2021 Vol.33(6)
Display Mode: |
2021, 33(6): 637-648.
doi: 10.21147/j.issn.1000-9604.2021.06.01
Abstract:
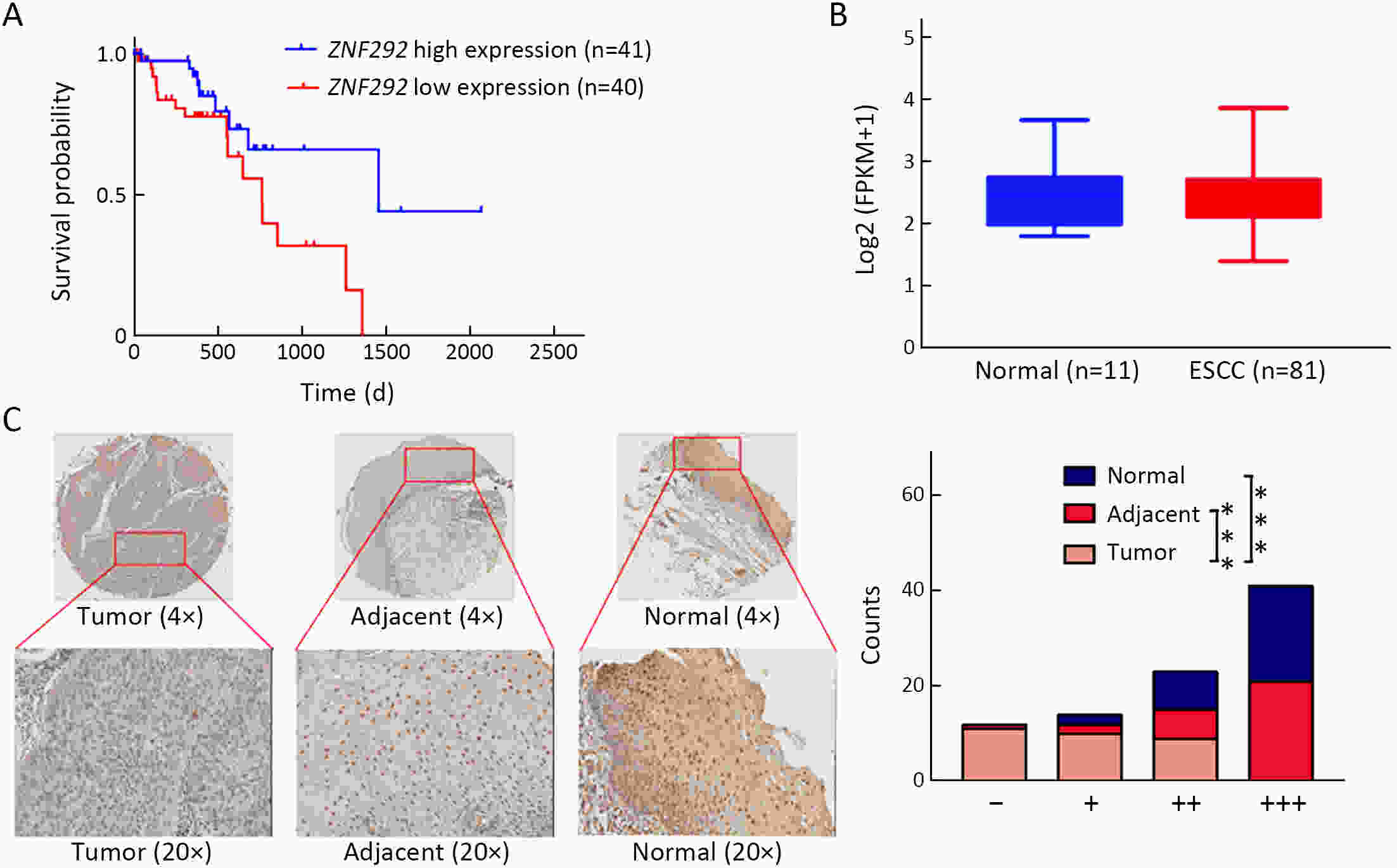
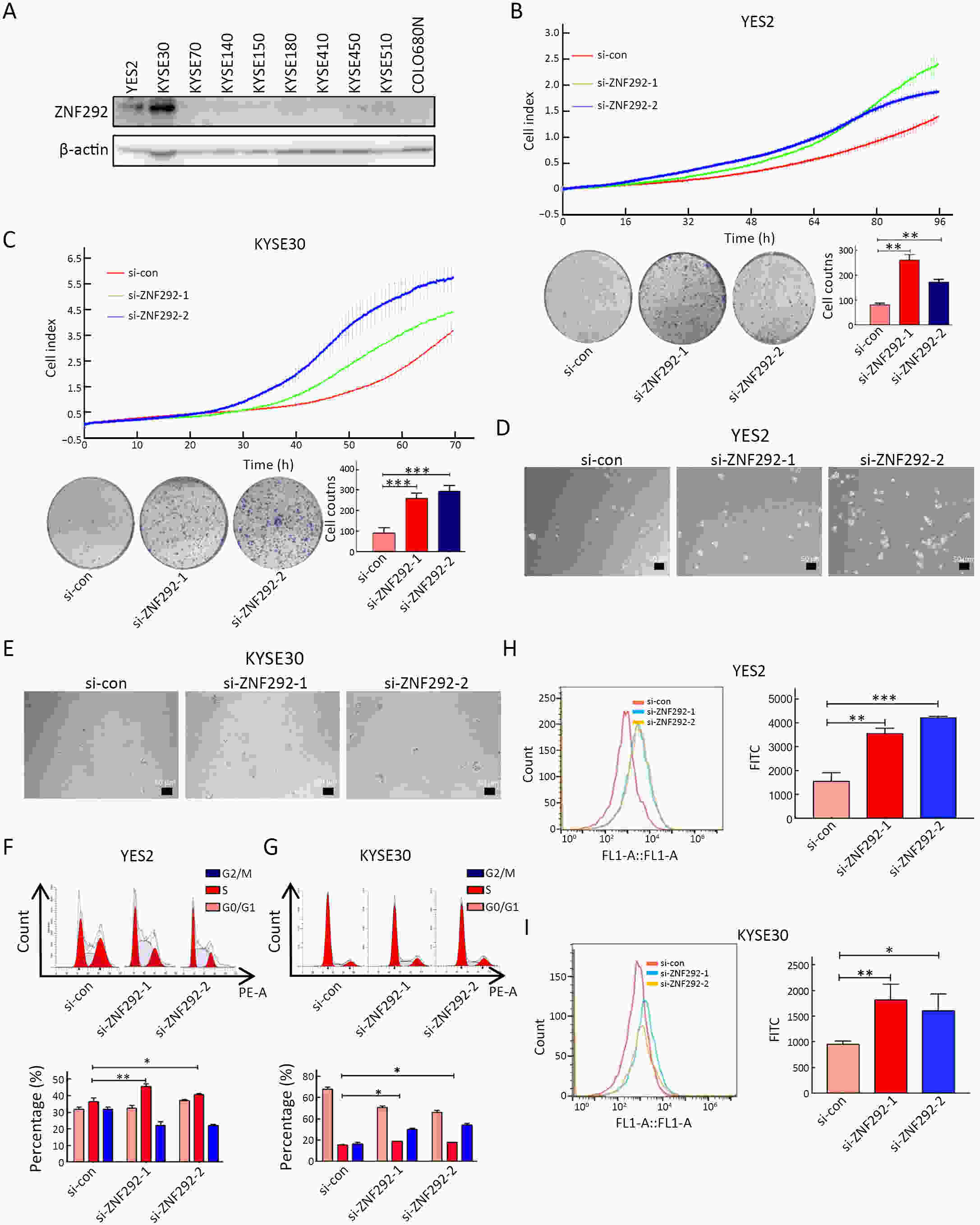
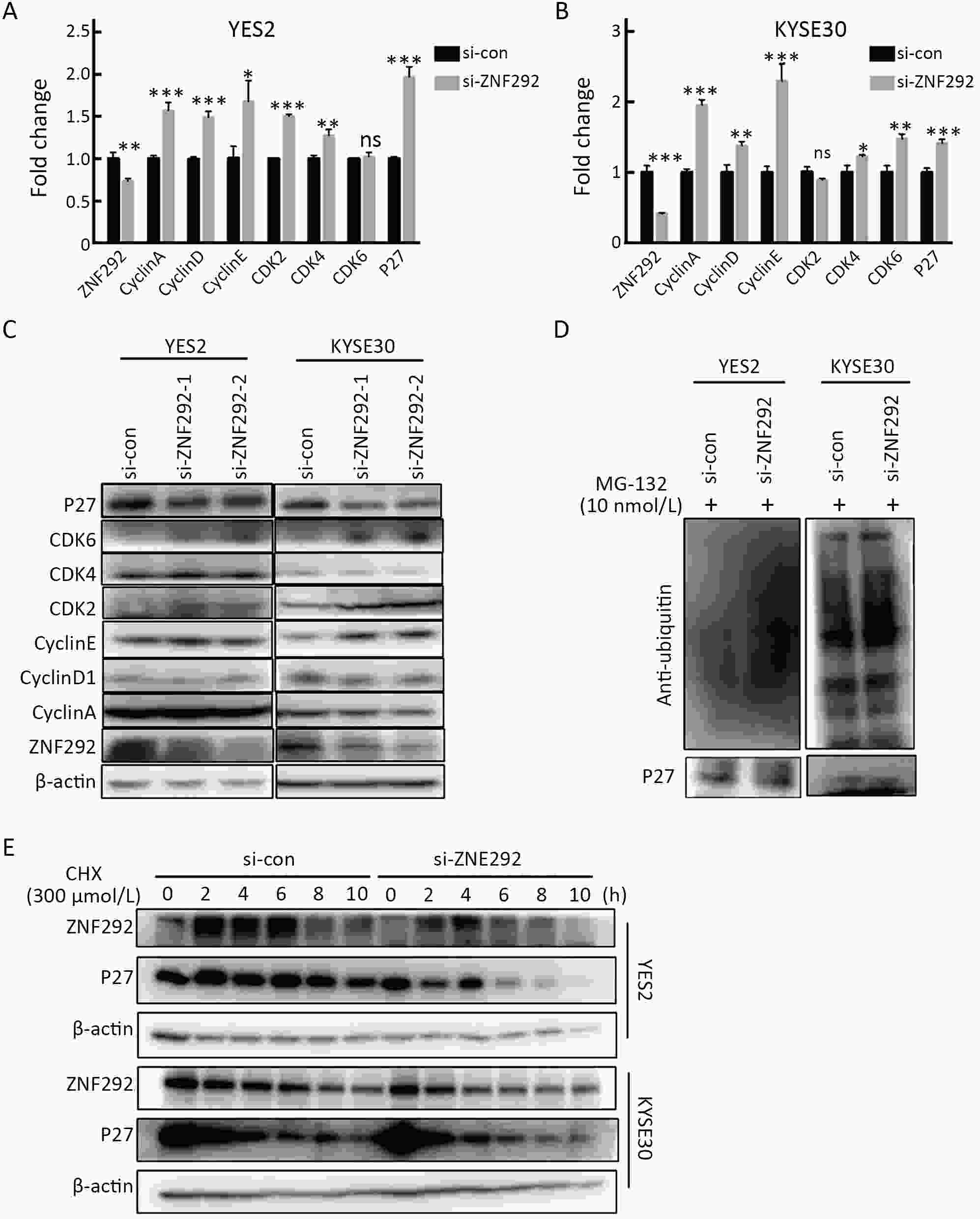
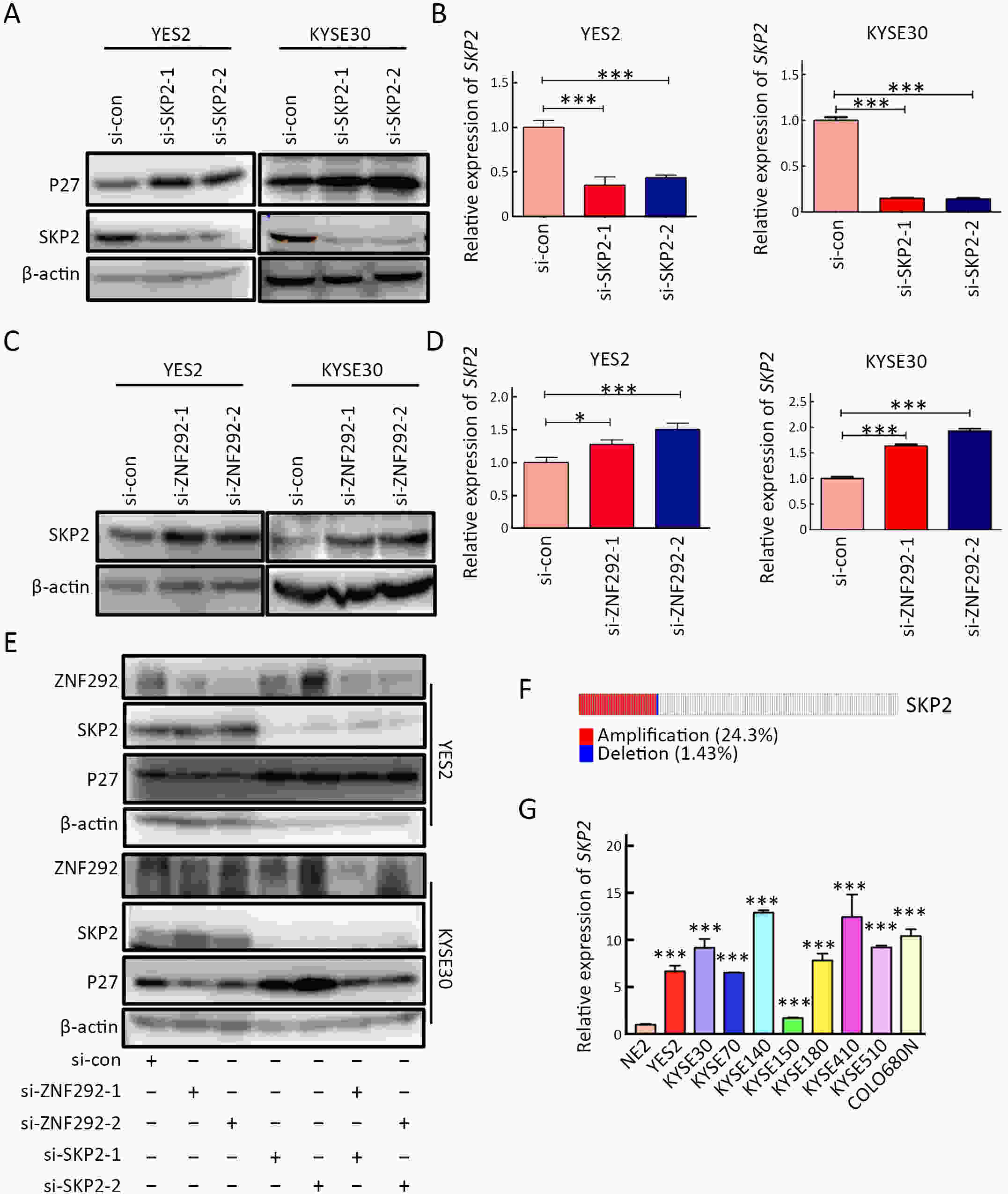
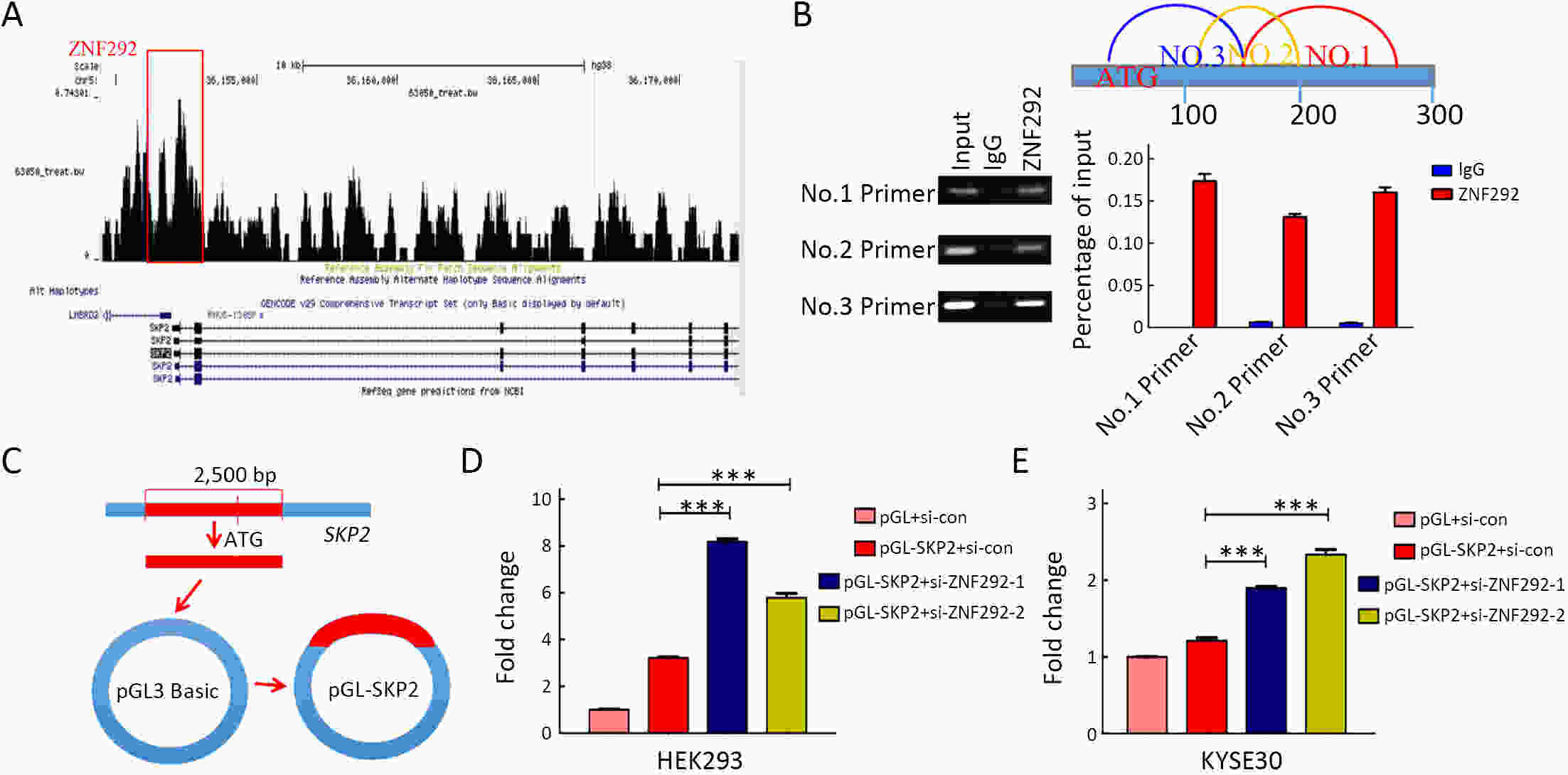
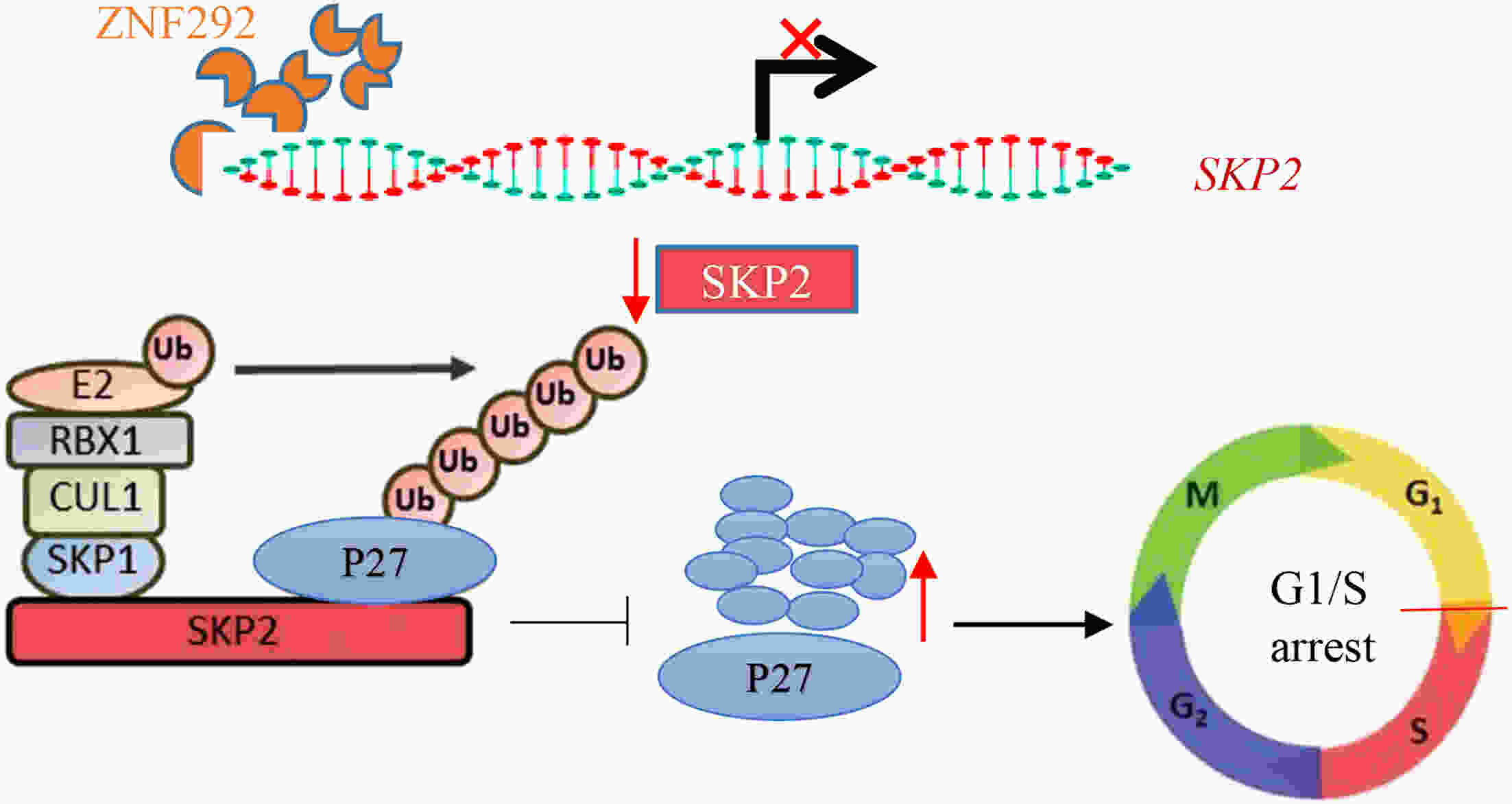
ObjectiveIncreasing evidence has demonstrated that ZNF292 plays a suppressive role in cancer, however, little is known about its function and exact mechanism in esophageal squamous cell carcinoma (ESCC). MethodsBioinformatic analysis and immunohistochemistry (IHC) were performed to analyze the role of ZNF292 in affecting the prognosis of ESCC. Cell proliferation and colony formation ability assays were performed to analyze cell growth after inferring the expression of ZNF292. Flow cytometry was used to analyze changes in the cell cycle upon the depletion of ZNF292. Quantitative real-time polymerase chain reaction (qRT-PCR) and western blot analysis were used to determine the alteration of cell cycle related RNAs and proteins after knocking down ZNF292. MG-132, cycloheximide (CHX) treatment experiments were performed to analyze the change and half-life time of P27 after knockdown of ZNF292. Chromatin immunoprecipitation (ChIP) and luciferase reporter assays were used to analyze the transcriptional regulation of SKP2 by ZNF292. ResultsWe report that low expression of ZNF292 is associated with poor prognosis, and ZNF292 emerges to be highly expressed in adjacent and normal tissues rather than tumor tissues in ESCC. Knockdown of ZNF292 significantly boosts cell growth and S phase entry in ESCC cells. ZNF292 depletion will decrease the expression and half-life time of P27, while knockdown of SKP2 will result in elevated expression of P27. ZNF292 can bind to the promoter region of SKP2, and knockdown of ZNF292 will boost the expression of SKP2. ConclusionsKnockdown of ZNF292 mediates G1/S cell cycle procession by activating SKP2/P27 signaling in ESCC cells. ZNF292 knockdown promotes SKP2 expression at the transcriptional level, thereby boosting P27 ubiquitin-degradation, and eventually facilitating the S phase entrance.
