2022 Vol.34(1)
Display Mode: |
2022, 34(1): 1-10.
doi: 10.21147/j.issn.1000-9604.2022.01.01
Abstract:
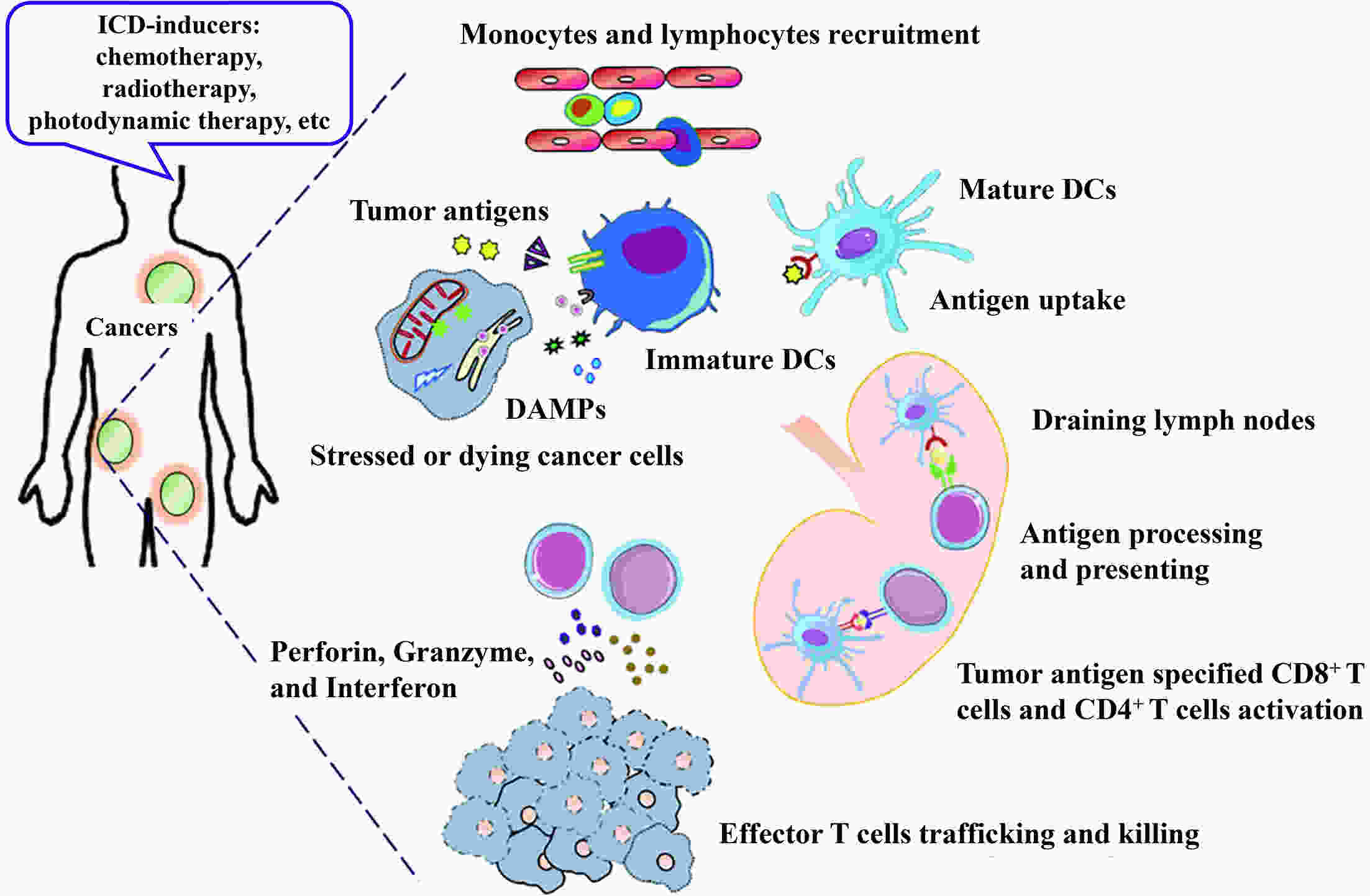
Immunotherapy has revolutionized cancer treatment and substantially improved patient outcomes with respect to multiple types of tumors. However, most patients cannot benefit from such therapies, mainly due to the intrinsic low immunogenicity of cancer cells (CCs) that allows them to escape recognition by immune cells of the body. Immunogenic cell death (ICD), which is a form of regulated cell death, engages in a complex dialogue between dying CCs and immune cells in the tumor microenvironment (TME), ultimately evoking the damage-associated molecular pattern (DAMP) signals to activate tumor-specific immunity. The ICD inducers mediate the death of CCs and improve both antigenicity and adjuvanticity. At the same time, they reprogram TME with a “cold-warm-hot” immune status, ultimately amplifying and sustaining dendritic cell- and T cell-dependent innate sensing as well as the antitumor immune responses. In this review, we discuss how to stimulate ICD based upon the biological properties of CCs that have evolved under diverse stress conditions. Additionally, we highlight how this dynamic interaction contributes to priming tumor immunogenicity, thereby boosting anticancer immune responses. We believe that a deep understanding of these ICD processes will provide a framework for evaluating its vital role in cancer immunotherapy.
