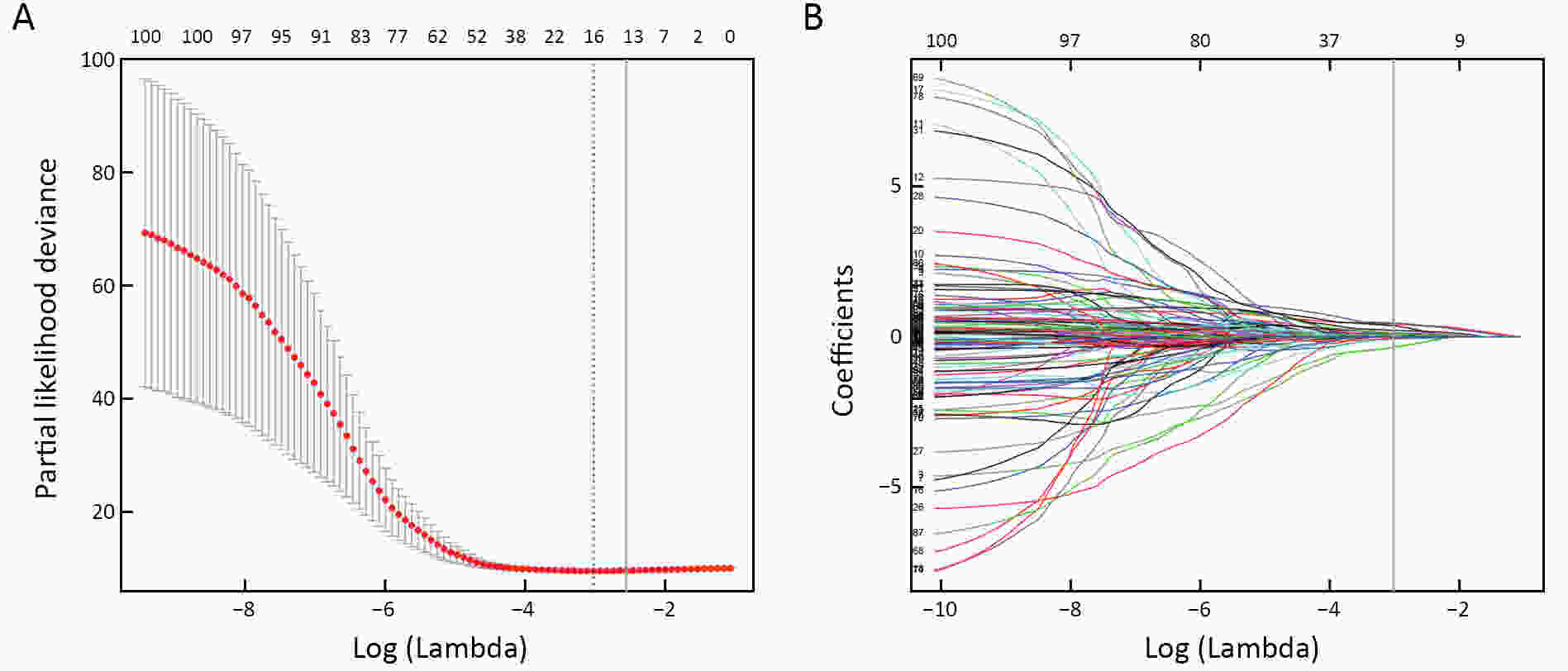
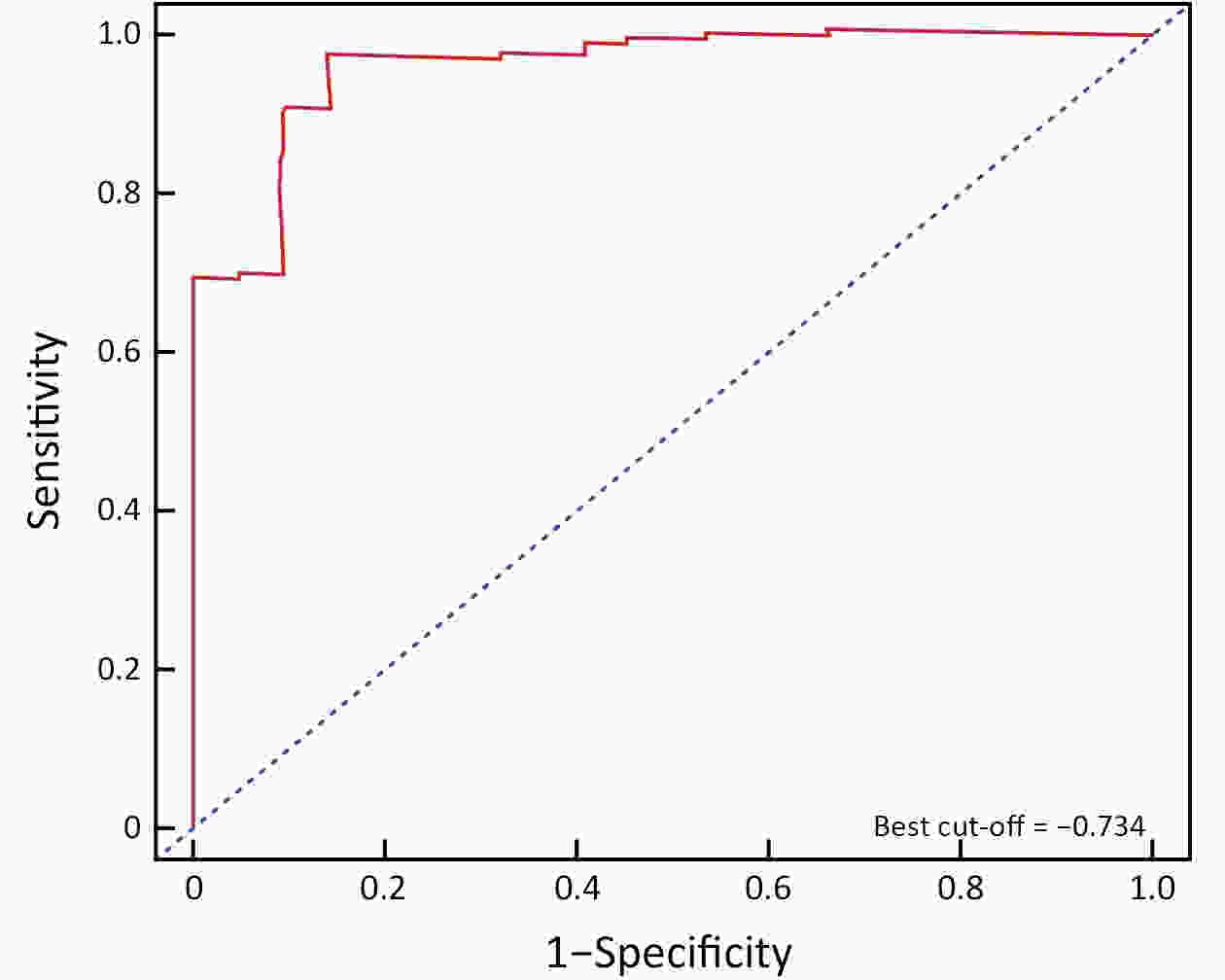
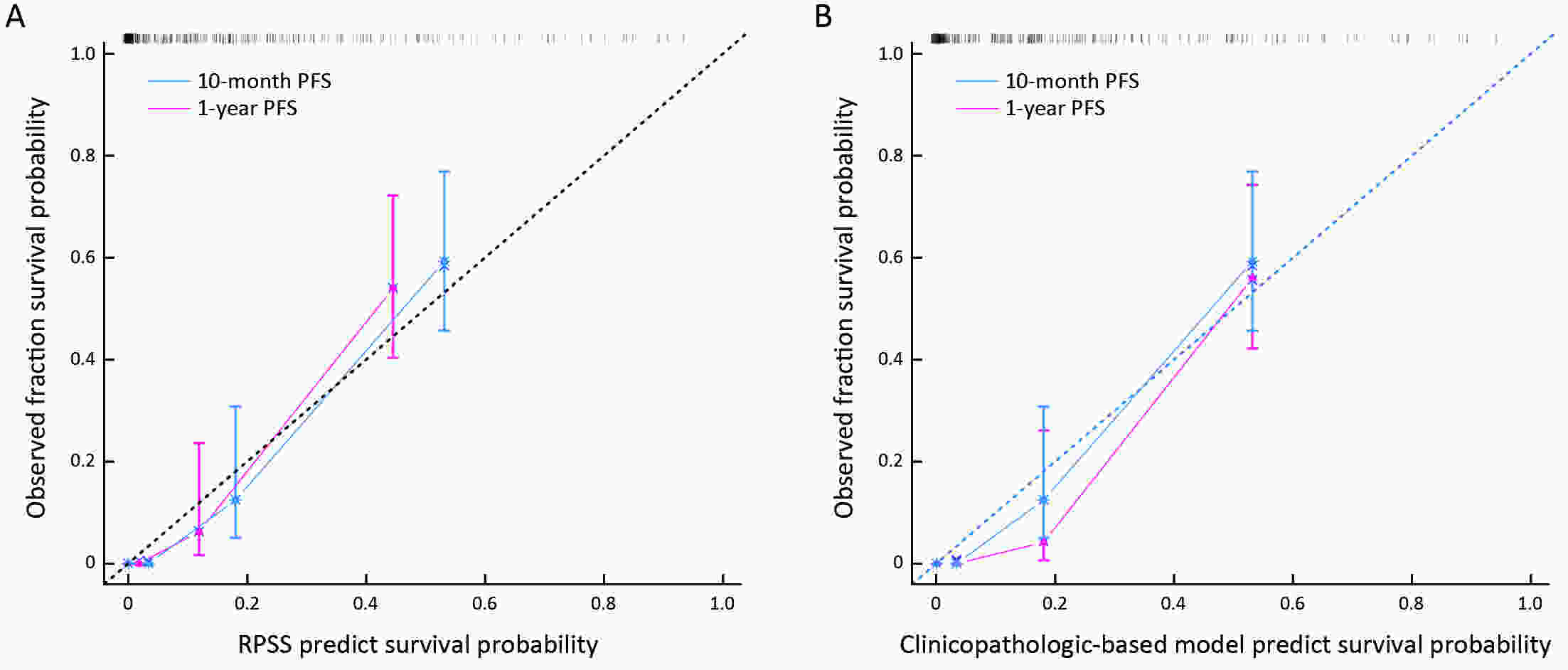
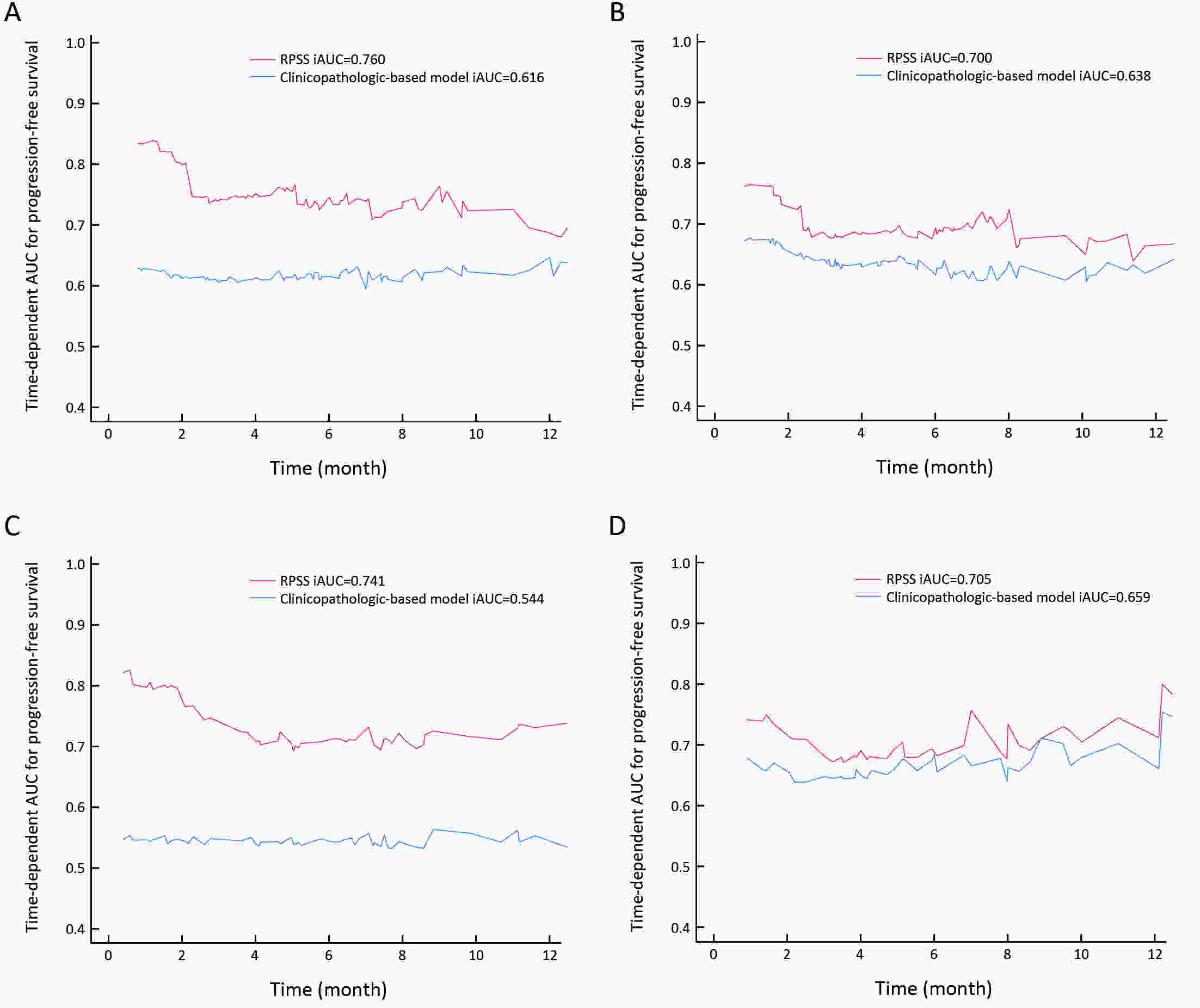
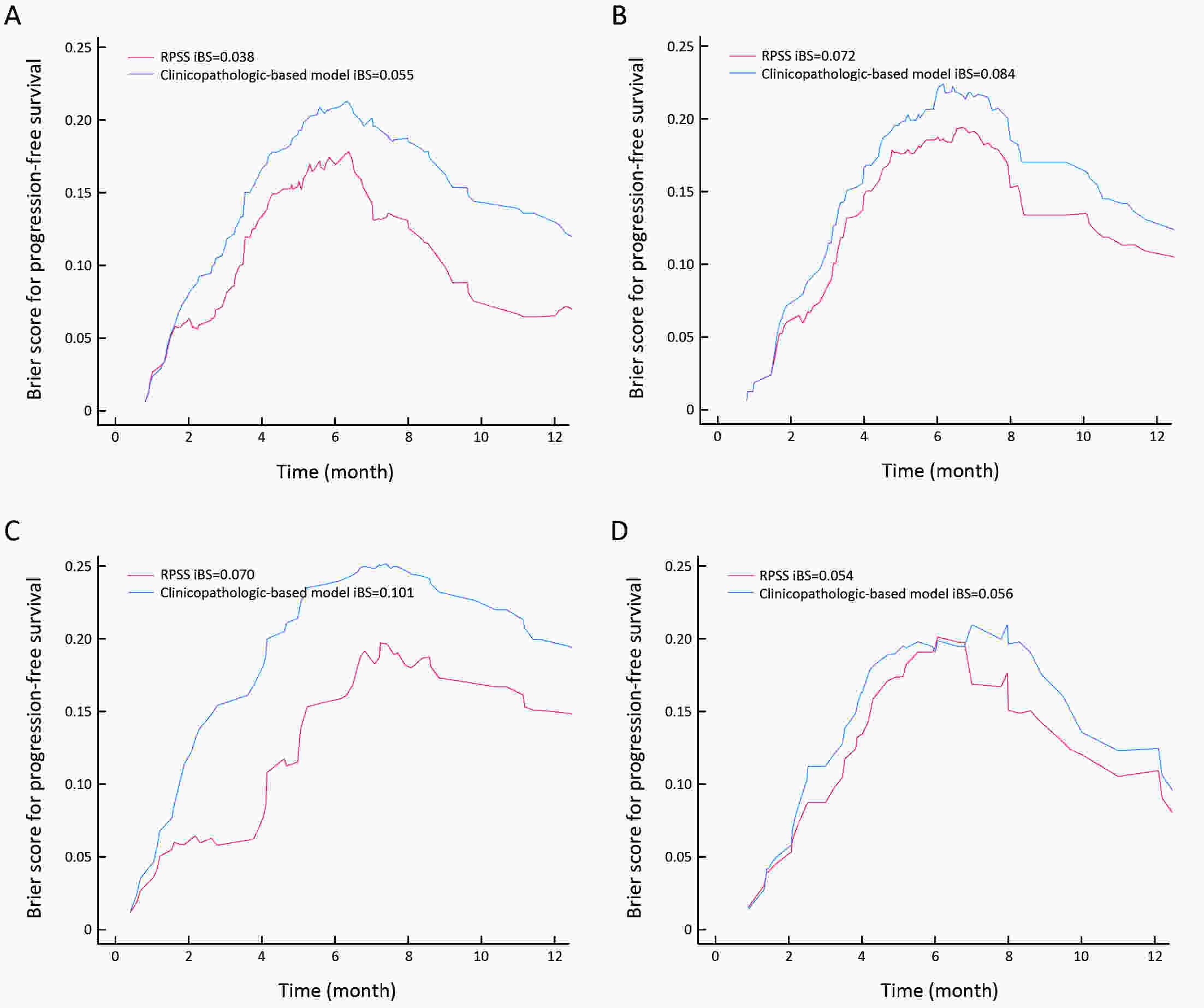
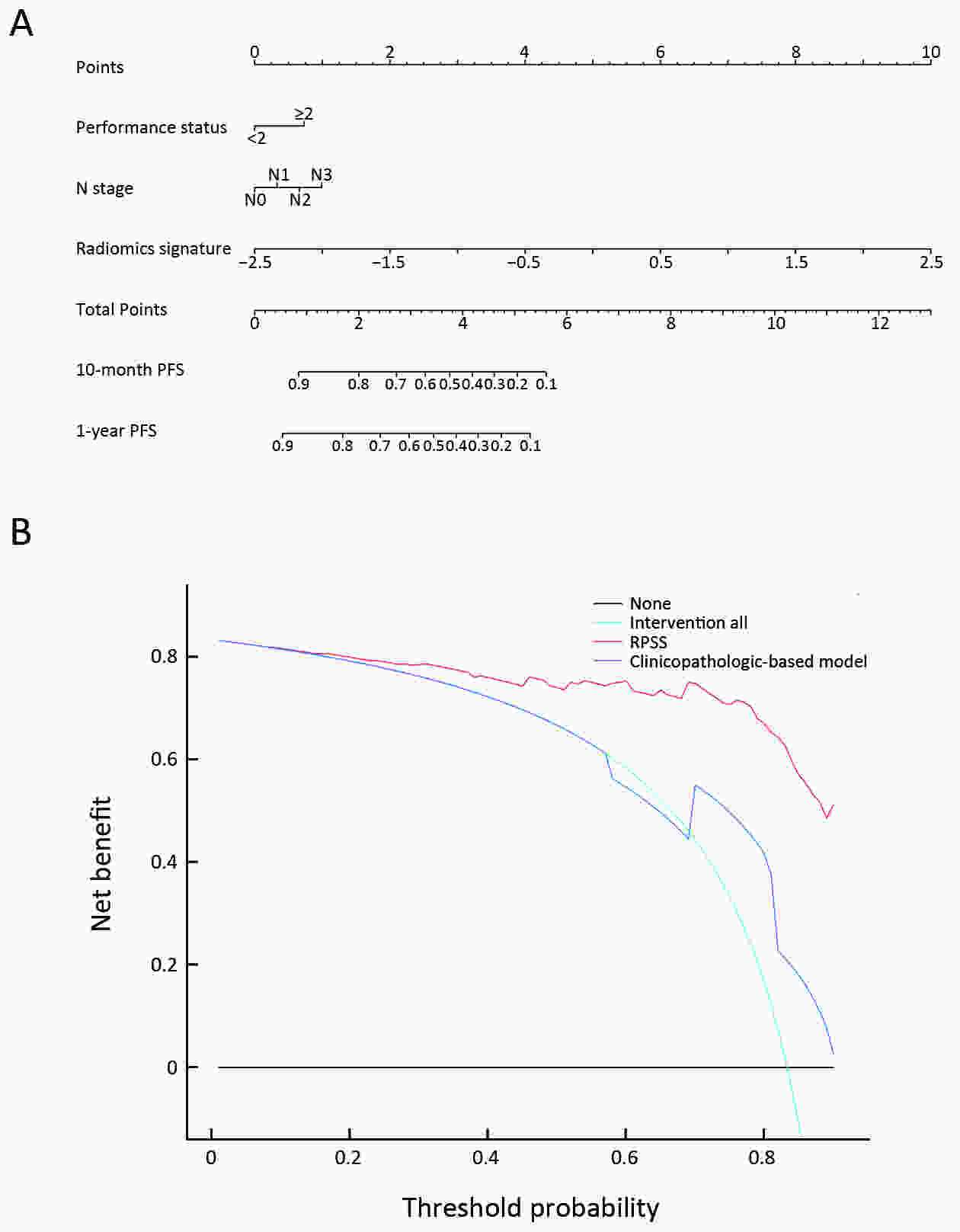
ObjectiveTo develop and validate a radiomics prognostic scoring system (RPSS) for prediction of progression-free survival (PFS) in patients with stage IV non-small cell lung cancer (NSCLC) treated with platinum-based chemotherapy. MethodsIn this retrospective study, four independent cohorts of stage IV NSCLC patients treated with platinum-based chemotherapy were included for model construction and validation (Discovery: n=159; Internal validation: n=156; External validation: n=81, Mutation validation: n=64). First, a total of 1,182 three-dimensional