2021, 33(6): 649-658.
doi: 10.21147/j.issn.1000-9604.2021.06.02
Abstract:
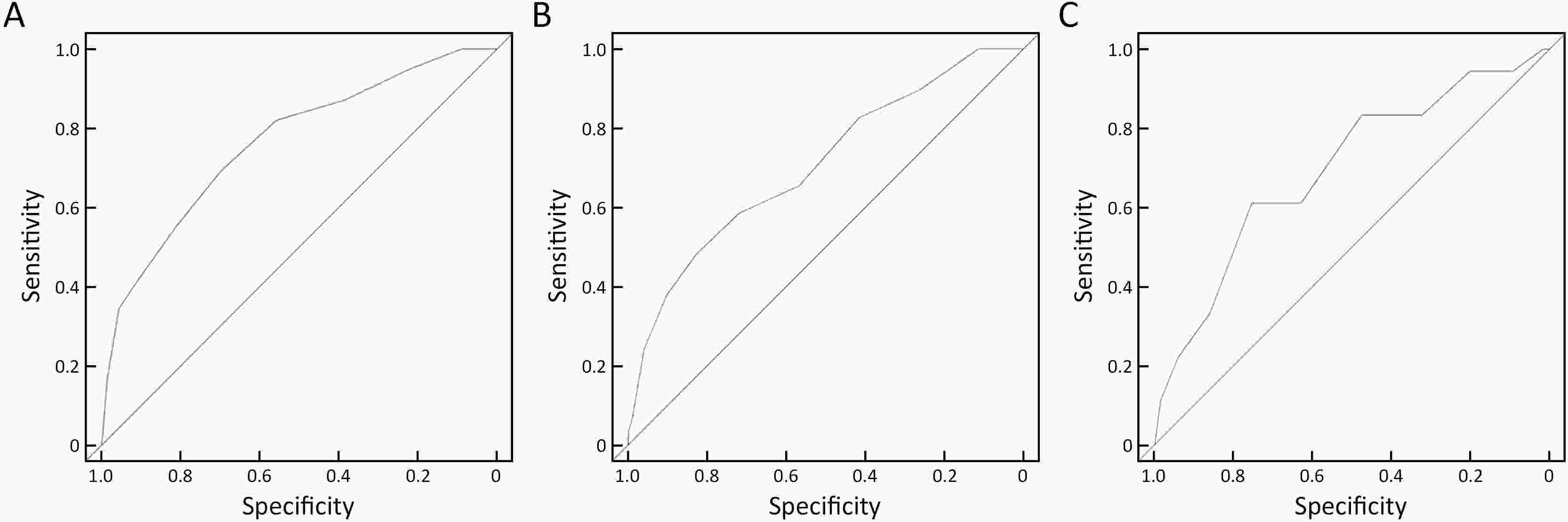
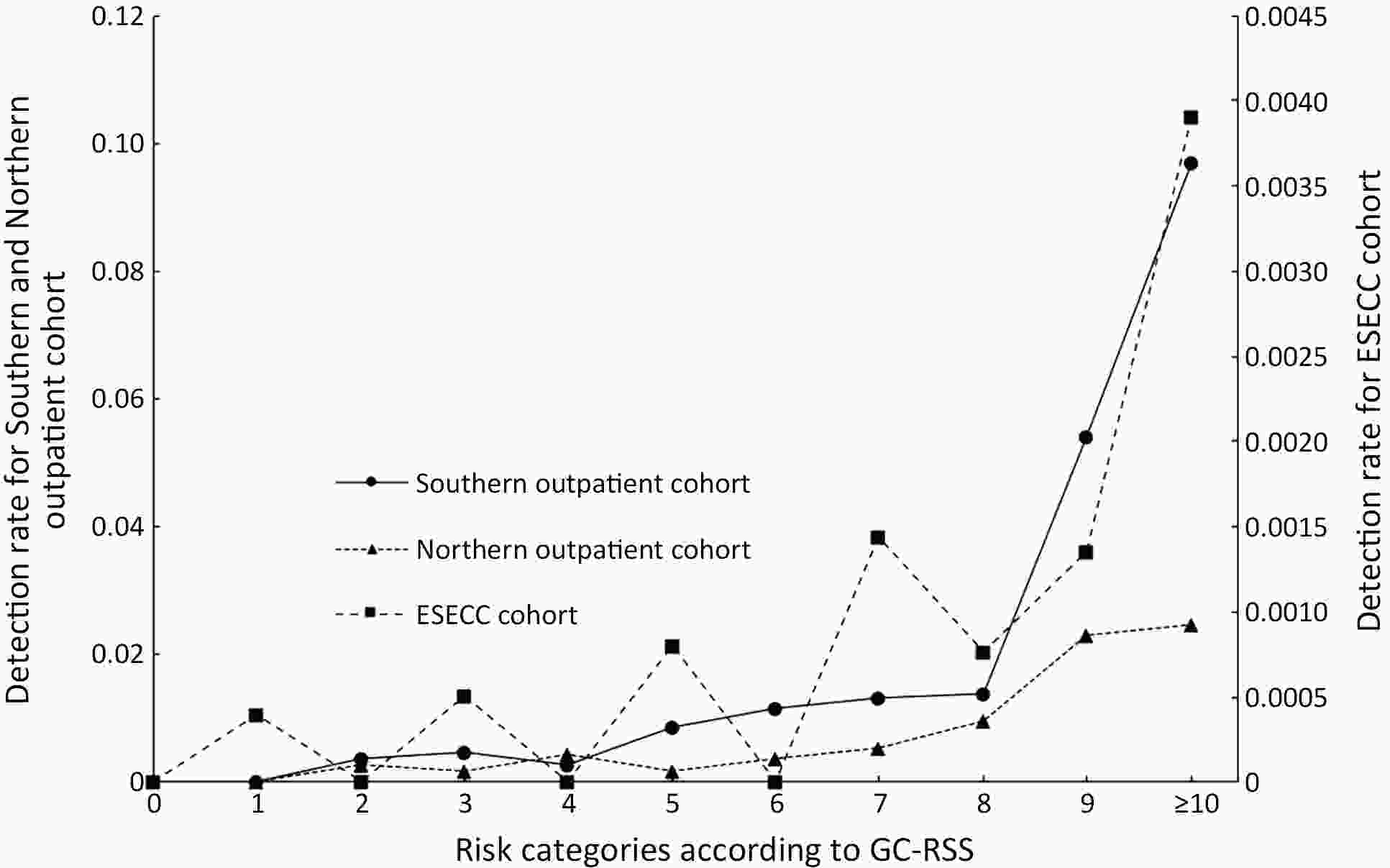
ObjectiveThis study aimed to develop and validate a risk scoring system to identify high-risk individuals carrying malignant lesions in stomach for tailored gastric cancer screening. MethodsA gastric cancer risk scoring system (GC-RSS) was developed based on questionnaire-based predictors for gastric cancer derived from systematic literature review. To assess the capability of this system for discrimination, risk scores for 8,214 and 7,235 outpatient subjects accepting endoscopic examination in two endoscopy centers, and 32,630 participants in a community-based cohort in China were calculated to plot receiver operating characteristic curves and generate area under the curve (AUC). To evaluate the performance of GC-RSS, the screening proportion, sensitivity and detection rate ratio compared to universal screening were used under different risk score cutoff values. ResultsGC-RSS comprised nine predictors including advanced age, male gender, low body mass index (<18.5 kg/m2), family history of gastric cancer, cigarette smoking, consumption of alcohol, preference for salty food, irregularity of meals and consumption of preserved food. This tool performed well in determining the risk of malignant gastric lesions with AUCs of 0.763, 0.706 and 0.696 in three validation sets. When subjects with risk scores ≥5 were evaluated with endoscopy, nearly 50% of these endoscopies could be saved with a detection rate of over 1.5 times achieved. When the cutoff was set at 8, only about 10% of subjects with the highest risk would be offered endoscopy, and detection rates for gastric cancer could be increased 2−4 fold compared to universal screening. ConclusionsAn effective questionnaire-based GC-RSS was developed and validated. This tool may play an important role in establishing a tailored screening strategy for gastric cancer in China.