Immunotherapy has revolutionized cancer treatment and substantially improved patient outcomes with respect to multiple types of tumors. However, most patients cannot benefit from such therapies, mainly due to the intrinsic low immunogenicity of cancer cells (CCs) that allows them to escape recognition by immune cells of the body. Immunogenic cell death (ICD), which is a form of regulated cell death, engages in a complex dialogue between dying CCs and immune cells in the tumor microenvironment (TME), ultimately evoking the damage-associated molecular pattern (DAMP) signals to activate tumor-specific immunity. The ICD inducers mediate the death of CCs and improve both antigenicity and adjuvanticity. At the same time, they reprogram TME with a “cold-warm-hot” immune status, ultimately amplifying and sustaining dendritic cell- and T cell-dependent innate sensing as well as the antitumor immune responses. In this review, we discuss how to stimulate ICD based upon the biological properties of CCs that have evolved under diverse stress conditions. Additionally, we highlight how this dynamic interaction contributes to priming tumor immunogenicity, thereby boosting anticancer immune responses. We believe that a deep understanding of these ICD processes will provide a framework for evaluating its vital role in cancer immunotherapy.
2022, 34(1): 11-27.
doi: 10.21147/j.issn.1000-9604.2022.01.02
Abstract:
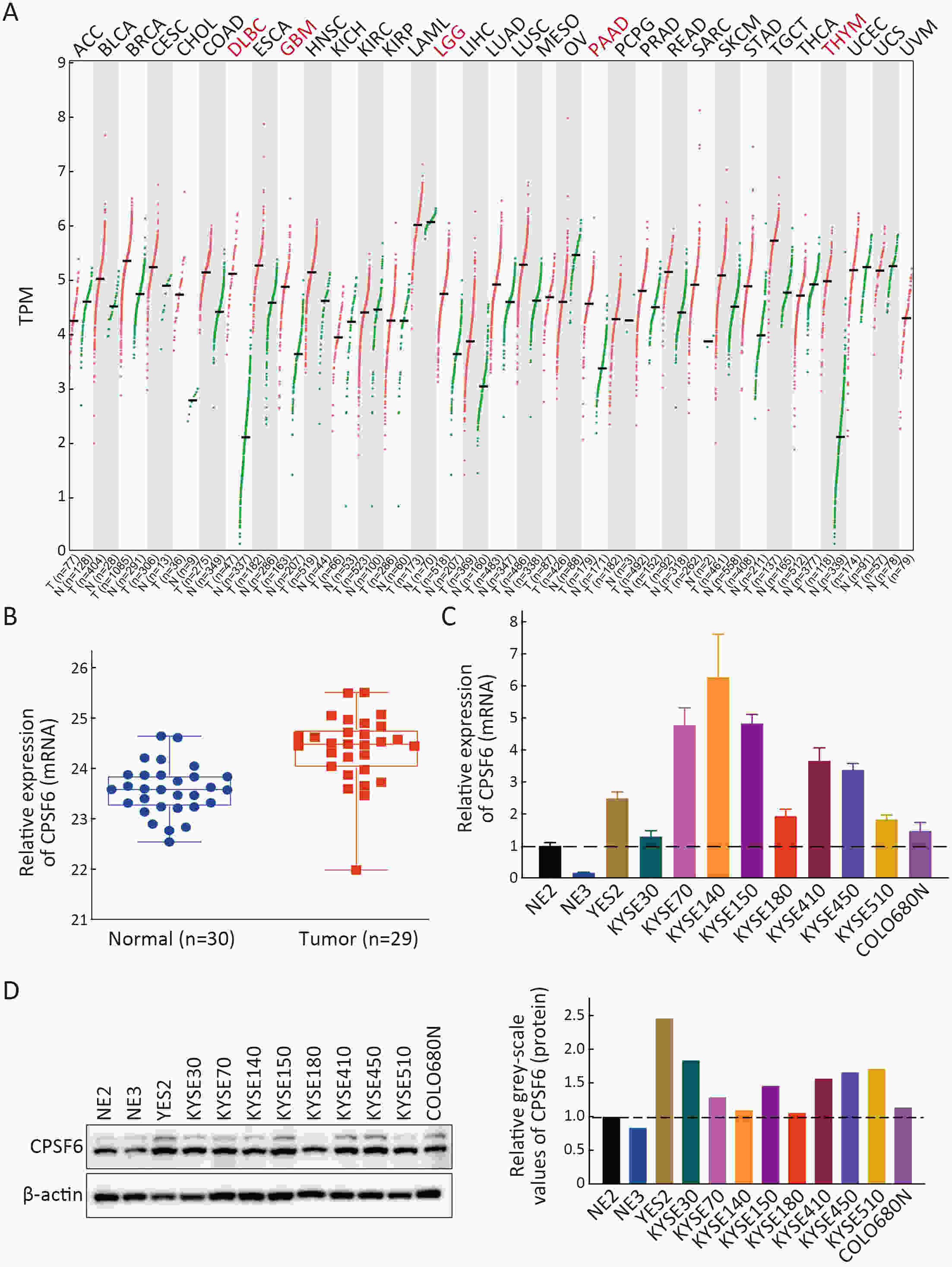
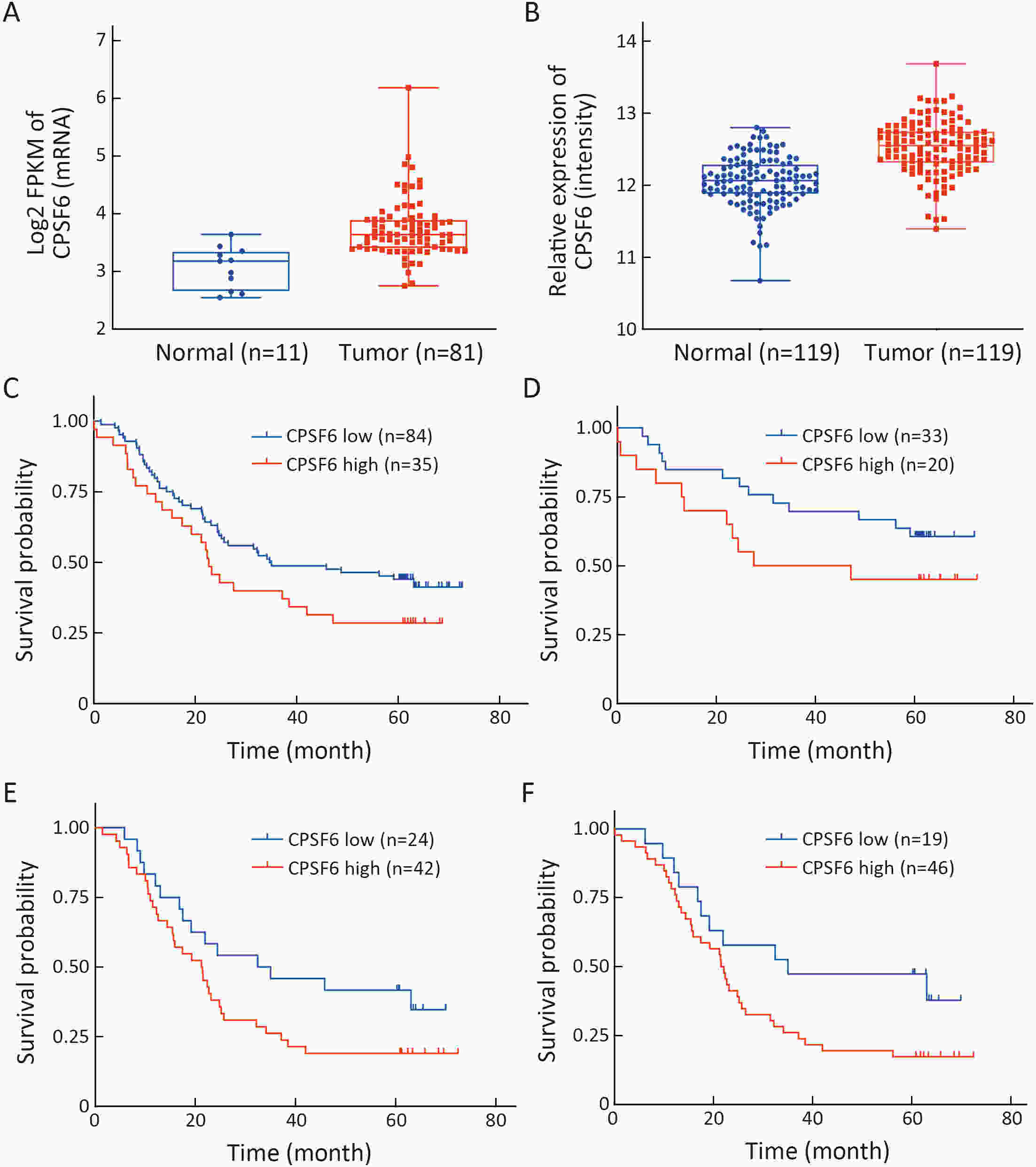
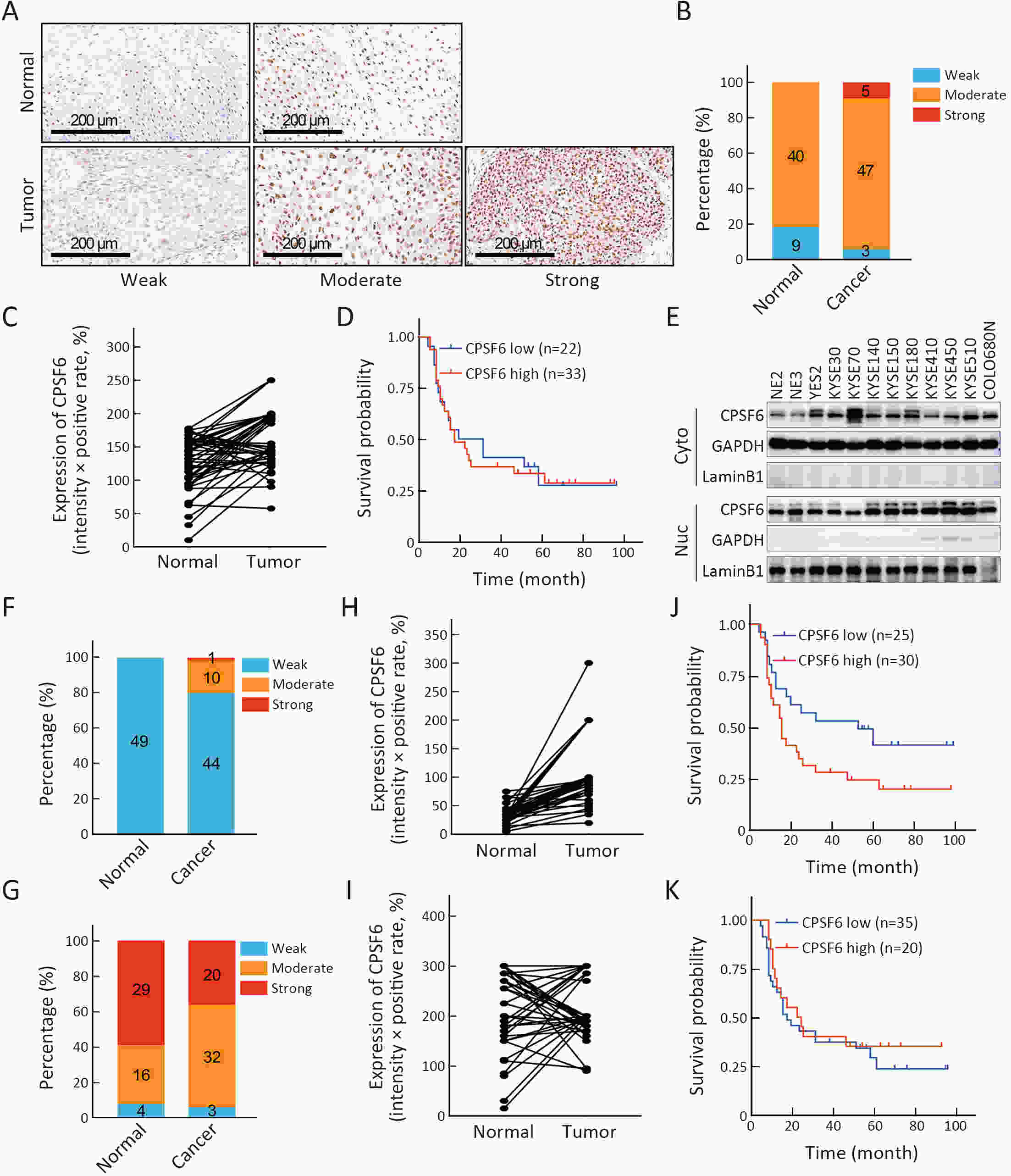
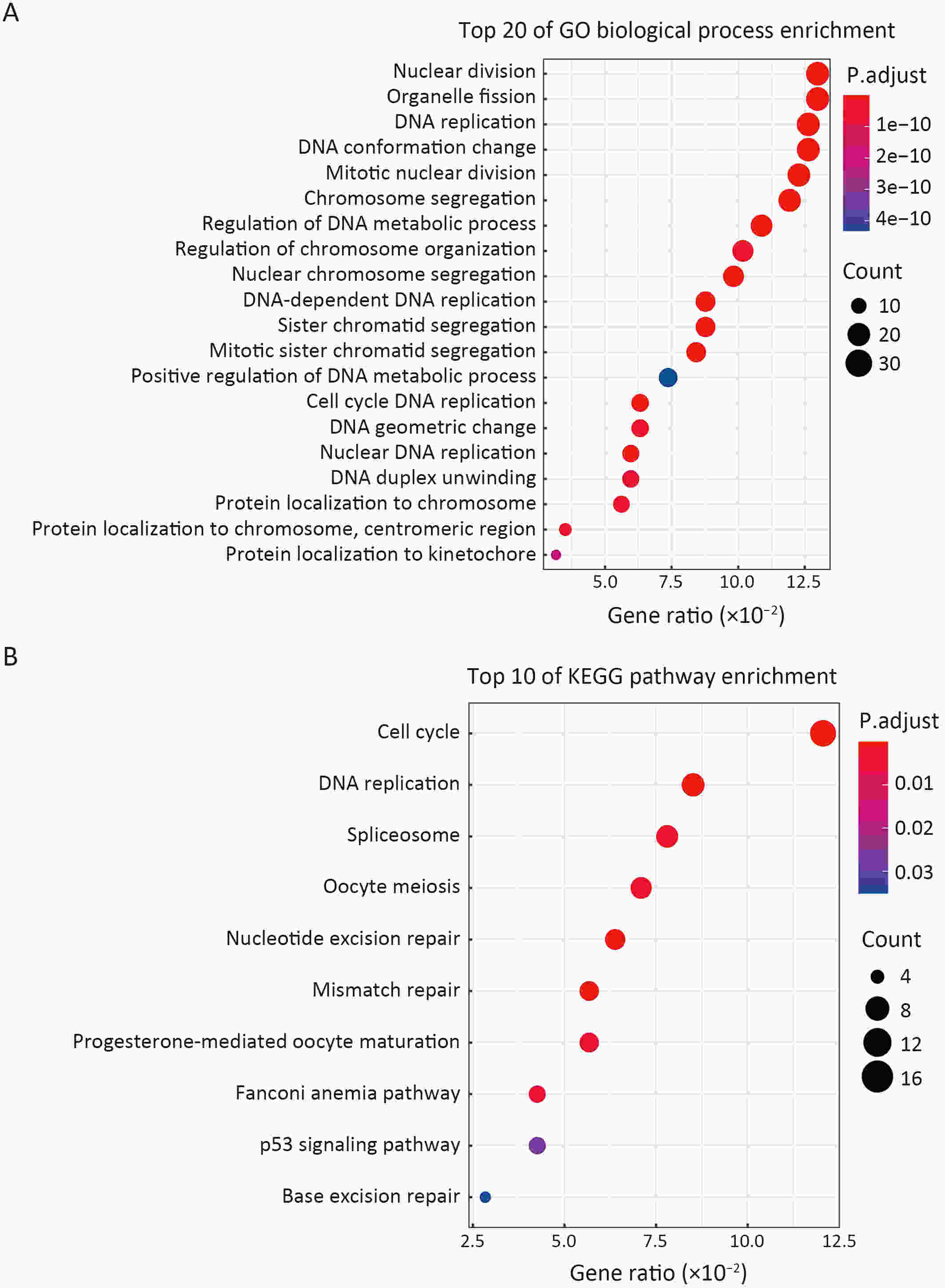
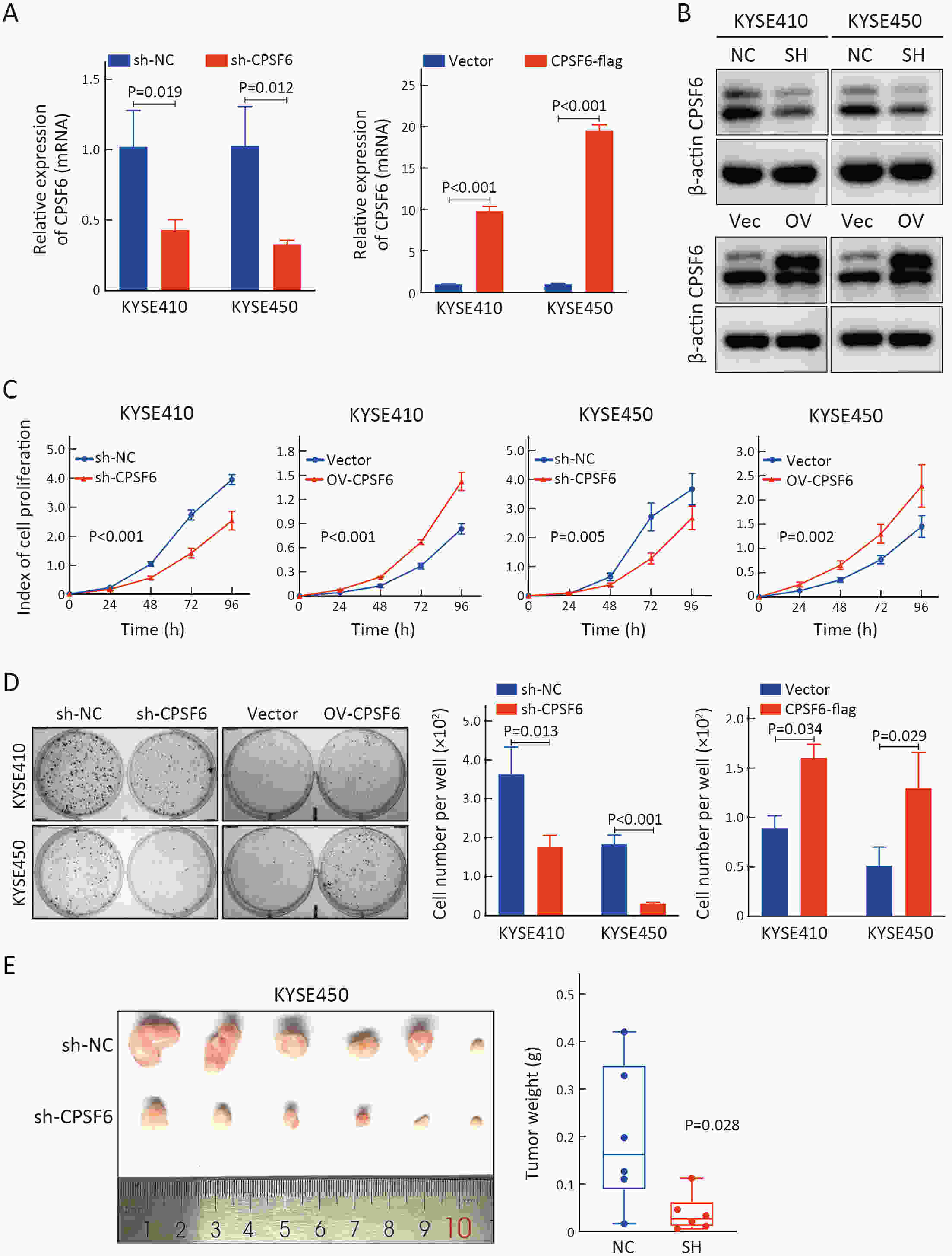
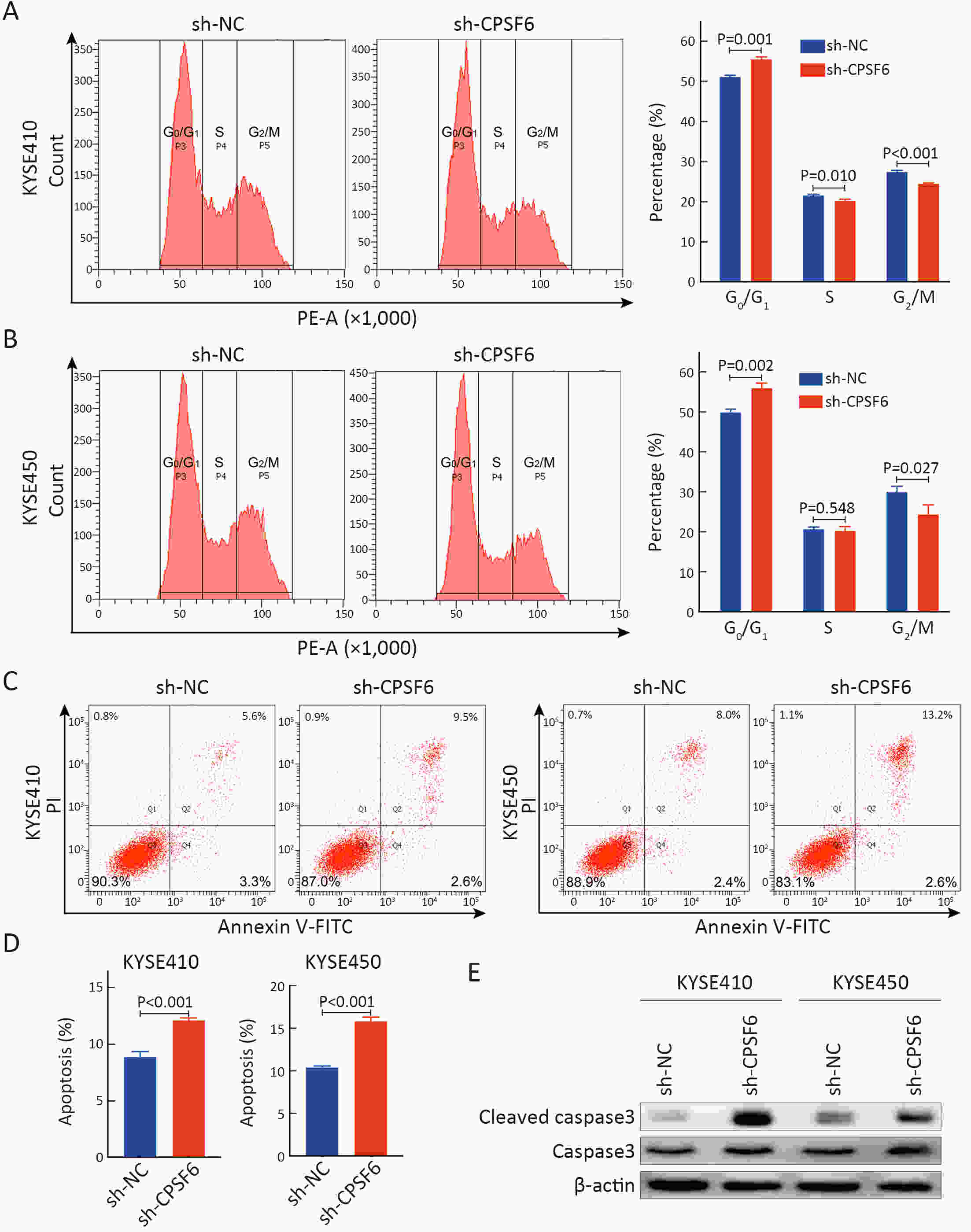