radiomics features were extracted from pre-treatment computed tomography (CT) images of each patient. Then, a radiomics signature was constructed using the least absolute shrinkage and selection operator method (LASSO) penalized Cox regression analysis. Finally, an individualized prognostic scoring system incorporating radiomics signature and clinicopathologic risk factors was proposed for PFS prediction. ResultsThe established radiomics signature consisting of 16 features showed good discrimination for classifying patients with high-risk and low-risk progression to chemotherapy in all cohorts (All P<0.05). On the multivariable analysis, independent factors for PFS were radiomics signature, performance status (PS), and N stage, which were all selected into construction of RPSS. The RPSS showed significant prognostic performance for predicting PFS in discovery [C-index: 0.772, 95% confidence interval (95% CI): 0.765−0.779], internal validation (C-index: 0.738, 95% CI: 0.730−0.746), external validation (C-index: 0.750, 95% CI: 0.734−0.765), and mutation validation (C-index: 0.739, 95% CI: 0.720−0.758). Decision curve analysis revealed that RPSS significantly outperformed the clinicopathologic-based model in terms of clinical usefulness (All P<0.05). ConclusionsThis study established a radiomics prognostic scoring system as RPSS that can be conveniently used to achieve individualized prediction of PFS probability for stage IV NSCLC patients treated with platinum-based chemotherapy, which holds promise for guiding personalized pre-therapy of stage IV NSCLC.