2021, 33(6): 659-670.
doi: 10.21147/j.issn.1000-9604.2021.06.03
Abstract:
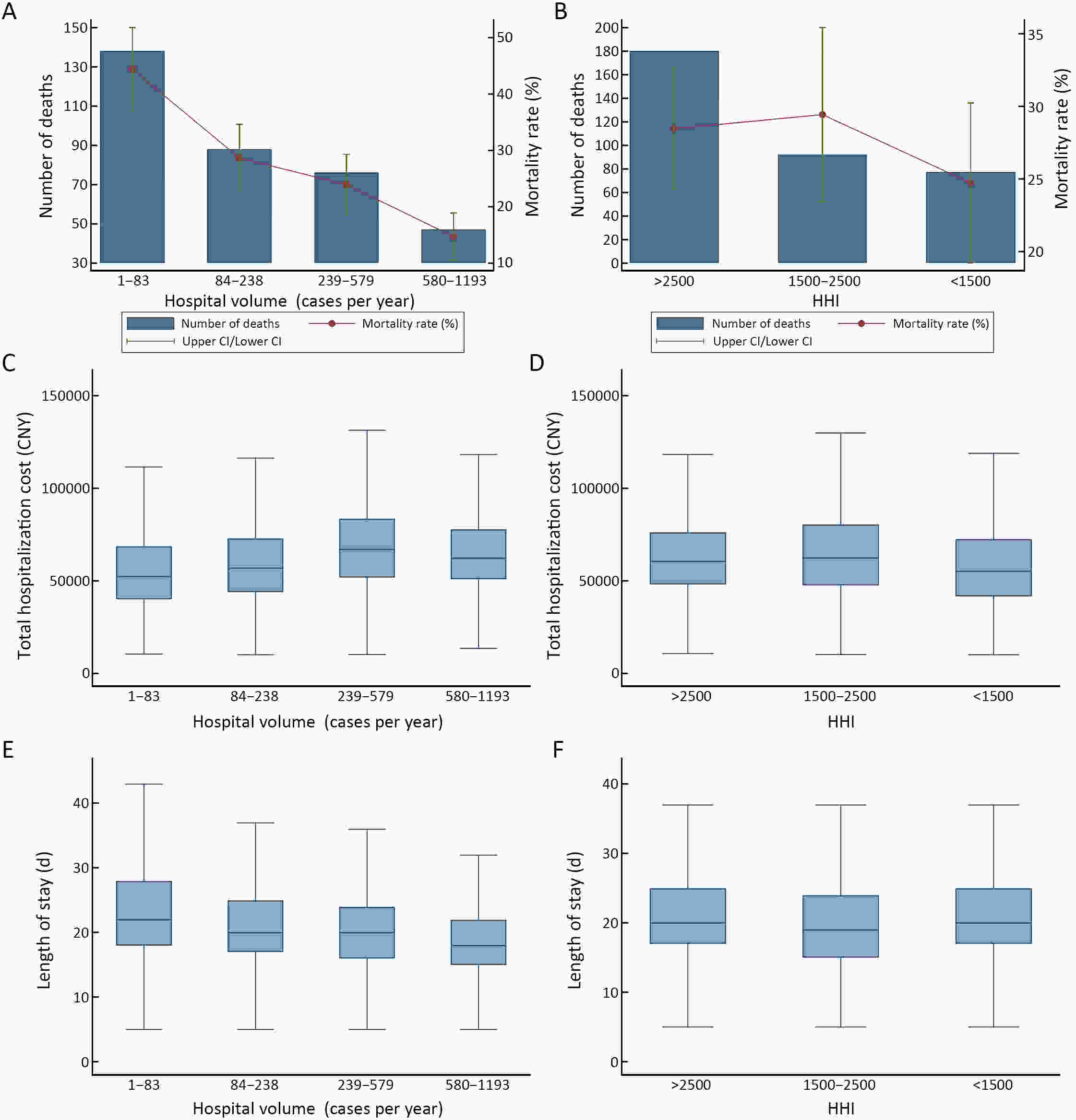
ObjectiveLimited evidence is available regarding the associations of centralization with gastric cancer patients’ quality of care in high surgical volume settings. The current study aimed to explore the effects of hospital volume and the Herfindahl-Hirschman index (HHI) on in-hospital mortality, total cost, and length of stay for Chinese gastrectomy patients in a nationwide database. MethodsWe extracted data on gastrectomy for gastric cancer from the Hospital Quality Monitoring System Database between 2013 and 2018. Hospital volume was divided into 4 quartiles: low (1−83 cases per year), medium (84−238 cases), high (239−579 cases), and very high (580−1,193 cases). The HHI was divided into 3 categories: highly concentrated (>2,500), moderately concentrated (1,500−2,500), and unconcentrated (<1,500). We used mixed-effects models to analyze the data while accounting for data clustering. ResultsWe analyzed 125,683 patients in 515 institutions. In the multivariable analyses, hospital volume was significantly associated with in-hospital mortality [medium vs. low: odds ratio (OR)=0.61, 95% confidence interval (95% CI)=0.43−0.84, P=0.003; high: OR=0.57, 95% CI=0.38−0.87, P=0.009; and very high: OR=0.33, 95% CI=0.18−0.61, P<0.001) and length of stay (high vs. low: β=−0.036, 95% CI=−0.071−−0.002, P=0.039) but not with total cost. Hospitals located in unconcentrated provinces had higher in-hospital mortality (OR=1.52, 95% CI=1.03−2.26, P=0.036) and longer lengths of stay (β=0.024, 95% CI=0.001−0.047, P=0.041) than hospitals located in highly concentrated provinces. ConclusionsCentralization of gastrectomy, measured by hospital volume and the HHI, was associated with decreased in-hospital mortality and shortened length of stay without increasing total cost. These results support the strategy of centralizing gastrectomy in high-volume settings.
2021, 33(6): 671-681.
doi: 10.21147/j.issn.1000-9604.2021.06.04
Abstract:
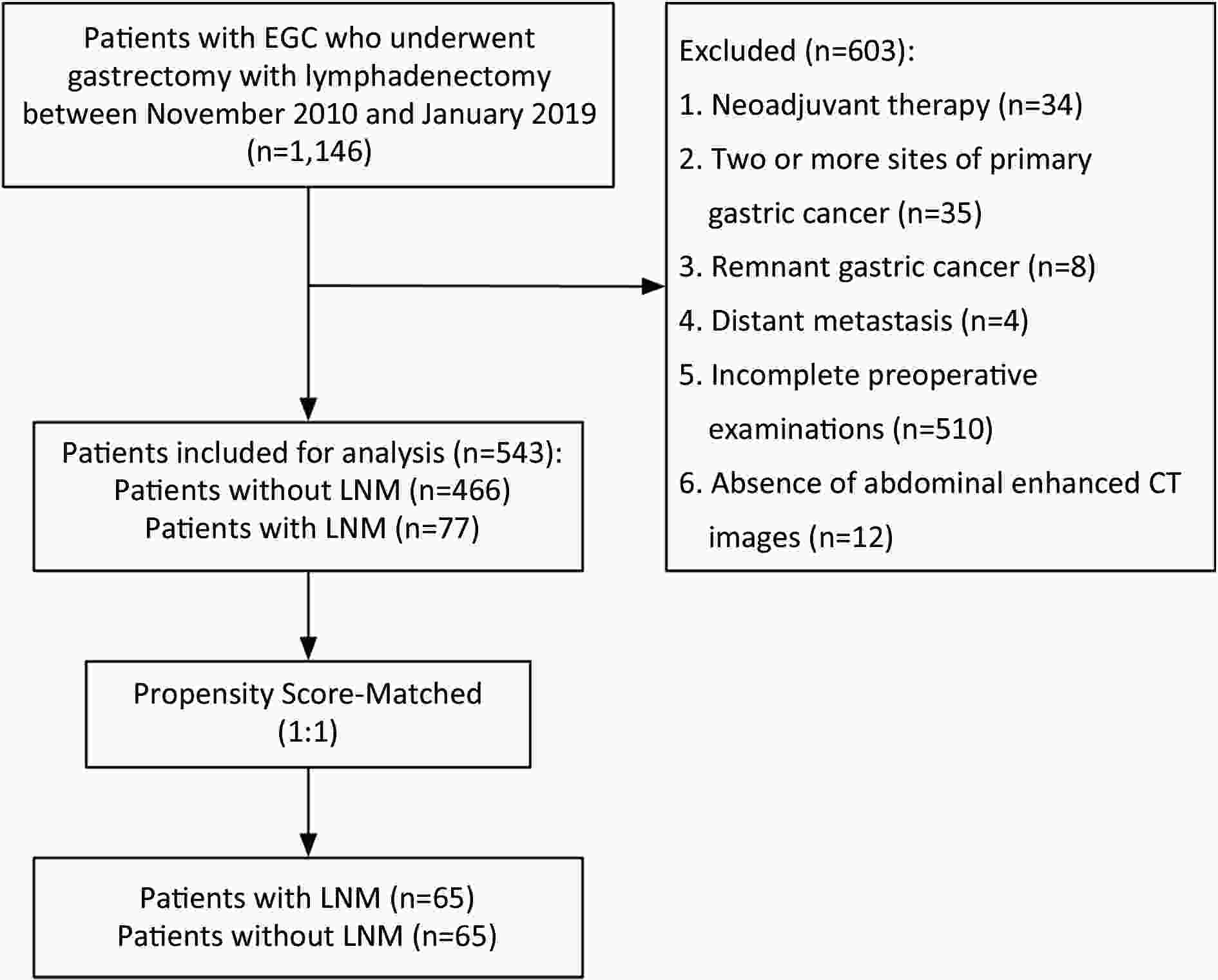
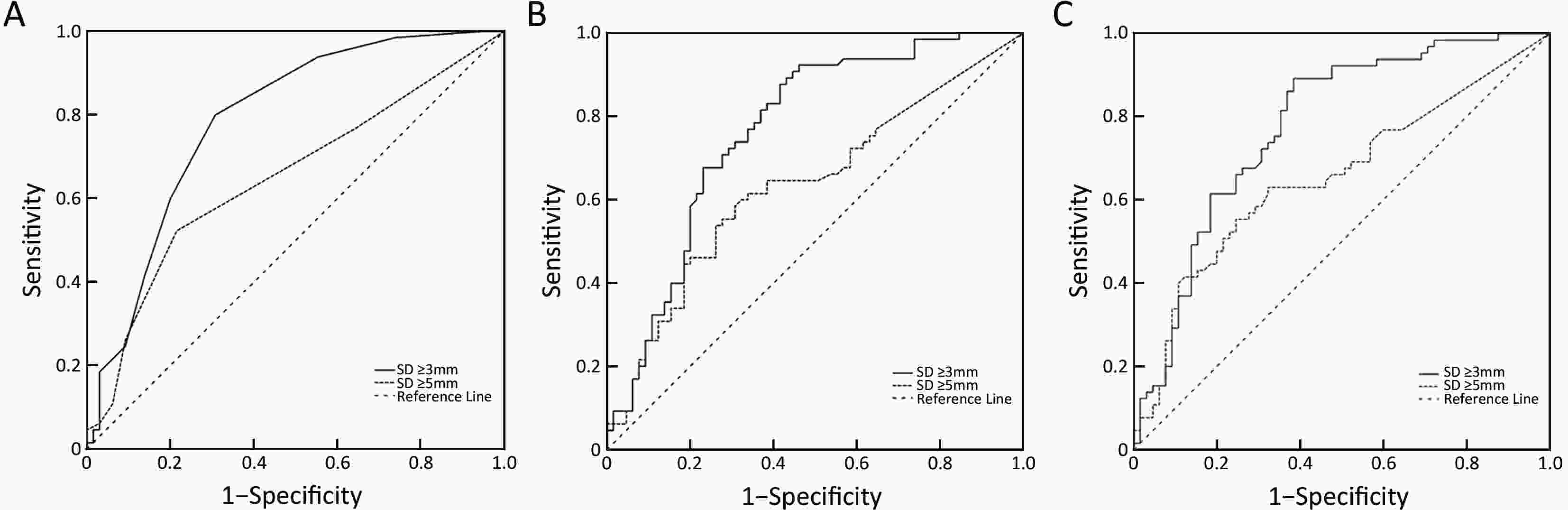
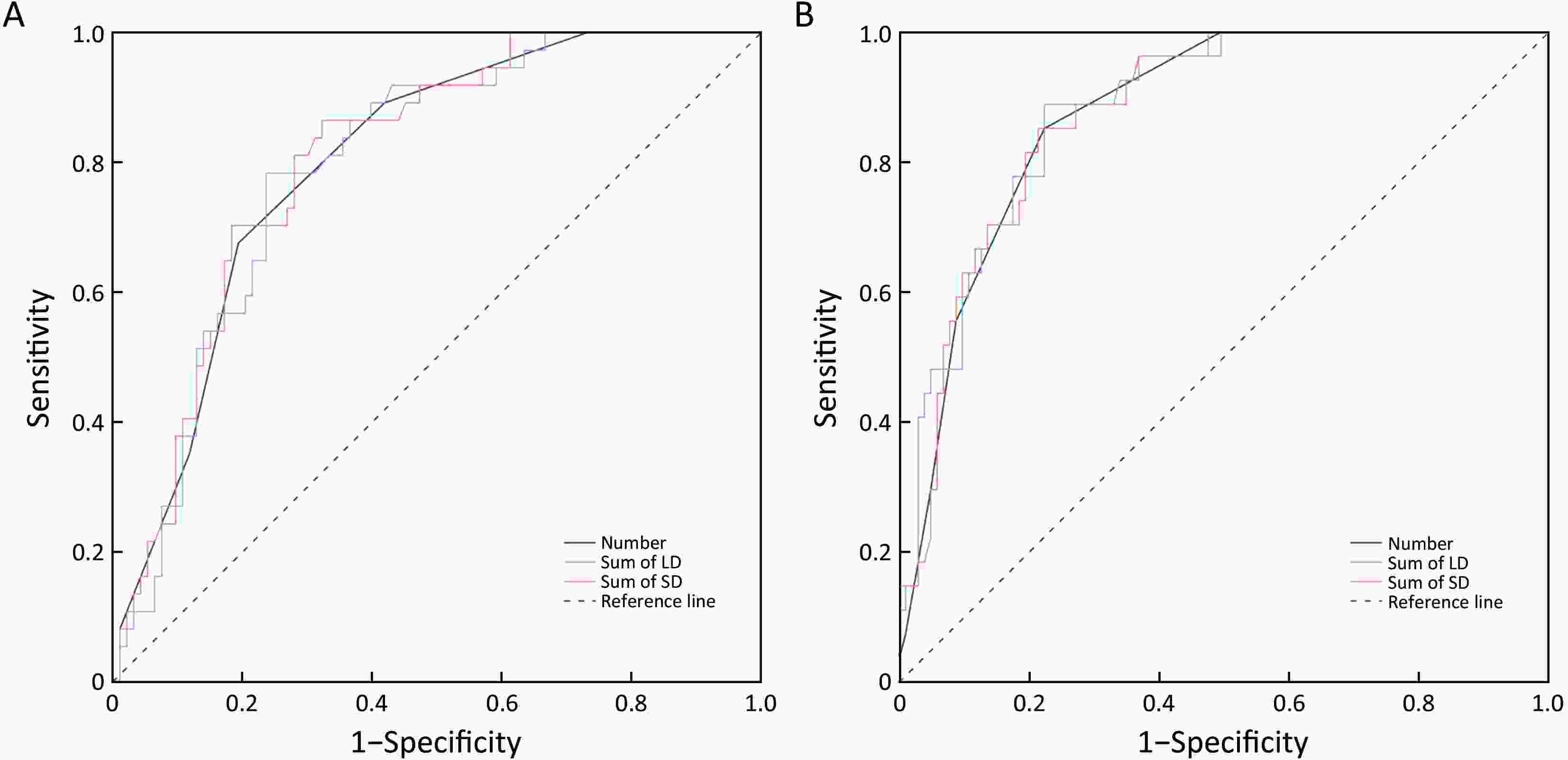
ObjectiveLymph node status is critical when selecting treatment methods for patients with early gastric cancer (EGC). The aim of this study was to assess the diagnostic value of computed tomography (CT) for detection of lymph node metastasis (LNM) in patients with EGC. MethodsWe retrospectively analyzed patients who had pathologically confirmed EGC between November 2010 and January 2019. After 1:1 propensity score matching, 65 patients with LNM and 65 patients without LNM were retained for comparison. The long diameter (LD) and short diameter (SD) of all visualized lymph nodes in all stations were recorded. The diagnostic value of LNM was assessed with receiver operating characteristic analysis. ResultsAmong 130 patients, we found a total of 558 lymph nodes on the CT images. Among the diagnostic indicators, the number, sum of LD and sum of SD of lymph nodes greater than 3 mm had better discrimination. The areas under the curve were all greater than 0.75. As for different regions, the optimal cutoff values of number, the sum of LD and sum of SD were determined as follows: overall, ≥4, 19.9 mm and 13.5 mm; left gastric artery basin, ≥3, 15.7 mm and 8.6 mm; right gastroepiploic artery basin, ≥2, 8.6 mm and 7.0 mm. ConclusionsCT is valuable for diagnosing LNM in EGC patients. The number, sum of LD and sum of SD of lymph nodes greater than 3 mm are preferable indicators. Different regional lymph nodes have different optimal criteria for predicting LNM in ECG patients.