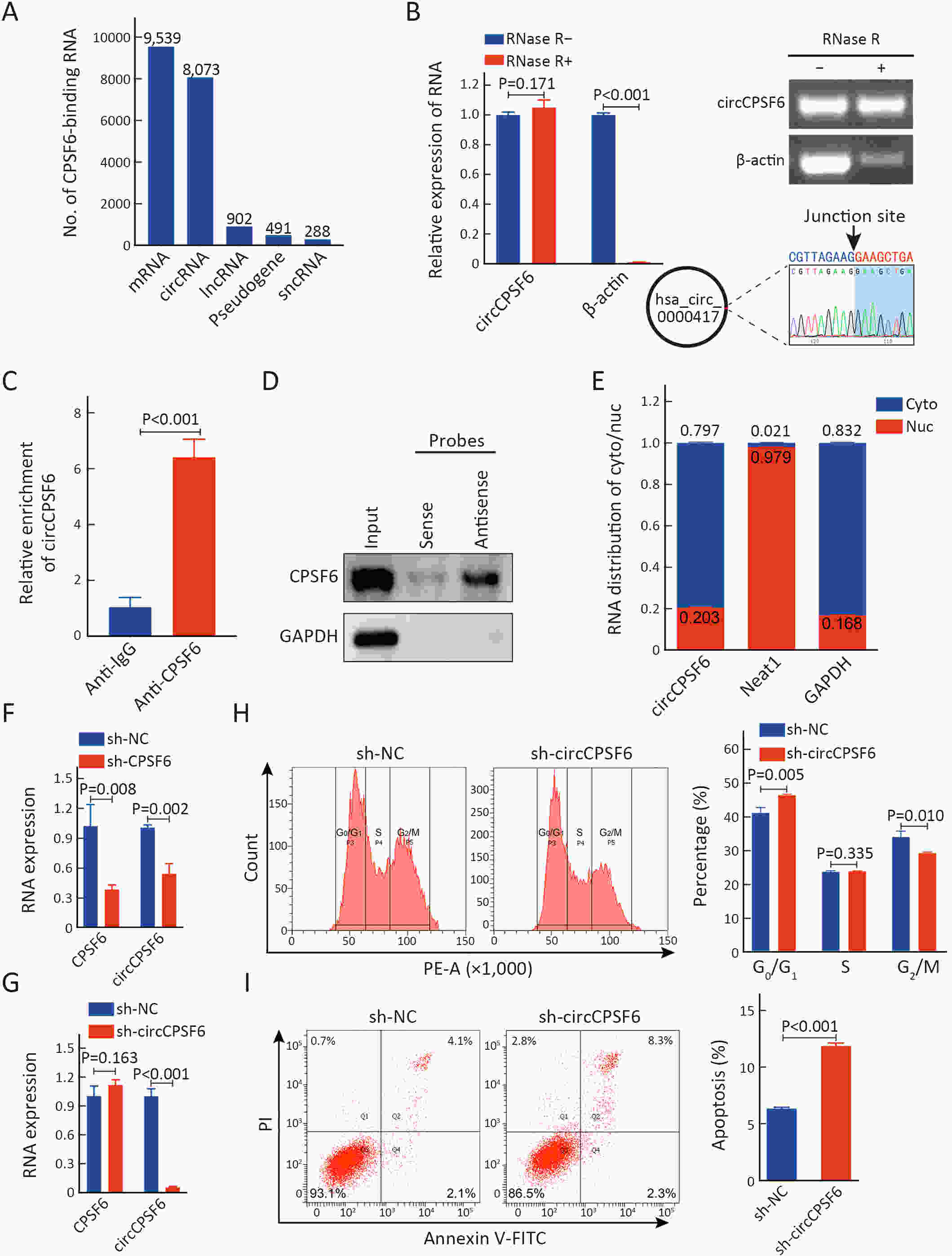
ObjectiveCleavage and polyadenylation specific factor 6 (CPSF6) has been documented as an oncoprotein in different types of cancer. However, functions of CPSF6 have not been investigated yet in esophageal squamous cell carcinoma (ESCC). Here, we aimed to investigate the potential clinical values and biological functions of CPSF6 in ESCC. MethodsFor determining the expression level of CPSF6 in ESCC patients, we analyzed published data, performed quantitative real-time polymerase chain reaction (RT-qPCR) and