2021, 33(5): 606-615.
doi: 10.21147/j.issn.1000-9604.2021.05.07
Abstract:
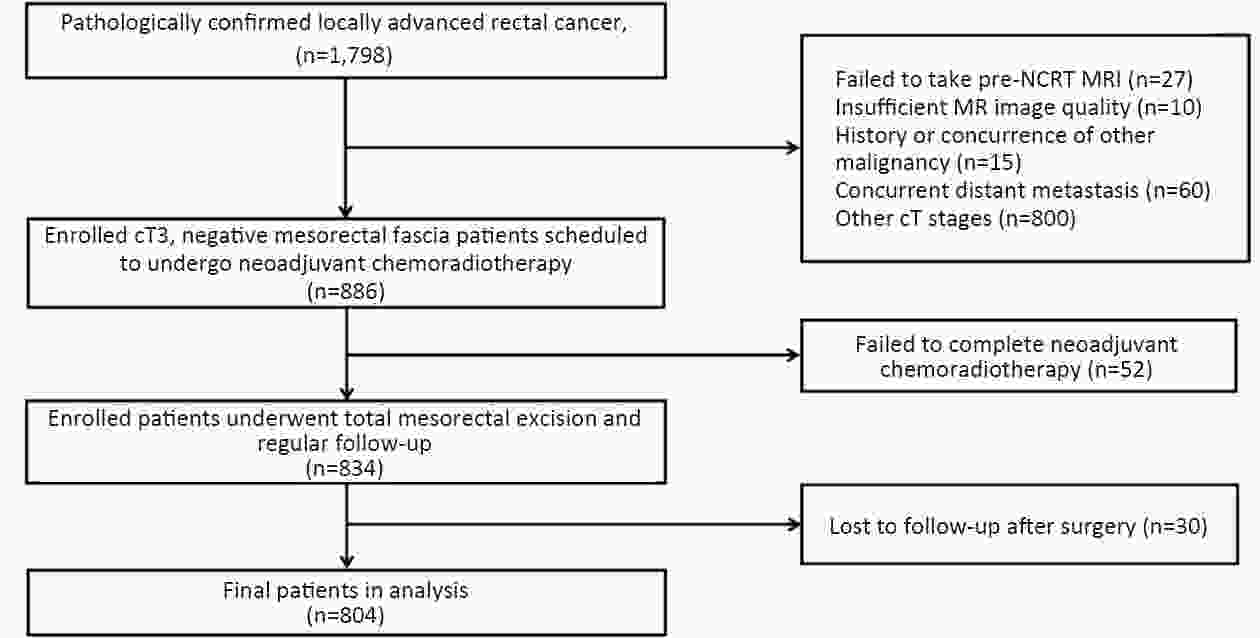
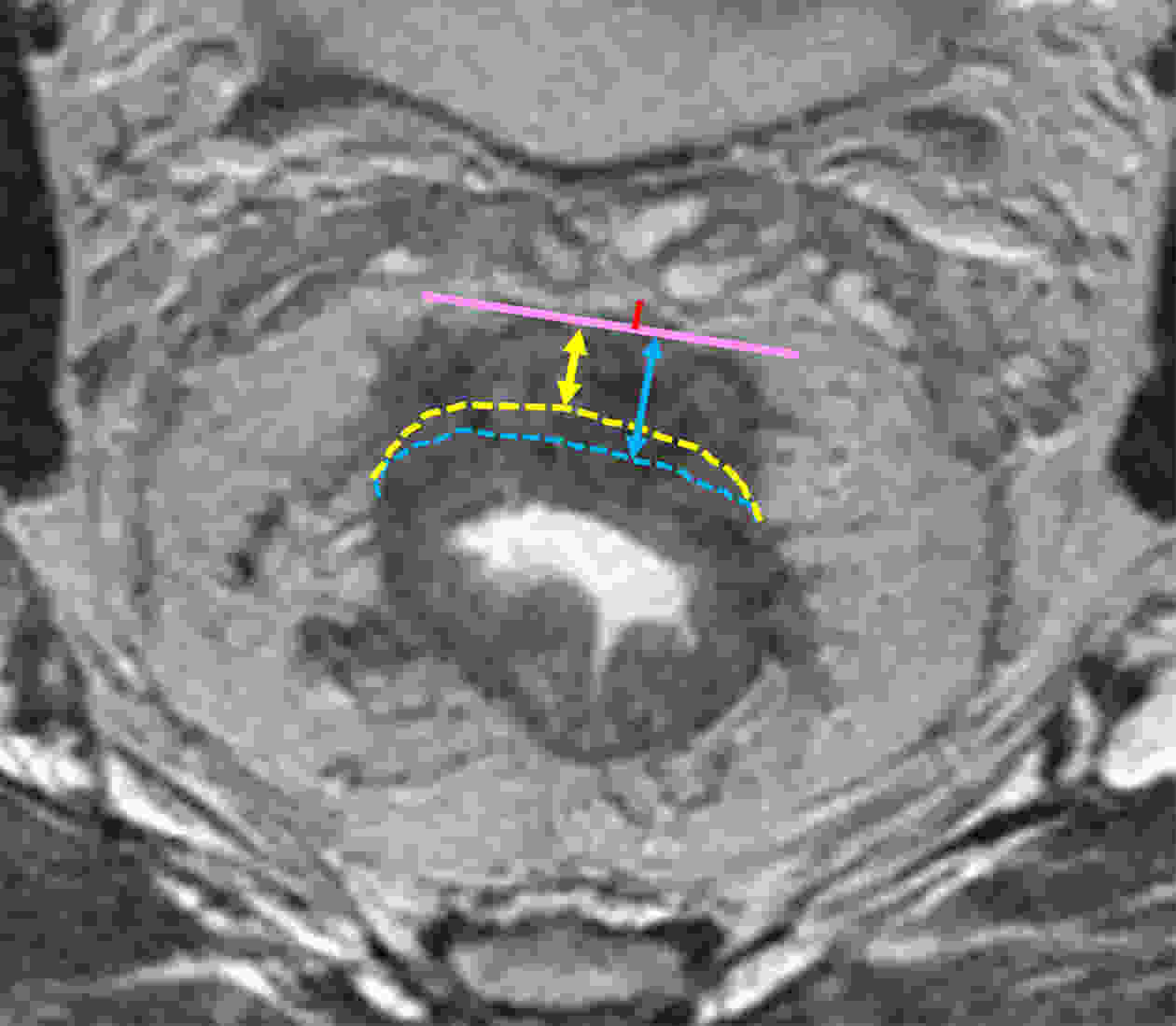
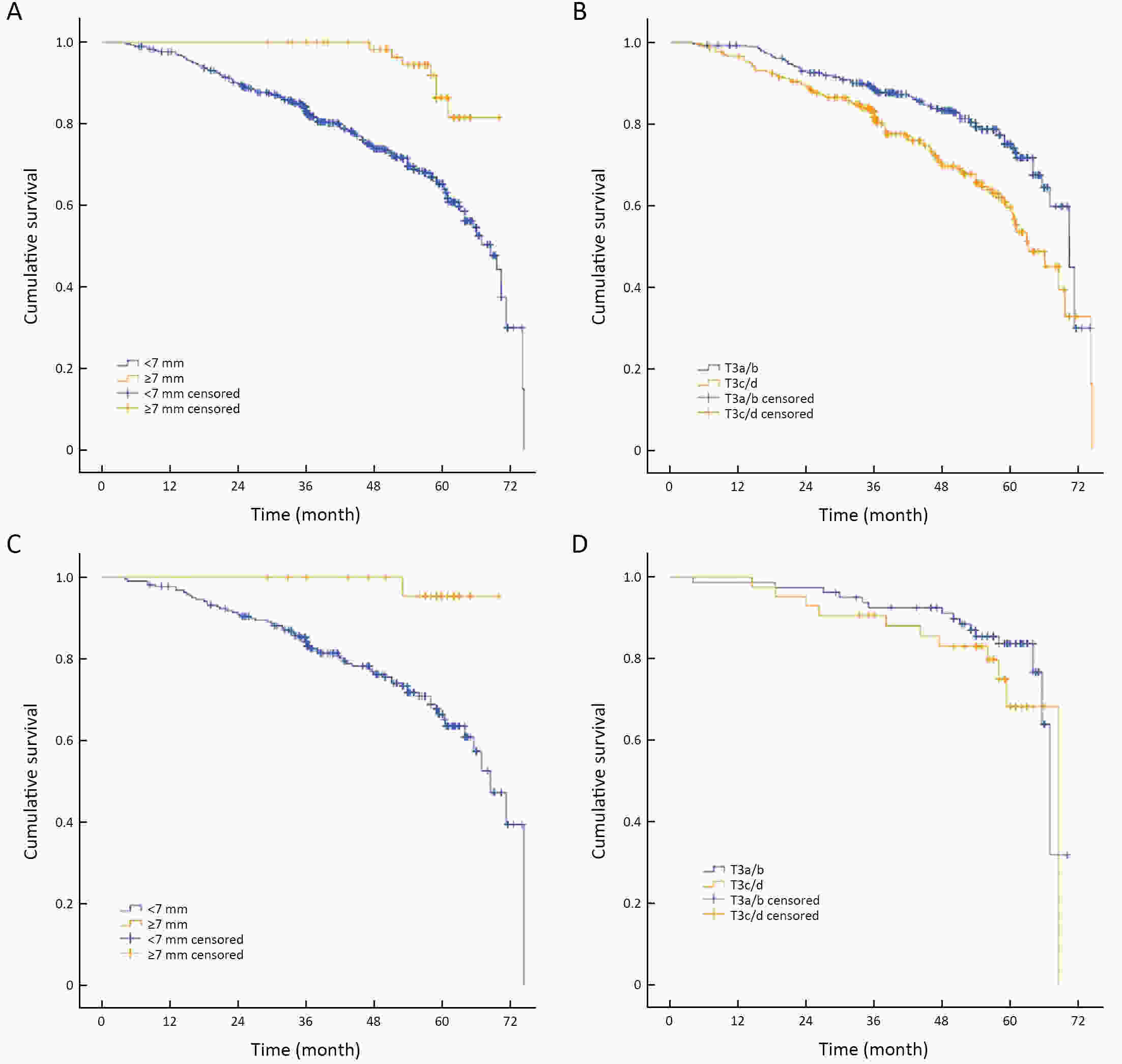
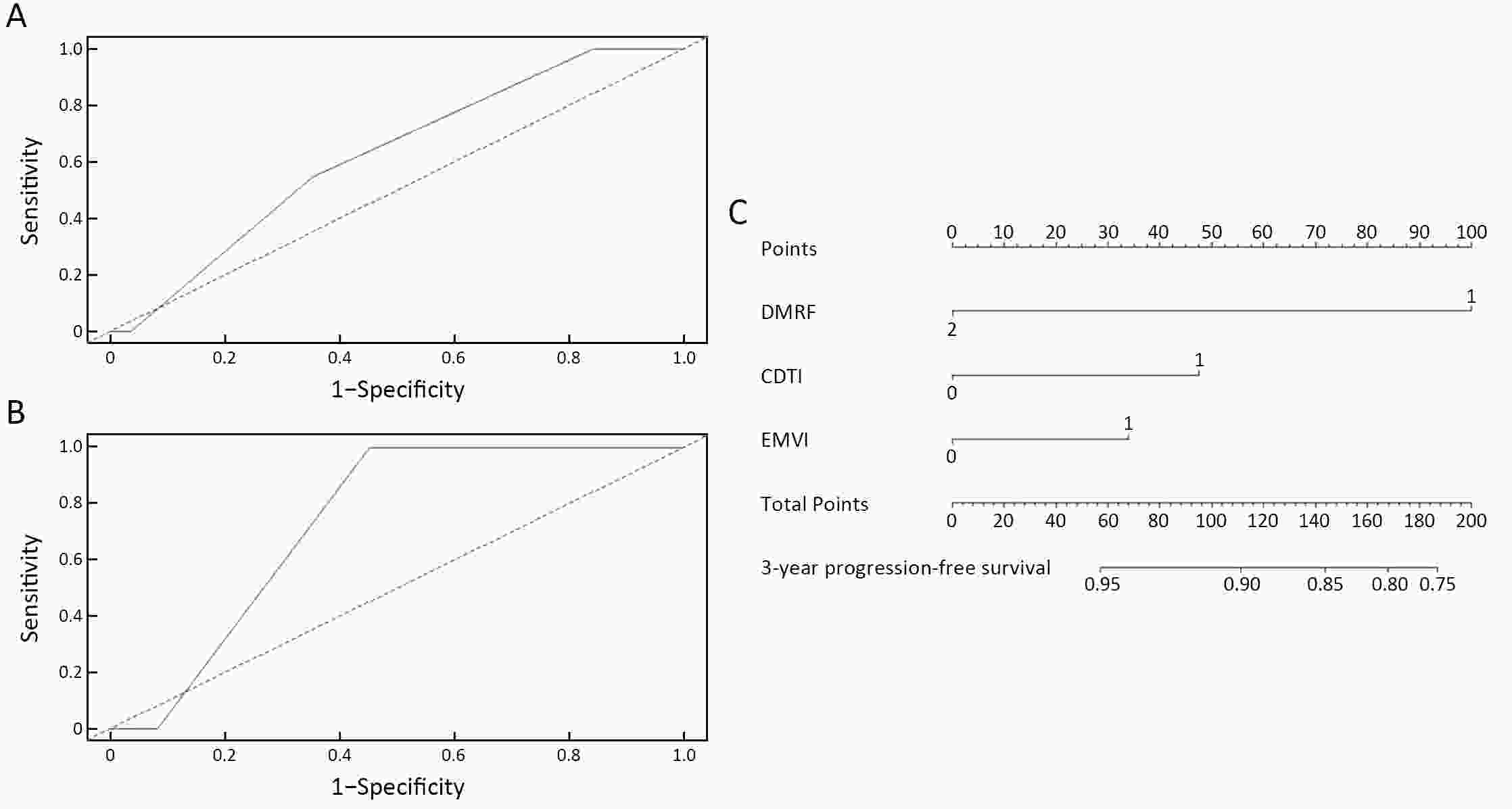
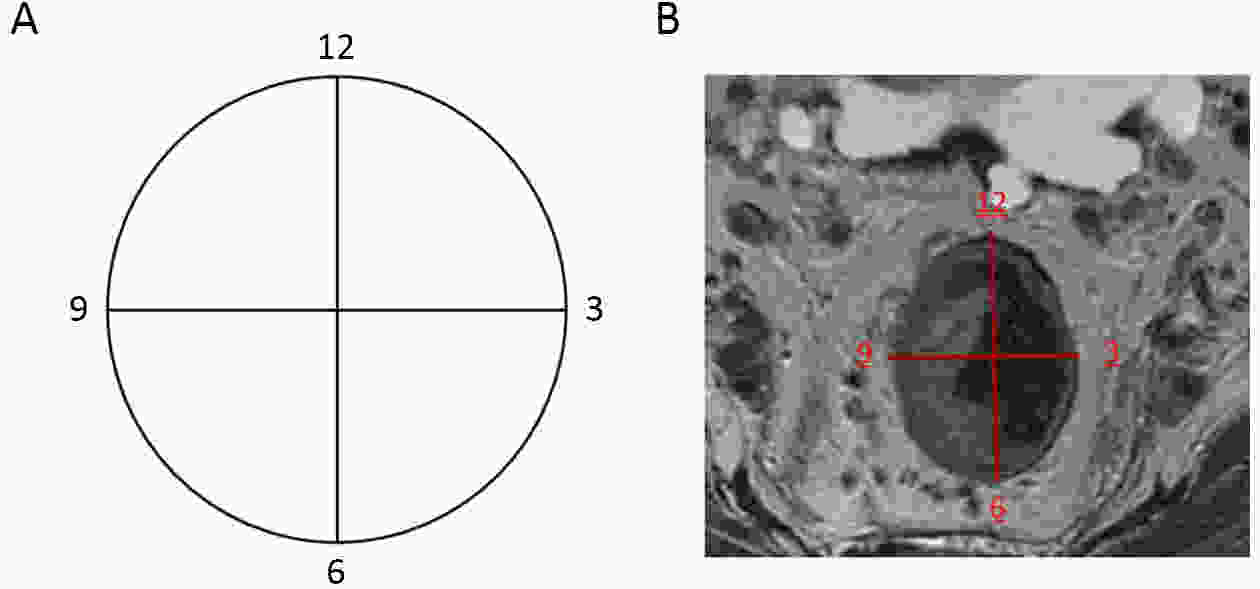
ObjectiveTo forward the magnetic resonance imaging (MRI) based distance between the deepest tumor invasion and mesorectal fascia (DMRF), and to explore its prognosis differentiation value in cT3 stage rectal cancer with comparison of cT3 substage. MethodsThis was a retrospective, multicenter cohort study including cT3 rectal cancer patients undergoing neoadjuvant chemoradiotherapy followed by radical surgery from January 2013 to September 2014. DMRF and cT3 substage were evaluated from baseline MRI. The cutoff of DMRF was determined by disease progression. Multivariate cox regression was used to test the prognostic values of baseline variables. ResultsA