2021, 33(6): 682-693.
doi: 10.21147/j.issn.1000-9604.2021.06.05
Abstract:
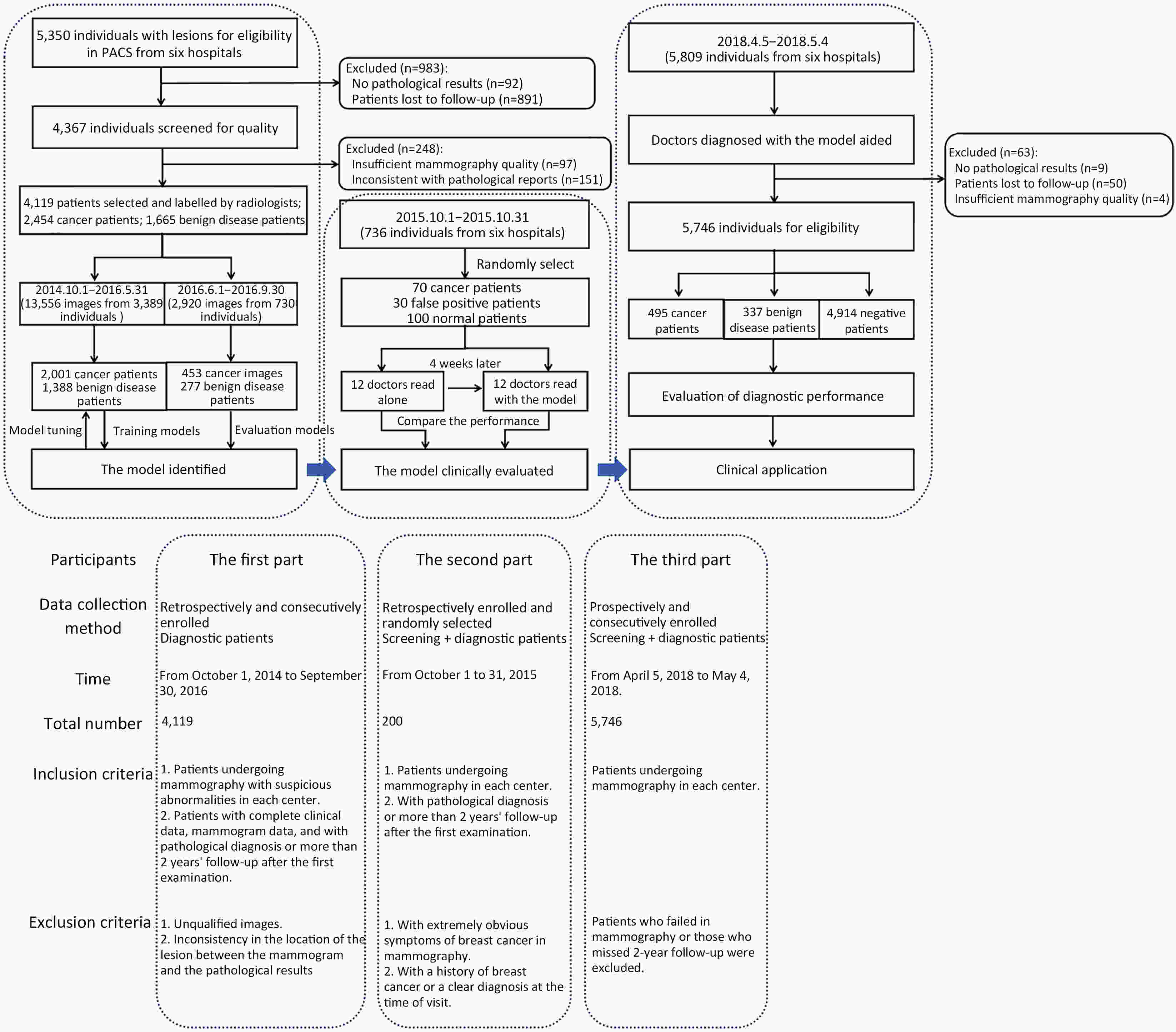
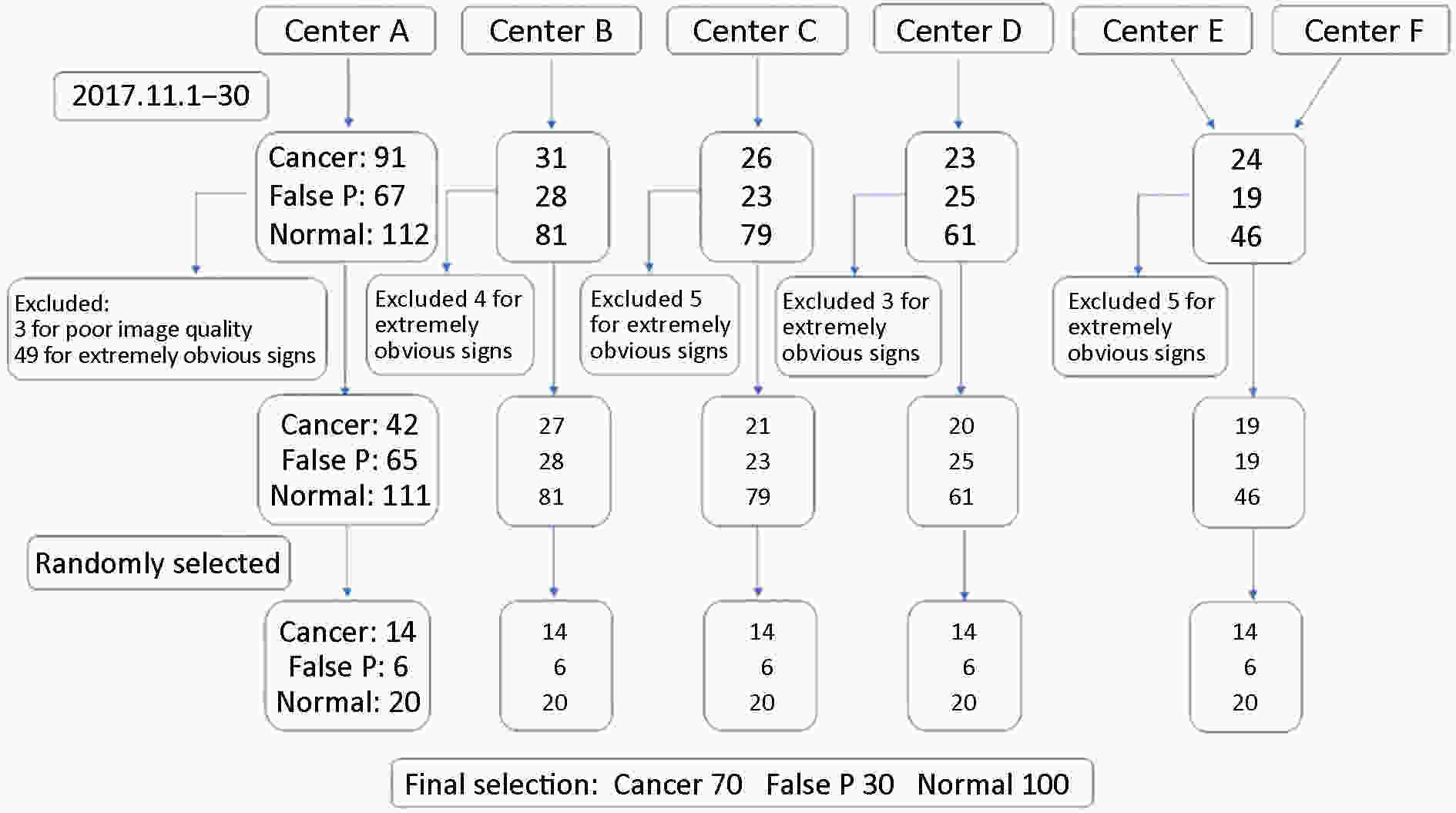
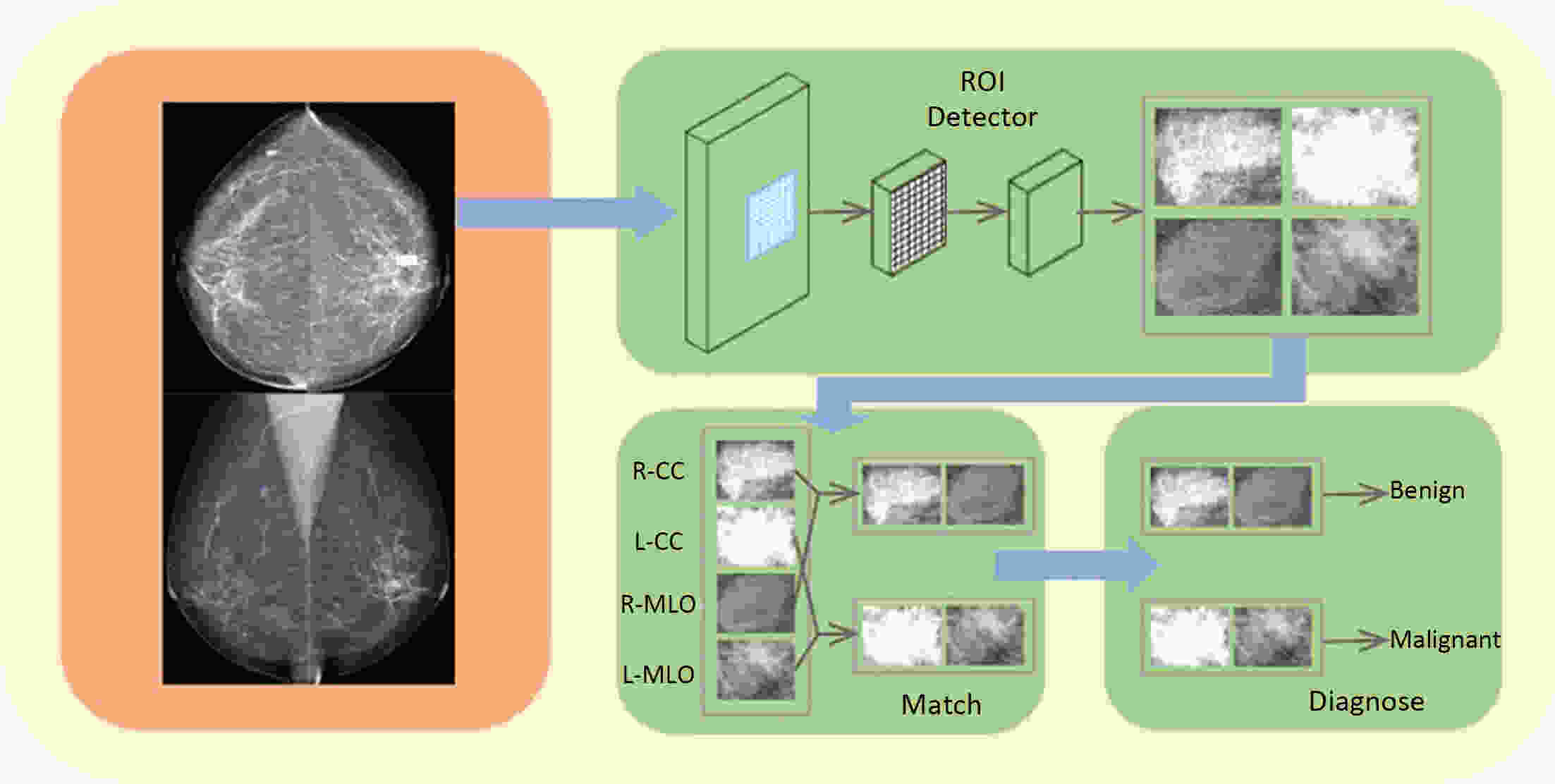
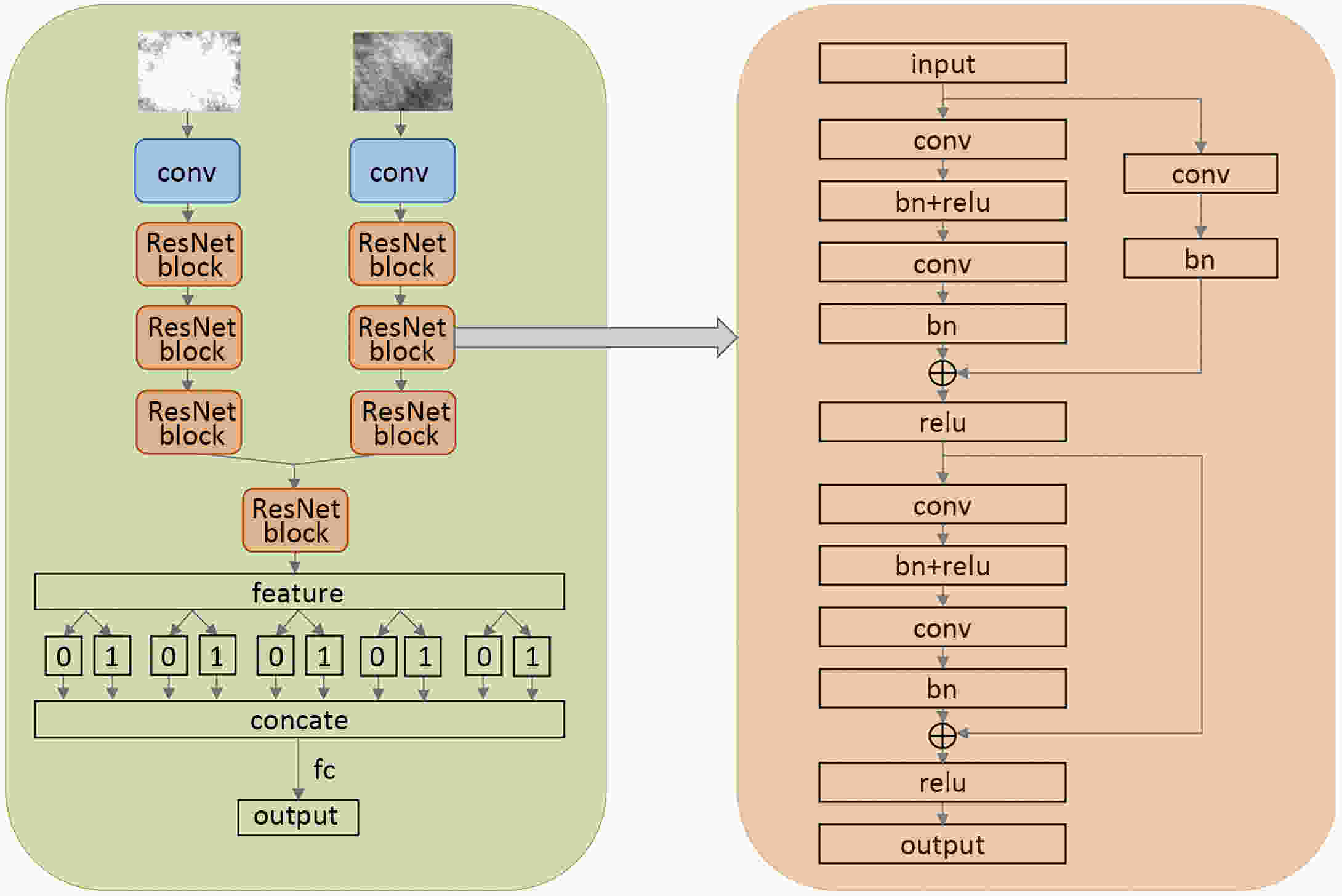
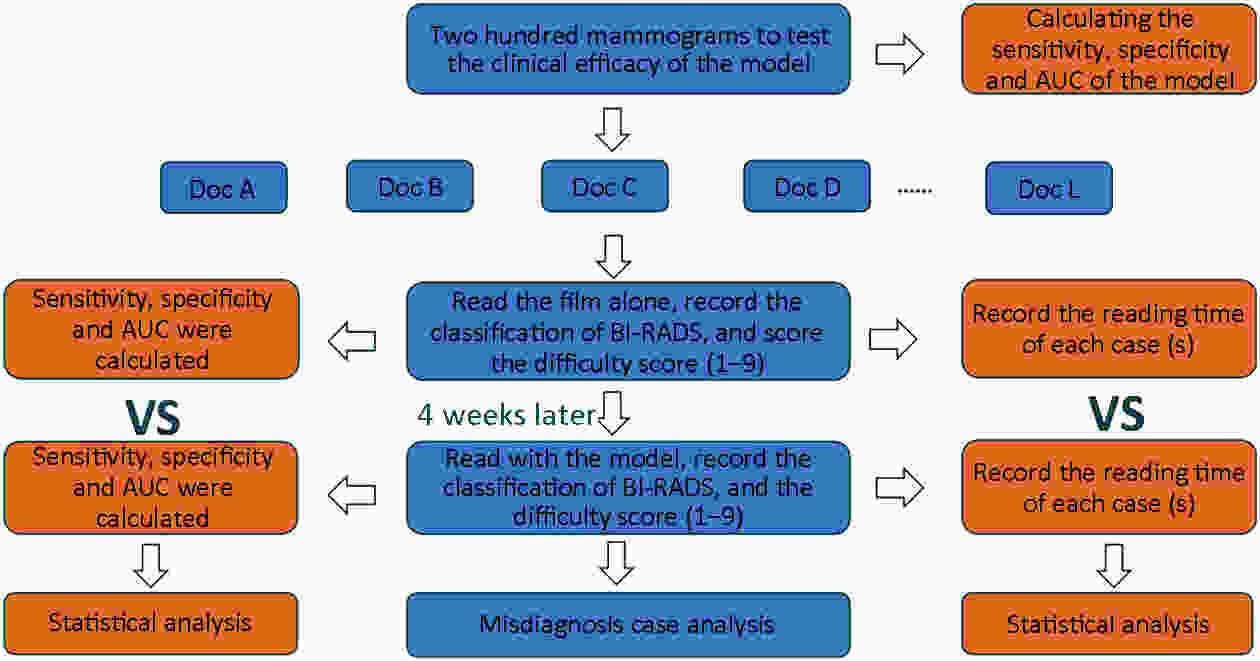
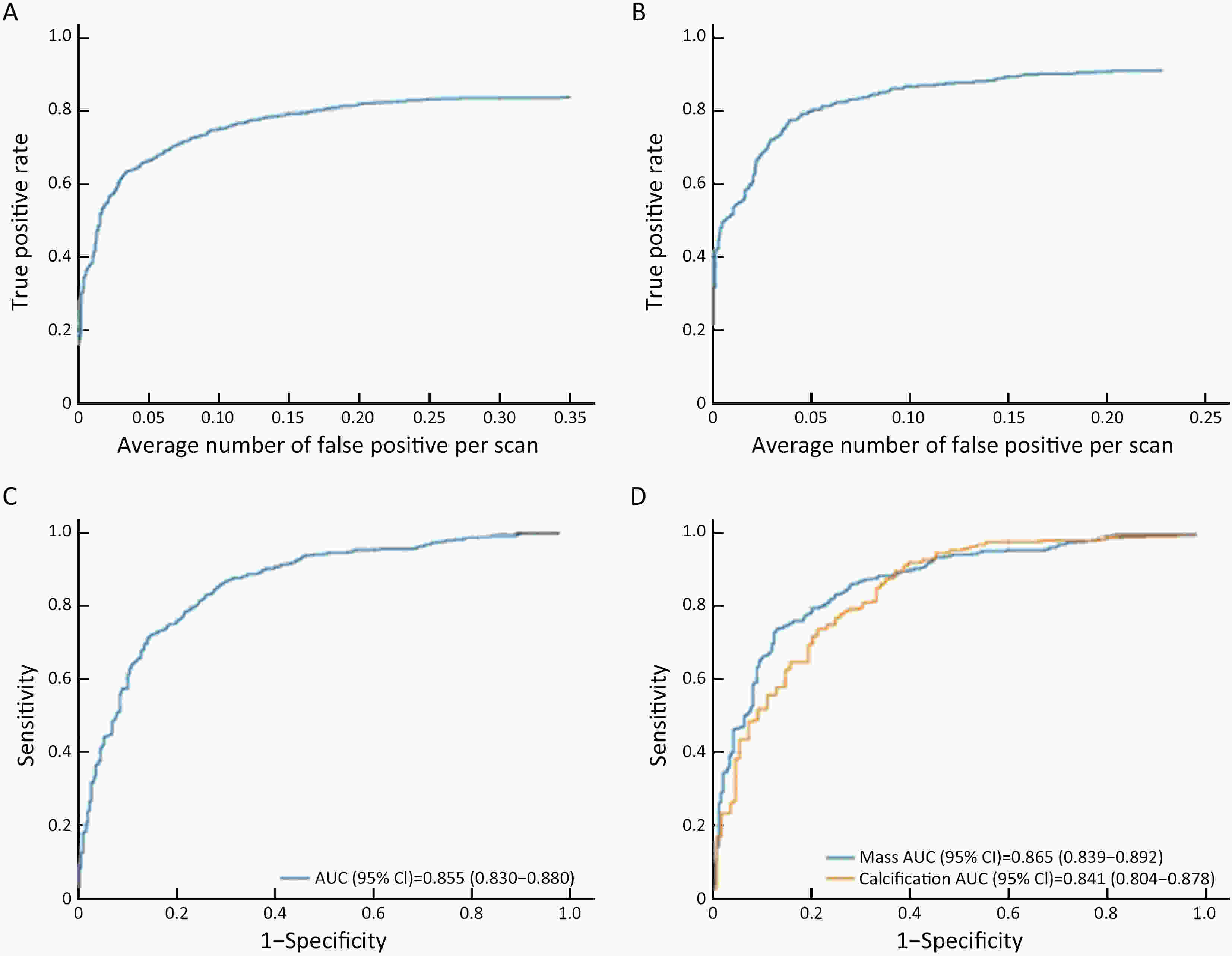
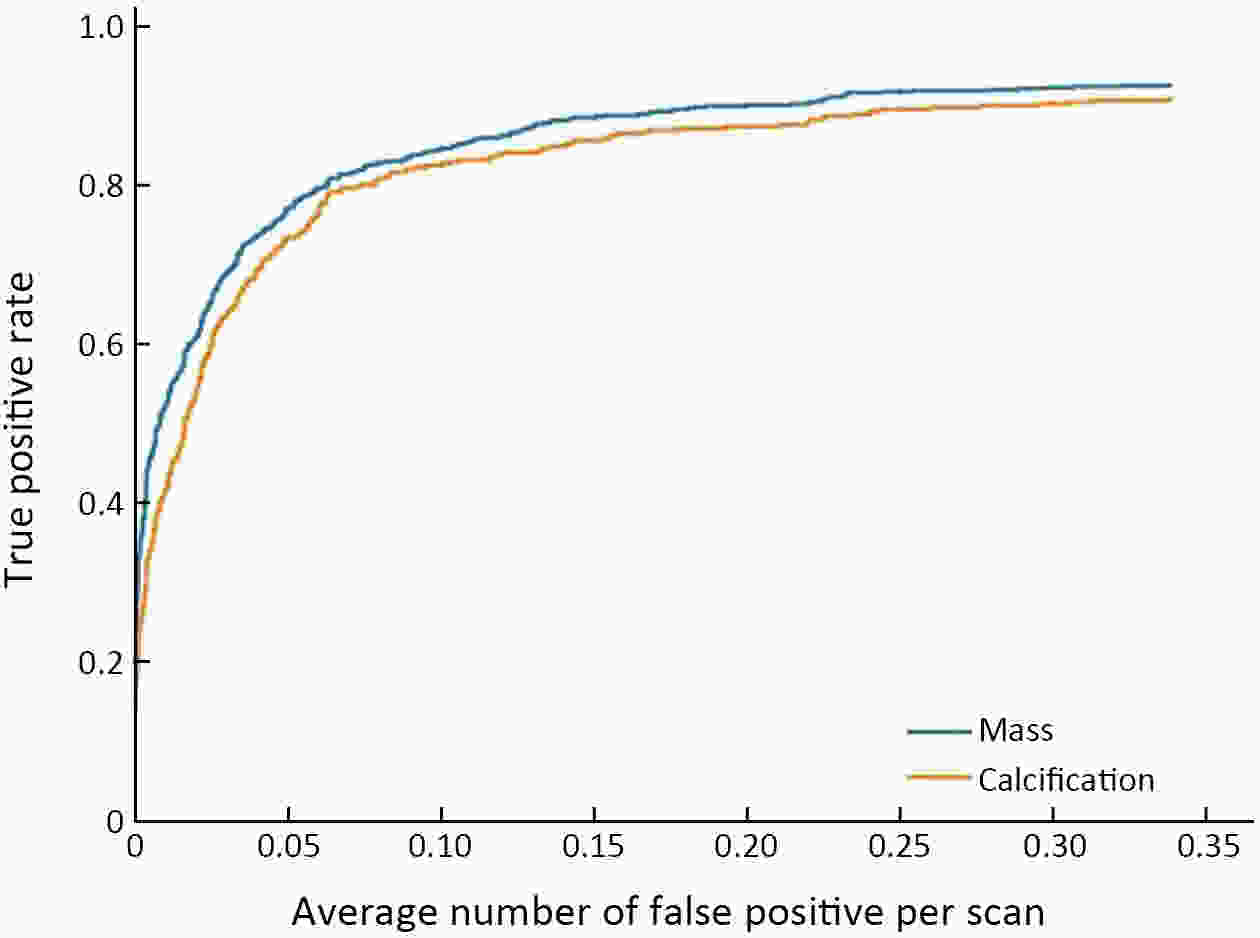
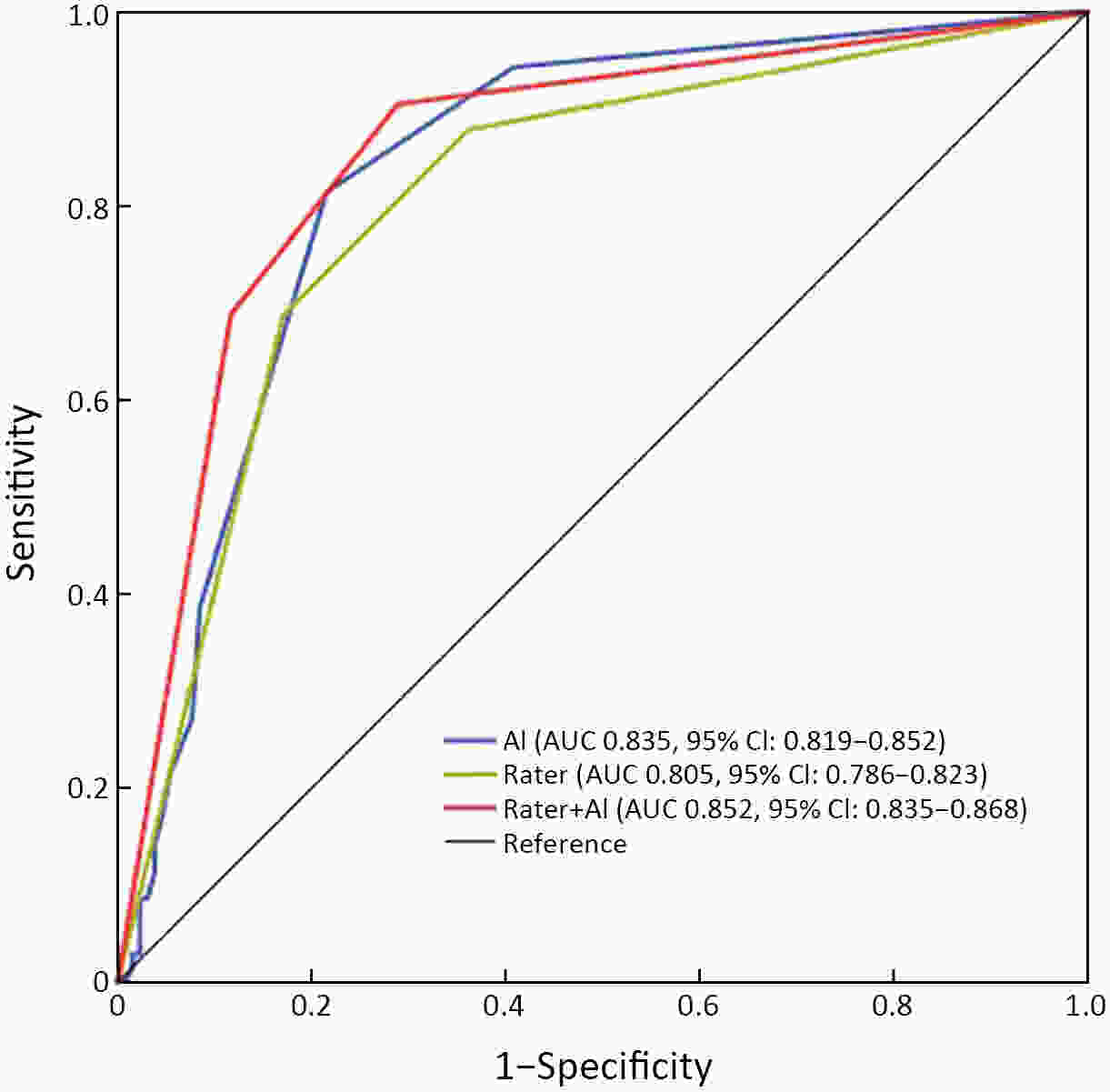
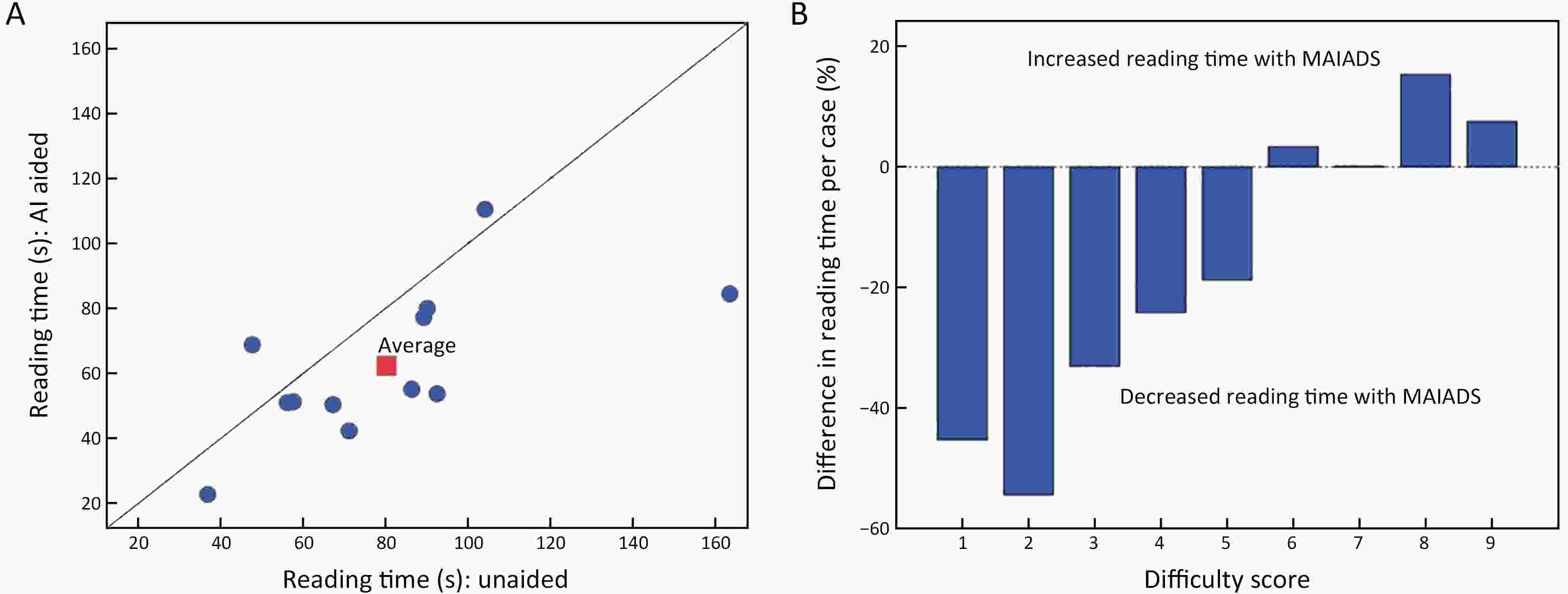
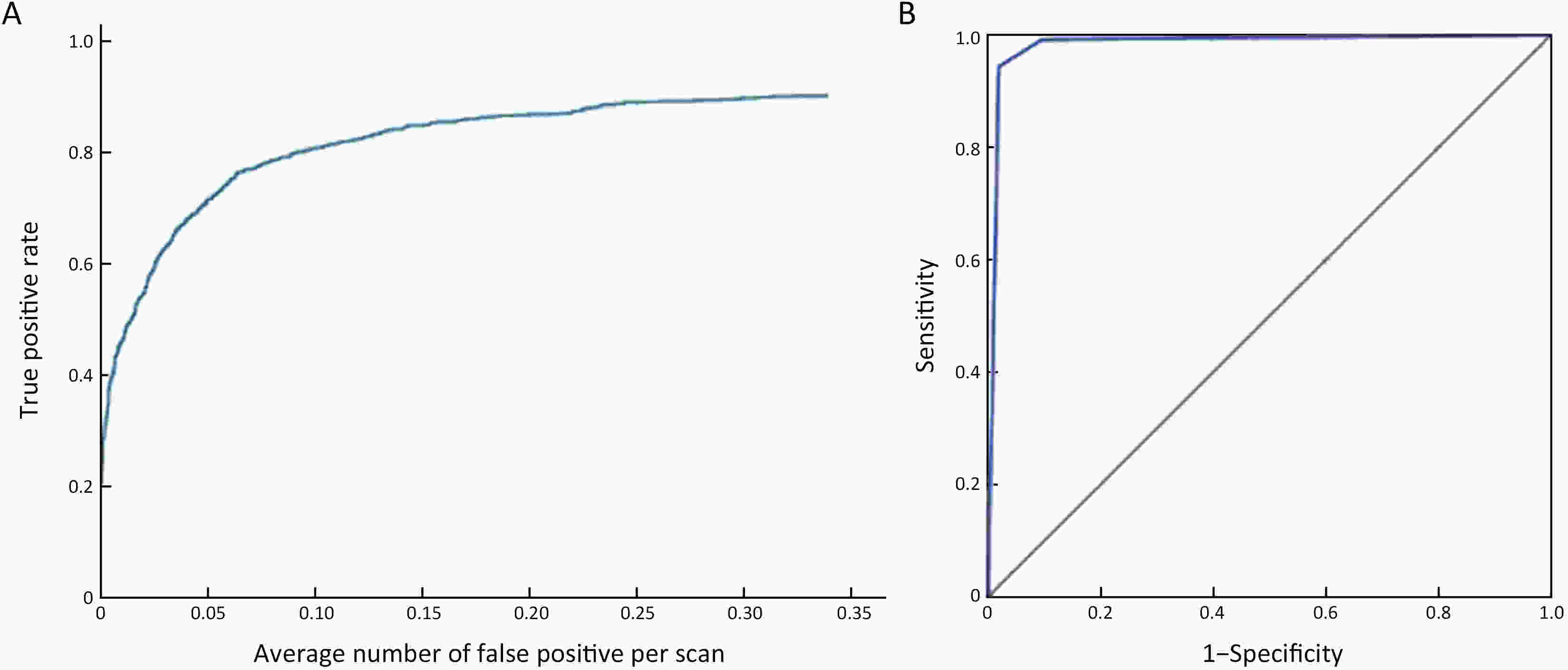
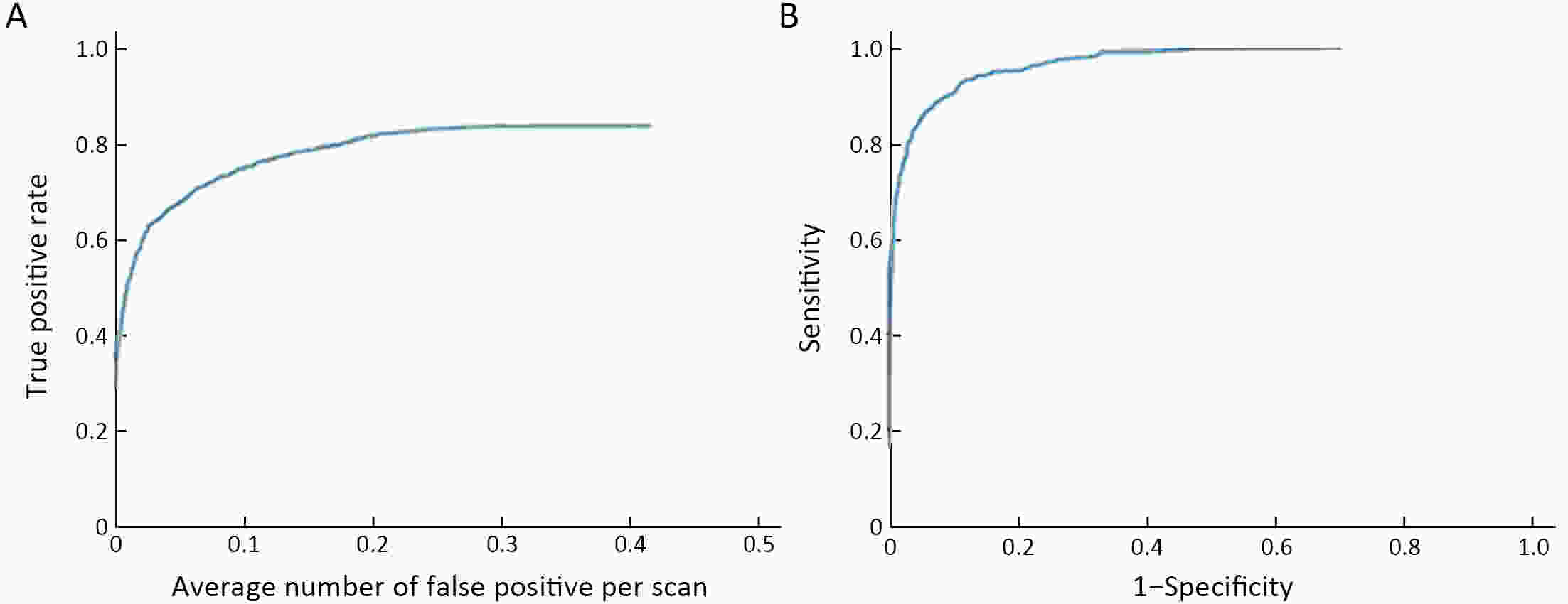
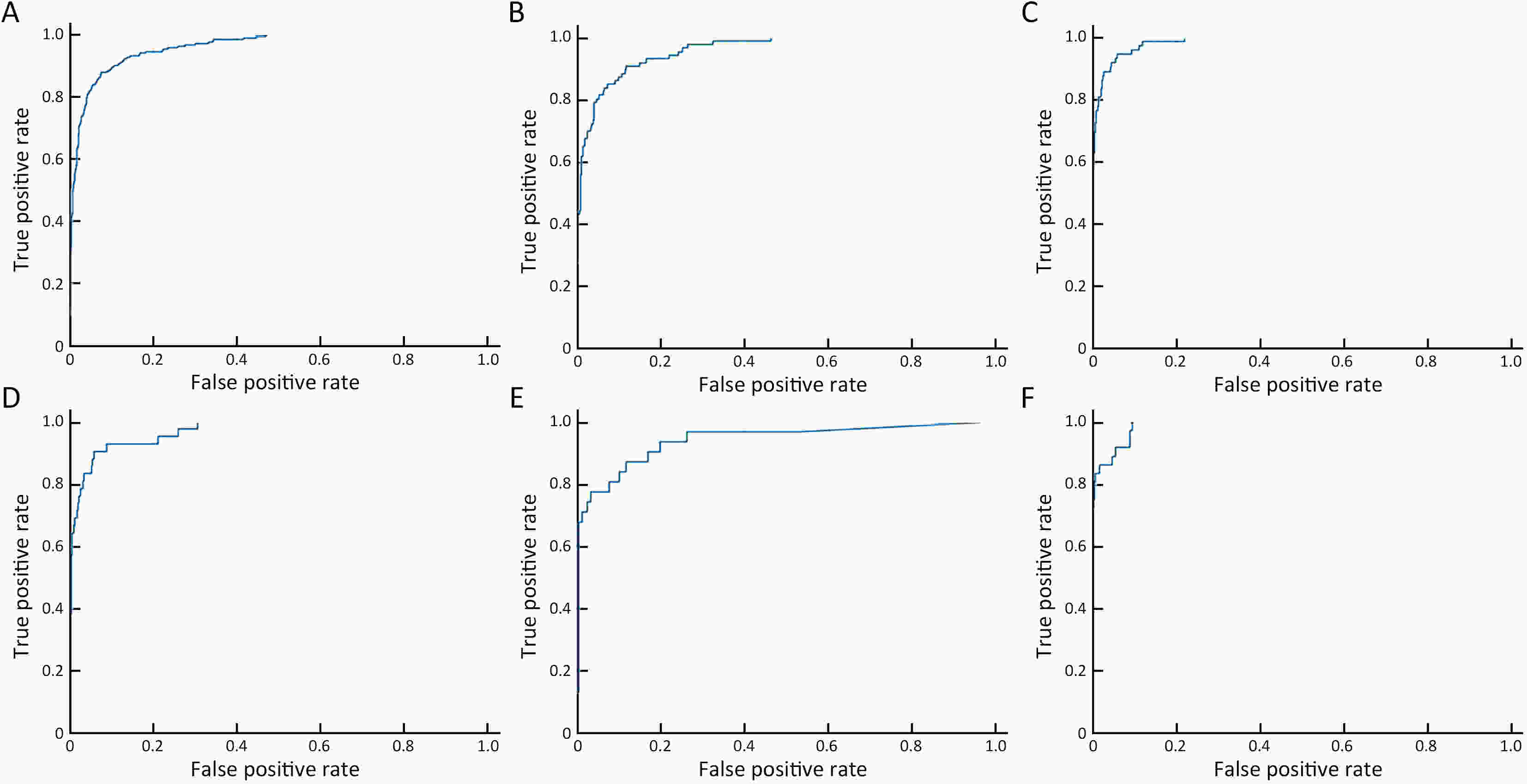
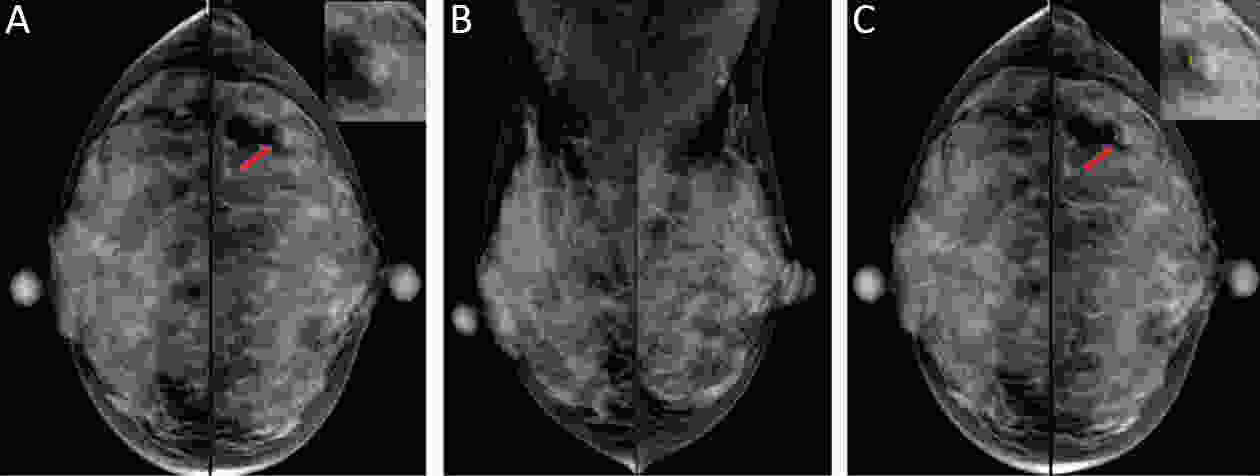
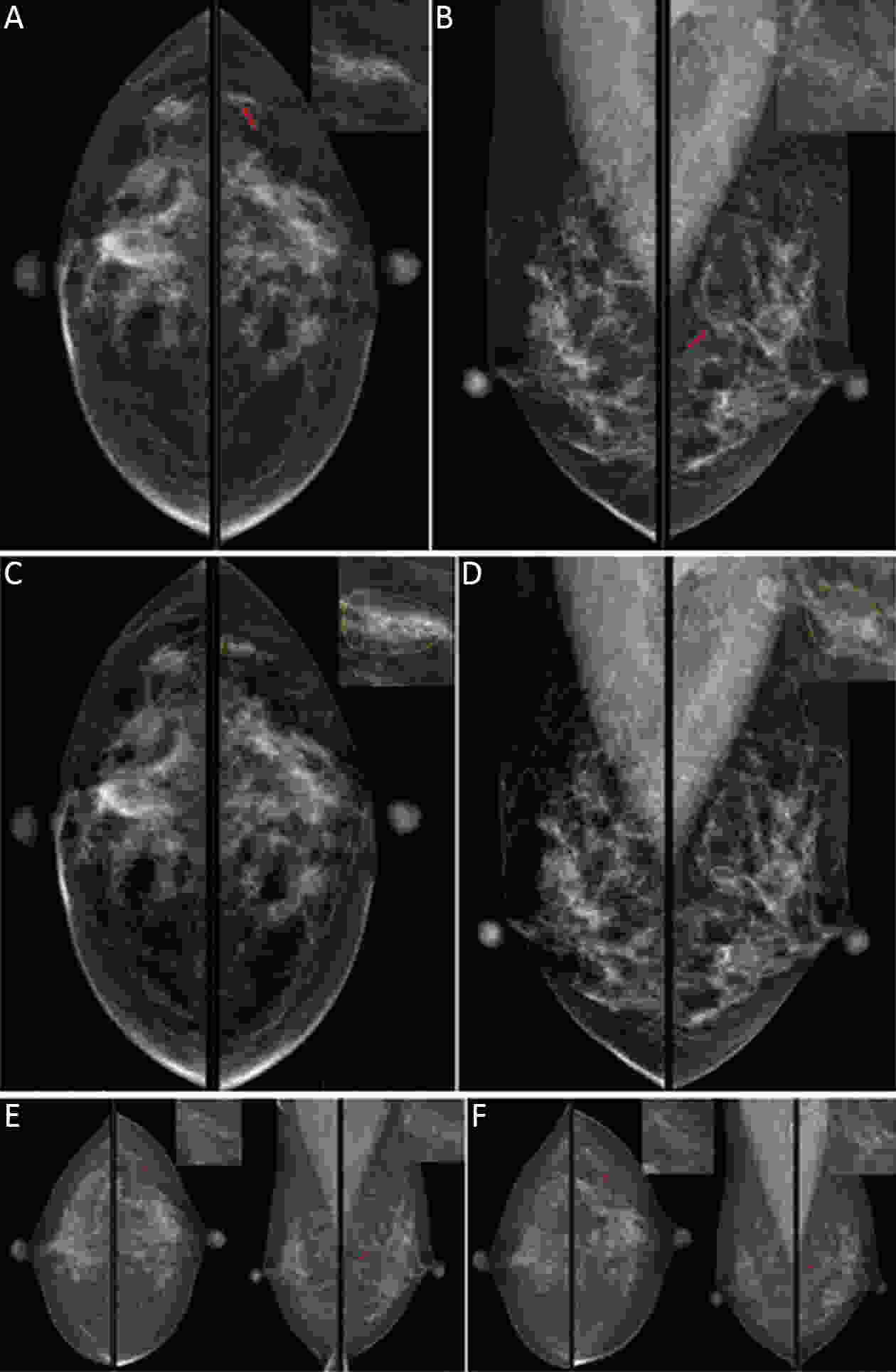
Objective Computer-aided diagnosis using deep learning algorithms has been initially applied in the field of mammography, but there is no large-scale clinical