total of 804 patients were included, of which 226 (28.1%) developed progression. A DMRF cutoff of 7 mm was chosen. DMRF category, the clock position of the deepest position of tumor invasion (CDTI) and extramural venous invasion (EMVI) were independent predictors for disease progression, and hazard ratios (HRs) were 0.26 [95% confidence interval (95% CI), 0.13−0.56], 1.88 (95% CI, 1.33−2.65) and 1.57 (95% CI, 1.13−2.18), respectively. cT3 substage was not a predictor for disease progression. ConclusionsThe measurement of DMRF value on baseline MRI can better distinguish cT3 rectal cancer prognosis rather than cT3 substage, and was recommended in clinical evaluation.
2021, 33(5): 616-626.
doi: 10.21147/j.issn.1000-9604.2021.05.08
Abstract:
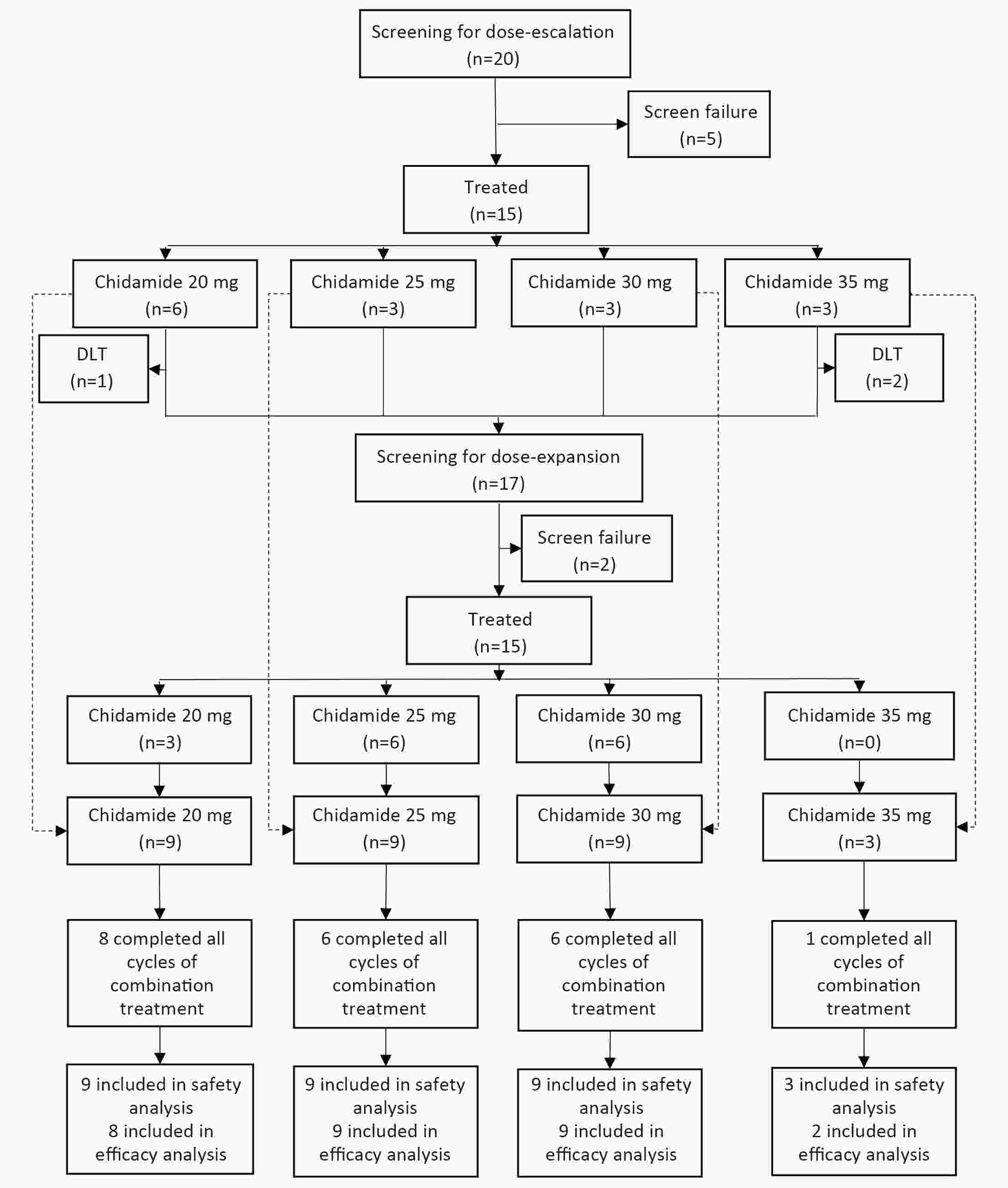
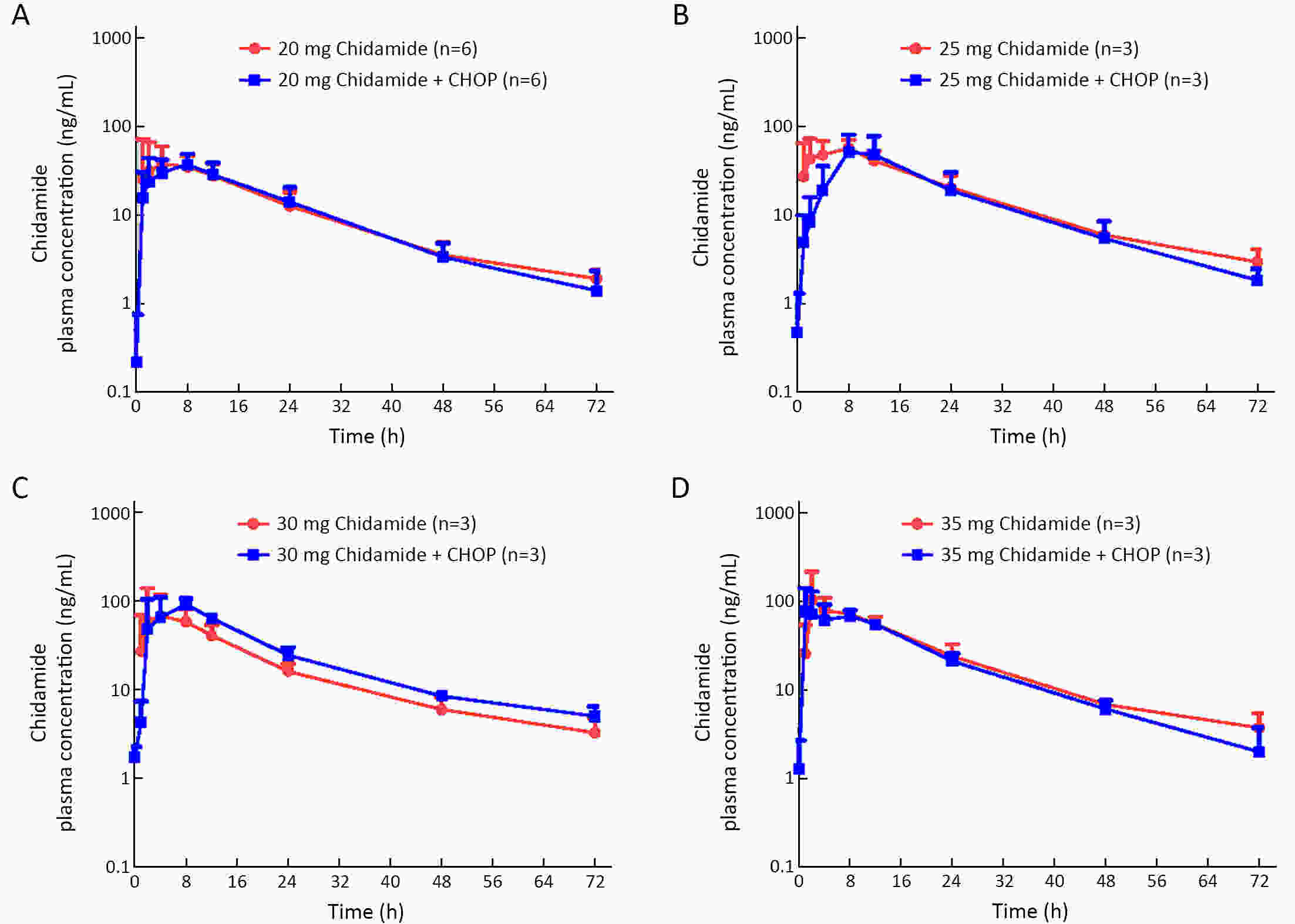
ObjectiveChidamide is an oral histone deacetylase subtype-selective inhibitor approved for relapsed or refractory peripheral T-cell lymphoma (PTCL). This phase 1b study evaluated the safety, pharmacokinetics, and preliminary efficacy of chidamide in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) for treatment-naïve PTCL patients. MethodsThis study was an open-label, multicenter trial composed of dose escalation and dose expansion. Patients received CHOP for six 21-d cycles and chidamide on d 1, 4, 8 and 11 in each cycle. Four dose levels of chidamide (20, 25, 30 and 35 mg) were evaluated. The primary objective was to evaluate the safety and tolerability of the combination regimen. ResultsA total of 30 patients were evaluated in this study: 15 in the dose-escalation part and 15 in the dose-expansion part. In the dose-escalation study, three patients were enrolled in the 35 mg chidamide cohort. One had dose-limiting toxicity with grade 3 vascular access complications, and one had grade 2 neutropenia with a sustained temperature >38 °C. Dose escalation was stopped at this chidamide dose level. The most common (≥10%) grade 3 or 4 adverse events (AEs) were leukopenia (90.0%), neutropenia (83.3%), vomiting (13.3%), thrombocytopenia (10.0%) and febrile neutropenia (10.0%). No significant changes in chidamide pharmacokinetic properties were observed before and after combination treatment. The objective response rate for the 28 patients evaluable for preliminary efficacy was 89.3% (25/28), with 16 (57.1%) achieving complete response or unconfirmed complete response. The estimated median progression-free survival was 14.0 months. In summary, we chose chidamide 30 mg as the recommended dose for phase 2. ConclusionsThe addition of chidamide to standard CHOP chemotherapy was tolerable with promising preliminary efficacy in previously untreated PTCL patients, which supports further clinical studies with this combination regimen for the frontline treatment of PTCL.