application. Methods This study proposed to develop and verify an artificial intelligence model based on mammography. Firstly, mammograms retrospectively collected from six centers were randomized to a training dataset and a validation dataset for establishing the model. Secondly, the model was tested by comparing 12 radiologists’ performance with and without it. Finally, prospectively enrolled women with mammograms from six centers were diagnosed by radiologists with the model. The detection and diagnostic capabilities were evaluated using the free-response receiver operating characteristic (FROC) curve and ROC curve. Results The sensitivity of model for detecting lesions after matching was 0.908 for false positive rate of 0.25 in unilateral images. The area under ROC curve (AUC) to distinguish the benign lesions from malignant lesions was 0.855 [95% confidence interval (95% CI): 0.830, 0.880]. The performance of 12 radiologists with the model was higher than that of radiologists alone (AUC: 0.852 vs. 0.805, P=0.005). The mean reading time of with the model was shorter than that of reading alone (80.18 s vs. 62.28 s, P=0.032). In prospective application, the sensitivity of detection reached 0.887 at false positive rate of 0.25; the AUC of radiologists with the model was 0.983 (95% CI: 0.978, 0.988), with sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 94.36%, 98.07%, 87.76%, and 99.09%, respectively. Conclusions The artificial intelligence model exhibits high accuracy for detecting and diagnosing breast lesions, improves diagnostic accuracy and saves time.
2021, 33(6): 694-707.