2021, 33(5): 627-636.
doi: 10.21147/j.issn.1000-9604.2021.05.09
Abstract:
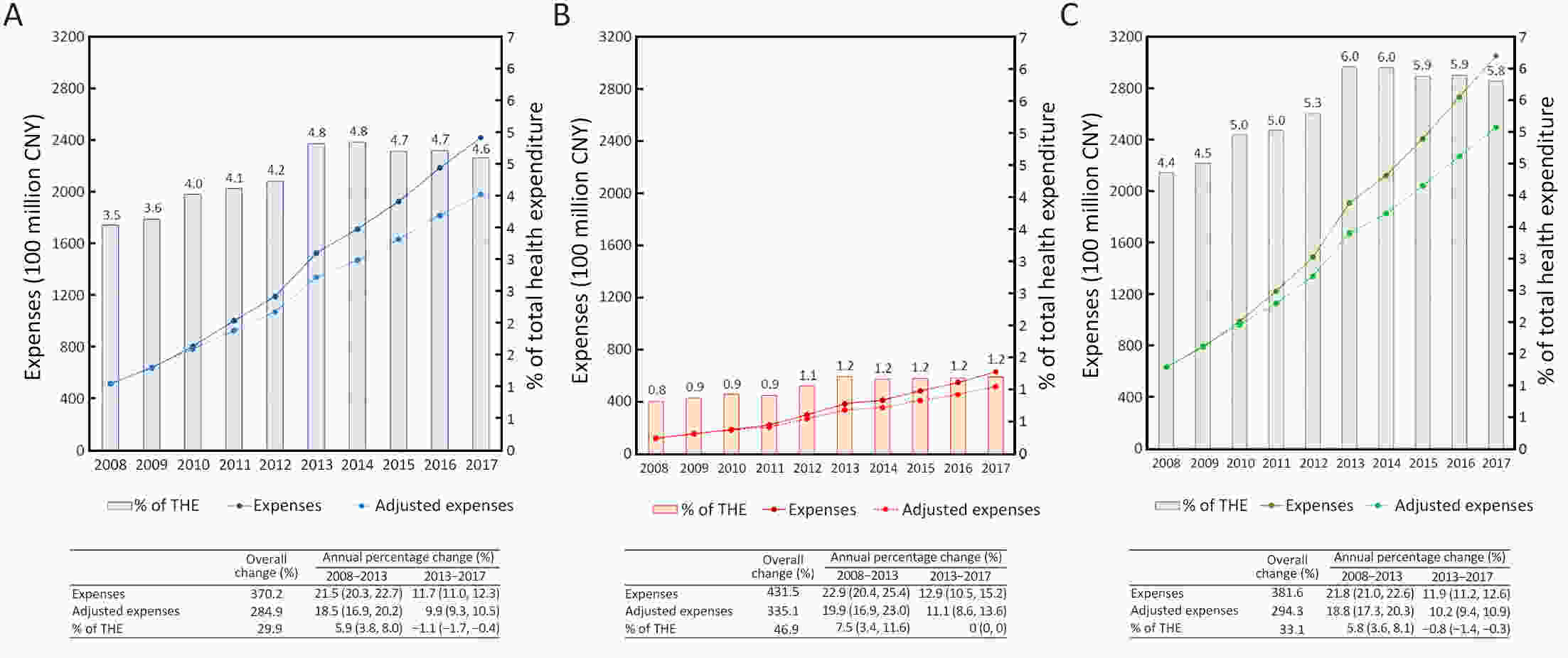
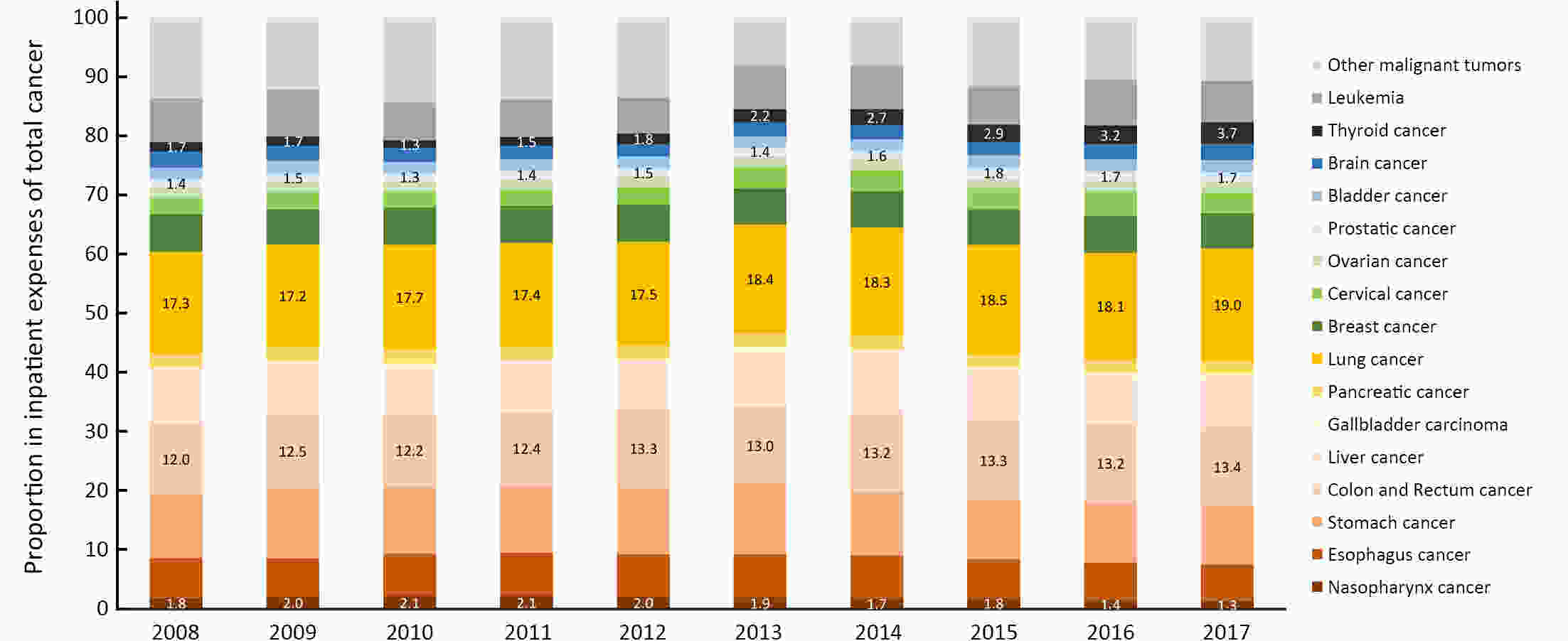
ObjectiveTo describe the contemporary trends in total, inpatient, and outpatient expenditure on major subtypes of cancer in different classifications of hospitals in mainland China. MethodsHome page of Inpatient Medical Records (HIMRs) and Hospital Annual Reports (HARs) were used to estimate hospital care expenditure on cancer. Inpatient payments and their share of cancer were calculated with the top-down method. Kriging spatial interpolation methods were used at the county level and summed at the province level. Outpatient expenditure was estimated with inpatient expenditure and the ratios of outpatient to inpatient payments in specialized cancer hospitals, stratified by province. Total expenditure on cancer was the sum of both payments. Log-linear regression was applied to estimate annual percentage change (APC) of expenditure. ResultsTotal expenses for cancer of Chinese residents reached up to 304.84 billion Chinese Yuan (CNY) in 2017, accounting for 5.8% of the total health expenses (THE). After adjusting for consumer price index (CPI), medical expenses for cancer have increased from 63.30 billion CNY in 2008 to 249.56 billion CNY in 2017 [APC: 15.2%, 95% confidence interval (95% CI): 13.4%−17.0%]. The APC was slightly higher than THE around 2013, while was lower after 2013. During 2008−2017, the ratio of inpatient to outpatient costs for cancer decreased from 4.3:1 to 3.8:1. The inpatient payments for cancer mainly happened in grade 3 general hospitals, East China, and among lung, colorectal, and stomach cancer; while the fastest increase was found in West China, and among thyroid, prostate, and colorectal cancer. ConclusionsDuring 2008−2017, the rapid growth trend of medical expenses for cancer has been effectively controlled with the continuous deepening of medical reform and improvements of residents’ health care. More attention should be paid to potential increases of medical costs caused by technological progress and demand release. Socialized and multi-channel insurance financing modes should be explored in the future.

 Abstract
Abstract FullText HTML
FullText HTML PDF 1280KB
PDF 1280KB