doi: 10.21147/j.issn.1000-9604.2021.06.06
Abstract:
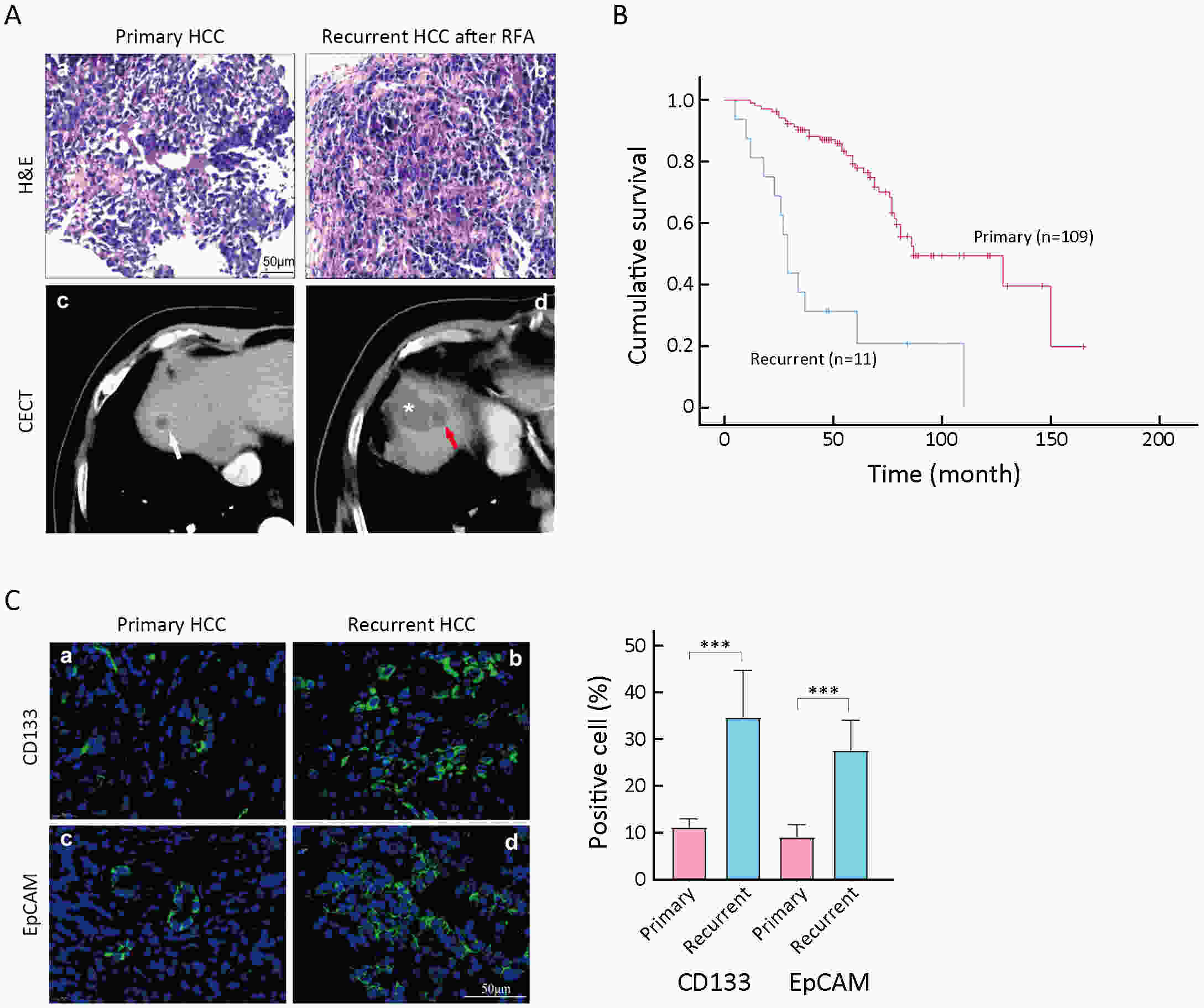
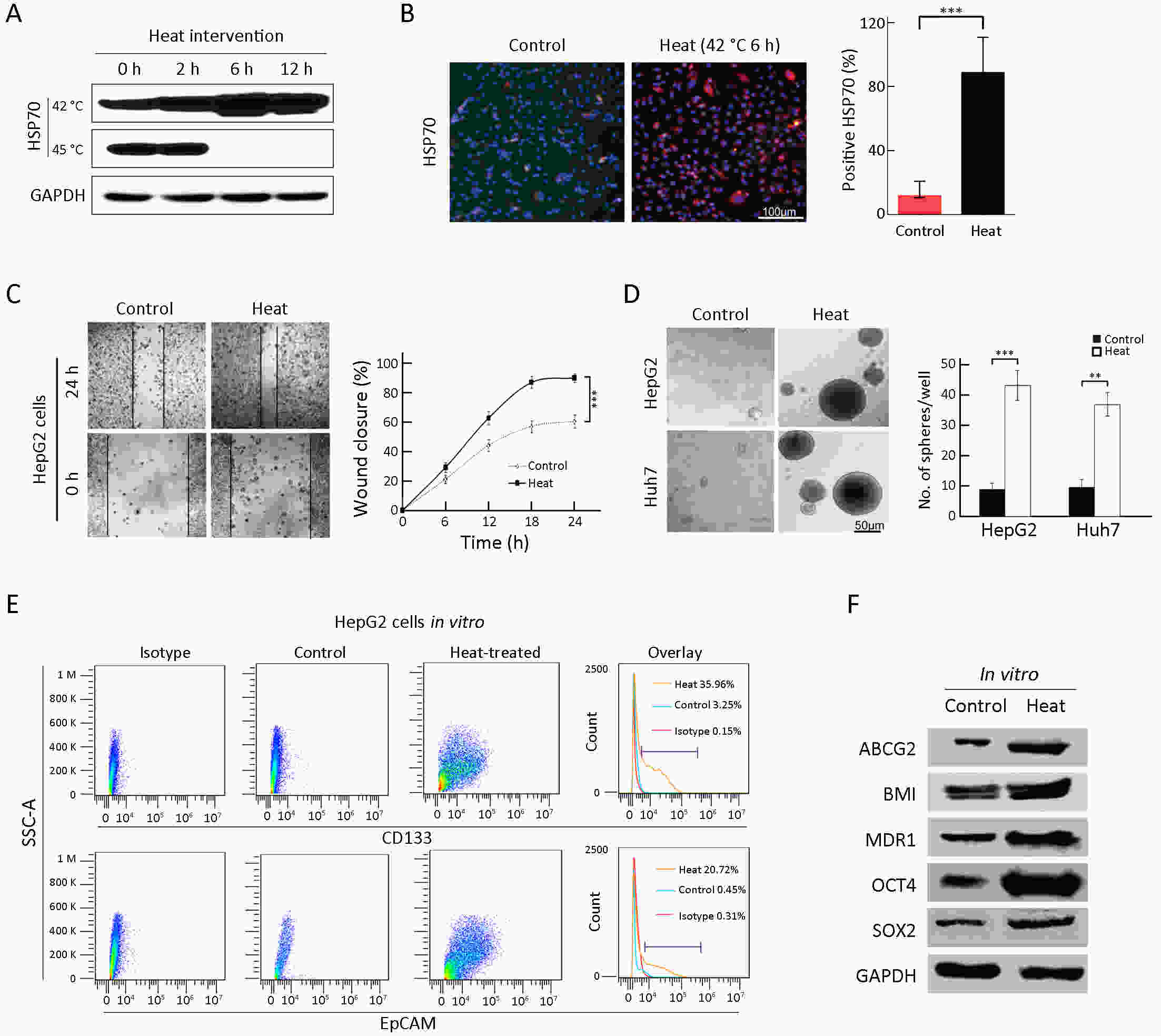
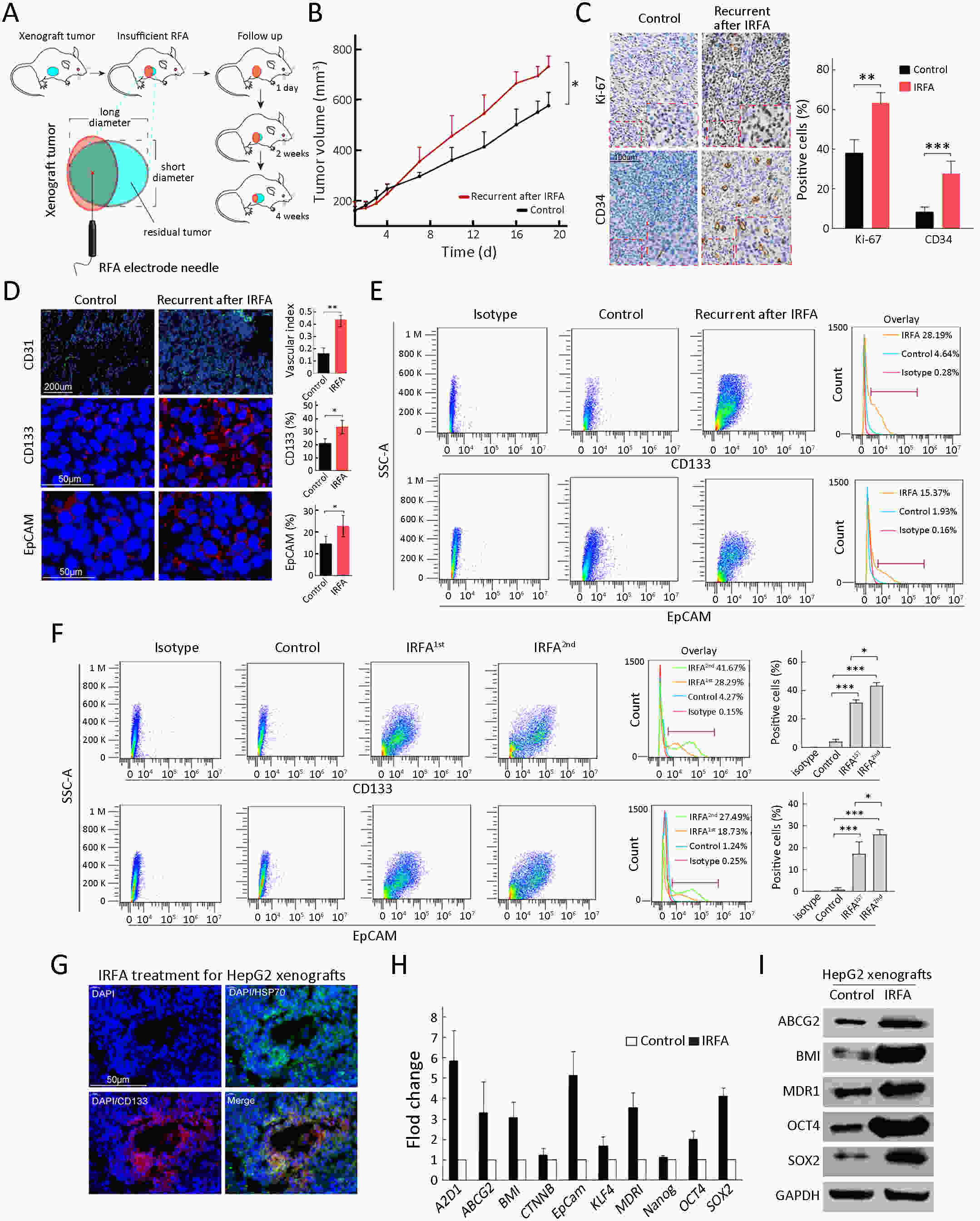
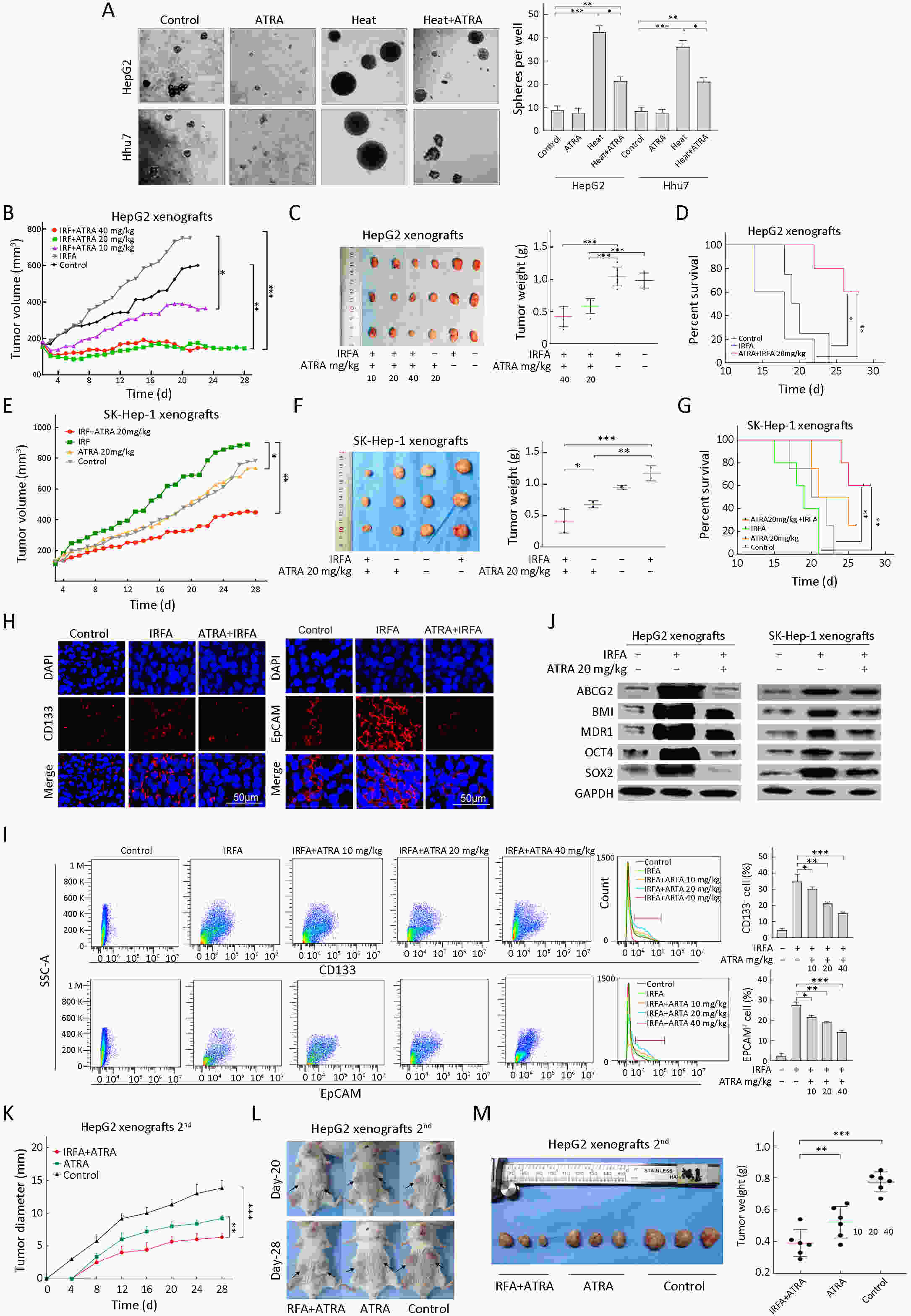
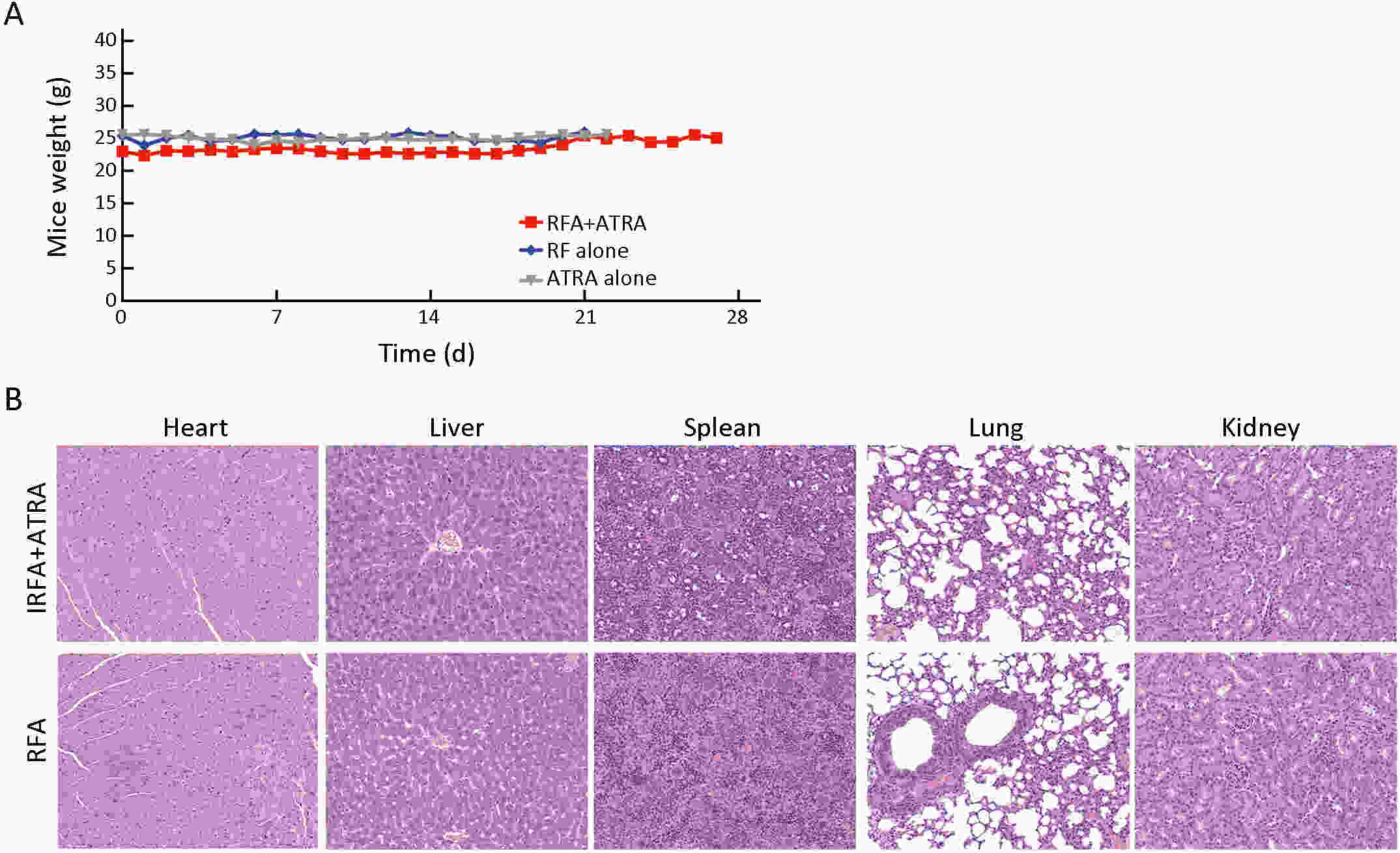
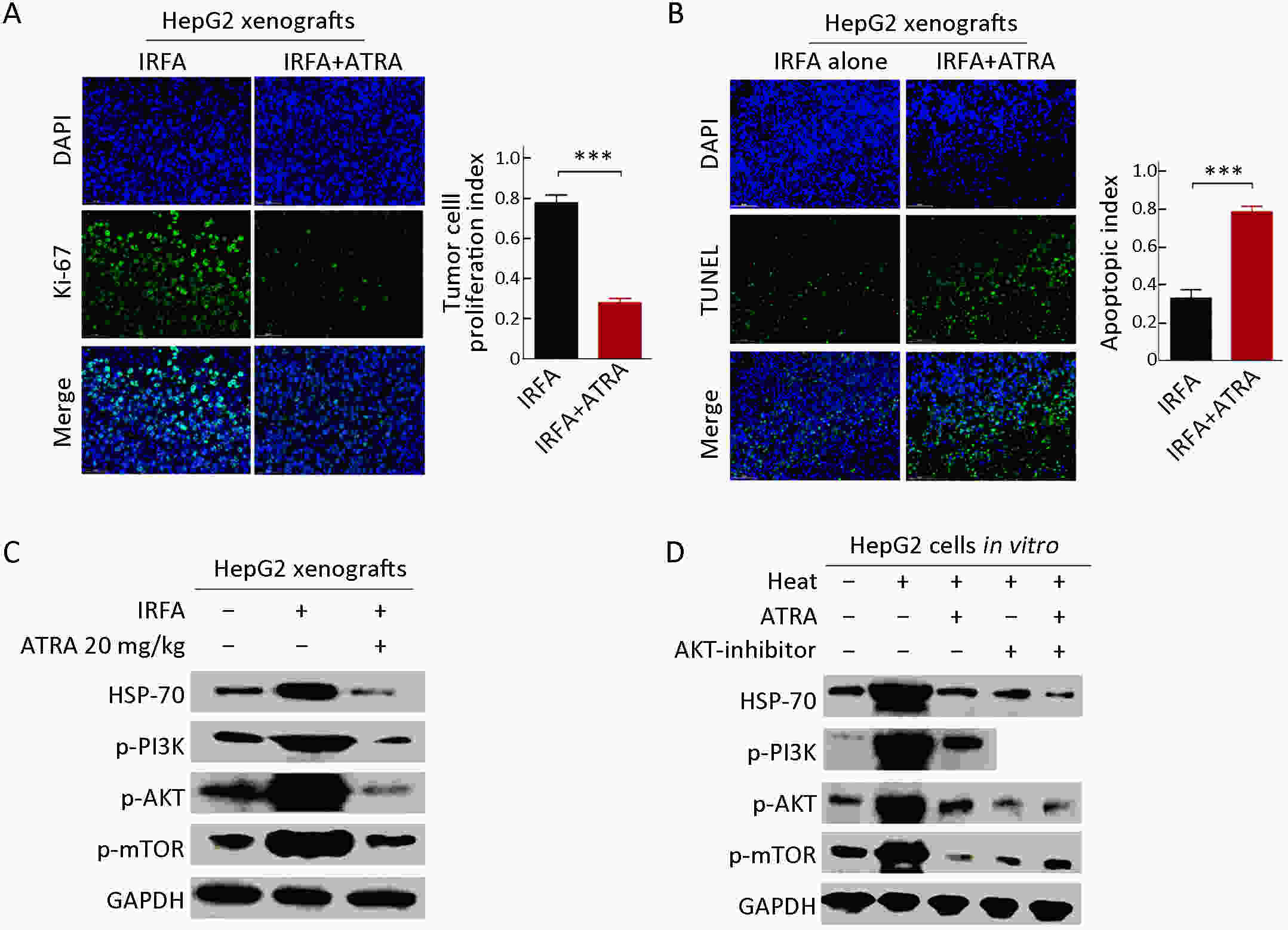
Objective Local recurrence of hepatocellular carcinoma (HCC) after radiofrequency ablation (RFA) treatment remains a serious problem. Tumor-initiating cells (TICs) are thought to be responsible for tumor relapse. Here, we investigated the effect of the TIC differentiation inducer, all-trans retinoic acid (ATRA), on RFA and explored the potential molecular mechanisms. Methods The proportions of CD133+ and epithelial cell adhesion molecule (EpCAM)+ TICs in recurrent HCC after RFA and primary HCC were first determined in clinic. Then, the effect of heat intervention or insufficient RFA (IRFA) on the malignant potential of HCC cells, including cell migration, sphere formation ability, tumor growth, the proportion of CD133+ and EpCAM+ TICs and expression of stem cell-related genes, was evaluated in vitro andin vivo. Finally, the effect of ATRA on the tumor growth and the proportion of TICs was evaluated. Results In clinical data, a higher proportion of CD133+ and EpCAM+ TICs was found in recurrent tumors than in primary tumors. In vitro heat intervention promoted the cell migration and sphere formation ability. Additionally, it increased the proportion of CD133+ and EpCAM+ TICs and the expression of stem cell-related genes. In addition, after IRFA the residual tumors in xenografts grew faster and had more TICs than untreated tumors. ATRA remarkably inhibited residual tumor growth after IRFA by elimination of TICs though the PI3K/AKT pathway. Combination treatment with ATRA resulted in longer survival outcomes in mouse xenografts than RFA alone. Conclusions ATRA, as a TIC differentiation inducer, could help to improve the effect of RFA treatment, which was partially attributed to its effect against TICs. The data indicated its potential as an alternative drug in the development of better therapeutic strategies for use in combination with RFA.
2021, 33(6): 708-718.
doi: 10.21147/j.issn.1000-9604.2021.06.07
Abstract:
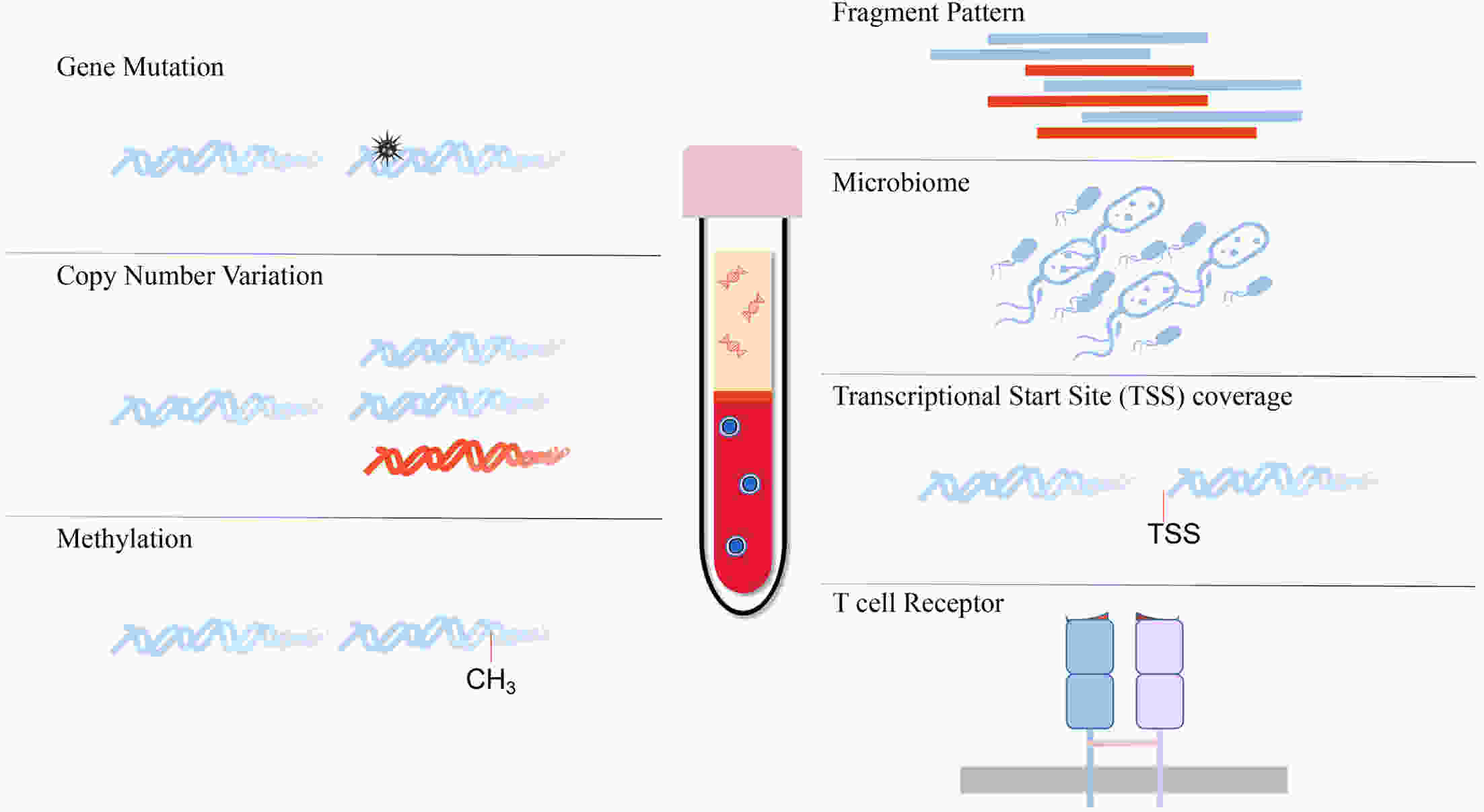
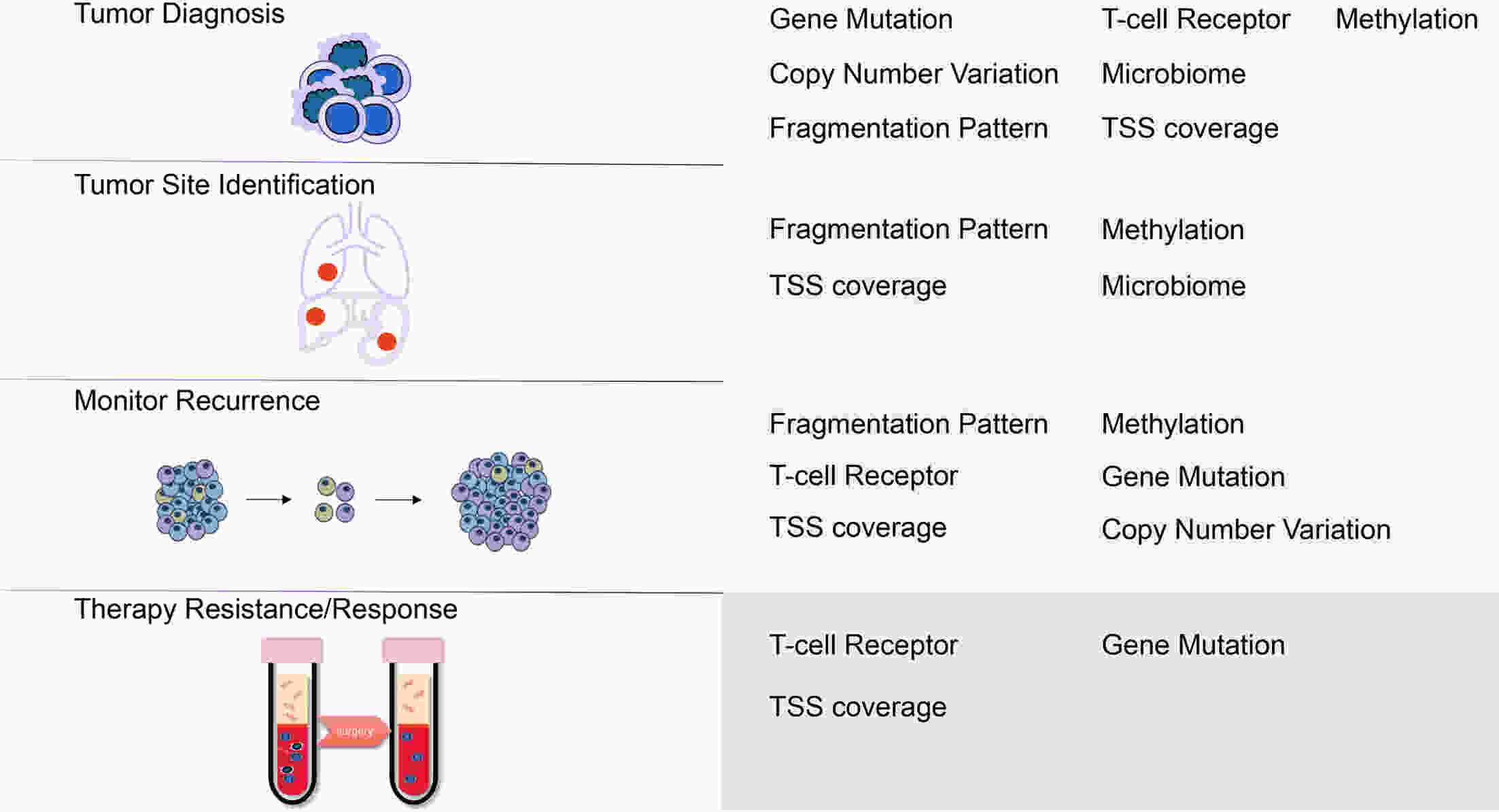
The profiling of plasma cell-free DNA (cfDNA) is becoming a valuable tool rapidly for tumor diagnosis, monitoring and prognosis. Diverse plasma cfDNA technologies have been in routine or emerging use, including analyses of mutations, copy number alterations, gene fusions and DNA methylation. Recently, new technologies in cfDNA analysis have been developed in laboratories, and potentially reflect the status of epigenetic modification, the immune microenvironment and the microbiome in tumor tissues. In this review, the authors discuss the principles, methods and effects of the current cfDNA assays and provide an overview of studies that may inform clinical applications in the near future.
The profiling of plasma cell-free DNA (cfDNA) is becoming a valuable tool rapidly for tumor diagnosis, monitoring and prognosis. Diverse plasma cfDNA technologies have been in routine or emerging use, including analyses of mutations, copy number alterations, gene fusions and DNA methylation. Recently, new technologies in cfDNA analysis have been developed in laboratories, and potentially reflect the status of epigenetic modification, the immune microenvironment and the microbiome in tumor tissues. In this review, the authors discuss the principles, methods and effects of the current cfDNA assays and provide an overview of studies that may inform clinical applications in the near future.
2021, 33(6): 719-723.
doi: 10.21147/j.issn.1000-9604.2021.06.08
Abstract:
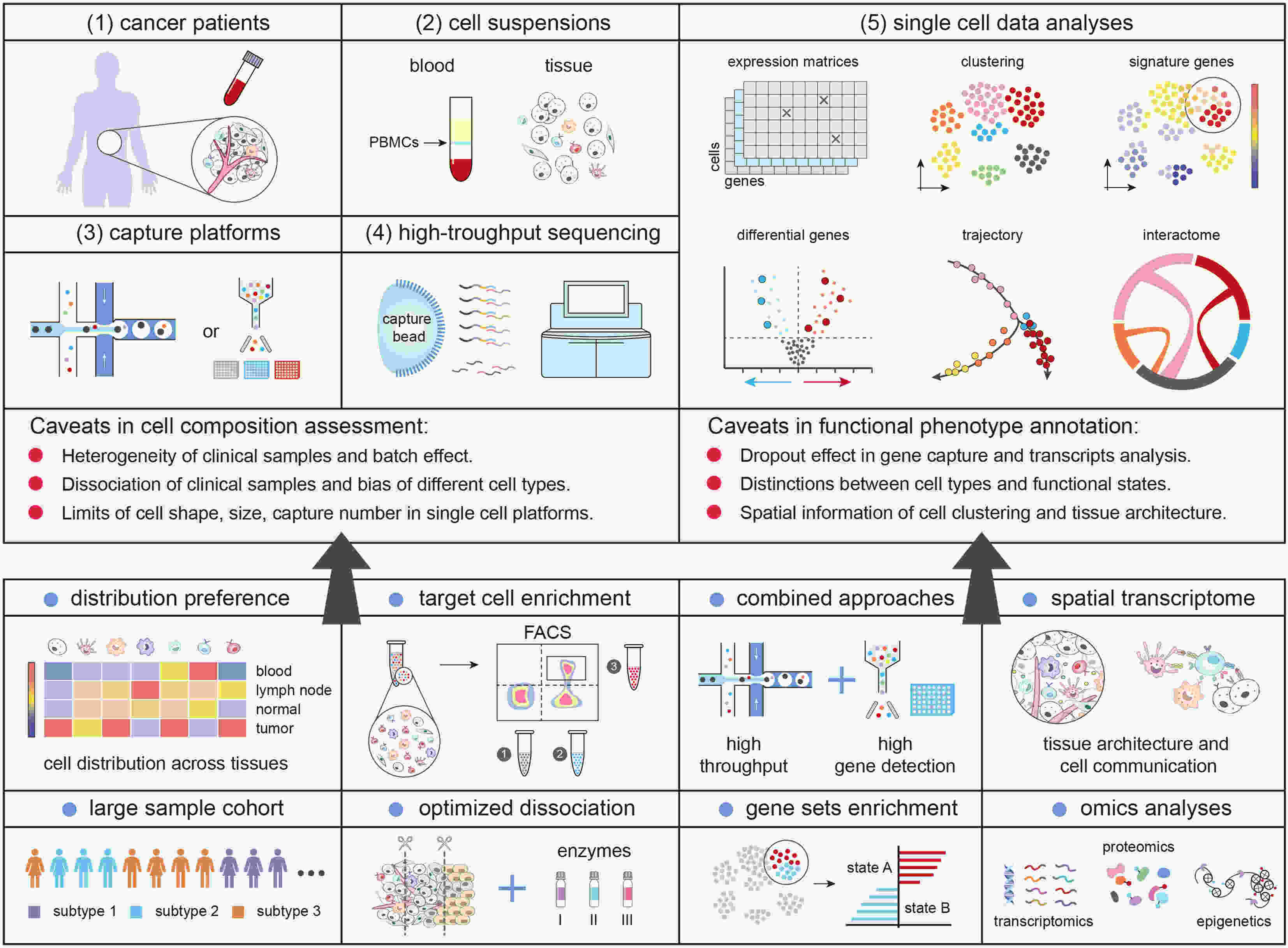
This decade has seen remarkable advances in the field of high-throughput single cell techniques. Single-cell RNA sequencing (scRNA-seq) has proven to be a powerful strategy to study the heterogeneity in clinical samples, providing an unbiased approach to uncover the characteristics in different cell subsets. To ensure the reproducibility and robustness of biological discoveries, researchers need to be aware of hidden caveats in tissue dissociation, cell capturing and transcripts measurement which may affect cell composition assessment and cellular function annotation. With measured interpretation of data and innovations in experimental and technical approaches, scRNA-seq can greatly unravel the heterogeneity in complex system and improve our understandings in tissue homeostasis and cancer biology.
This decade has seen remarkable advances in the field of high-throughput single cell techniques. Single-cell RNA sequencing (scRNA-seq) has proven to be a powerful strategy to study the heterogeneity in clinical samples, providing an unbiased approach to uncover the characteristics in different cell subsets. To ensure the reproducibility and robustness of biological discoveries, researchers need to be aware of hidden caveats in tissue dissociation, cell capturing and transcripts measurement which may affect cell composition assessment and cellular function annotation. With measured interpretation of data and innovations in experimental and technical approaches, scRNA-seq can greatly unravel the heterogeneity in complex system and improve our understandings in tissue homeostasis and cancer biology.
2021, 33(6): 724-724.
doi: 10.21147/j.issn.1000-9604.2021.06.09
Abstract:

 Abstract
Abstract FullText HTML
FullText HTML PDF 5850KB
PDF 5850KB