immunohistochemistry assays. Kaplan-Meier curves and log-rank tests were used for survival analyses. GO and KEGG analyses were done for CPSF6-related genes. Cell proliferation, colony formation and xenograft assays were conducted to verify the effects of CPSF6 on ESCC. In addition, cell cycle and apoptosis assays were also performed to manifest the functions of CPSF6 and circCPSF6. RNA pulldown and radioimmunoprecipitation (RIP) assays were used for confirming the interaction between circCPSF6 (hsa_circ_0000417) and CPSF6 protein. The regulatory relationship between CPSF6 protein and circCPSF6 was determined by RT-qPCR. ResultsWe found that CPSF6 was upregulated in ESCC tissues and overexpression of cytoplasmic CPSF6 was associated with poor prognosis. GO and KEGG analyses suggested that CPSF6 could mainly affect cell division in ESCC. Further experiments manifested that CPSF6 promoted cell proliferation and colony formation in vitro. Xenograft assay showed that knockdown of CPSF6 significantly decreased tumor growth rate in vivo. Subsequently, we verified that depletion of CPSF6 led to cell cycle arrest and apoptosis. Finally, we validated that CPSF6, as a circRNA-binding protein, interacted with and regulated its circular isoform circCPSF6 (hsa_circ_0000417), of which depletion also resulted in cell cycle arrest and cell apoptosis in ESCC. ConclusionsThese findings gave us insight that overexpression of cytoplasmic CPSF6 protein is associated with poor prognosis in ESCC and CPSF6 may function as an oncoprotein, at least in part, through regulating circCPSF6 expression.
2022, 34(1): 28-39.
doi: 10.21147/j.issn.1000-9604.2022.01.03
Abstract:
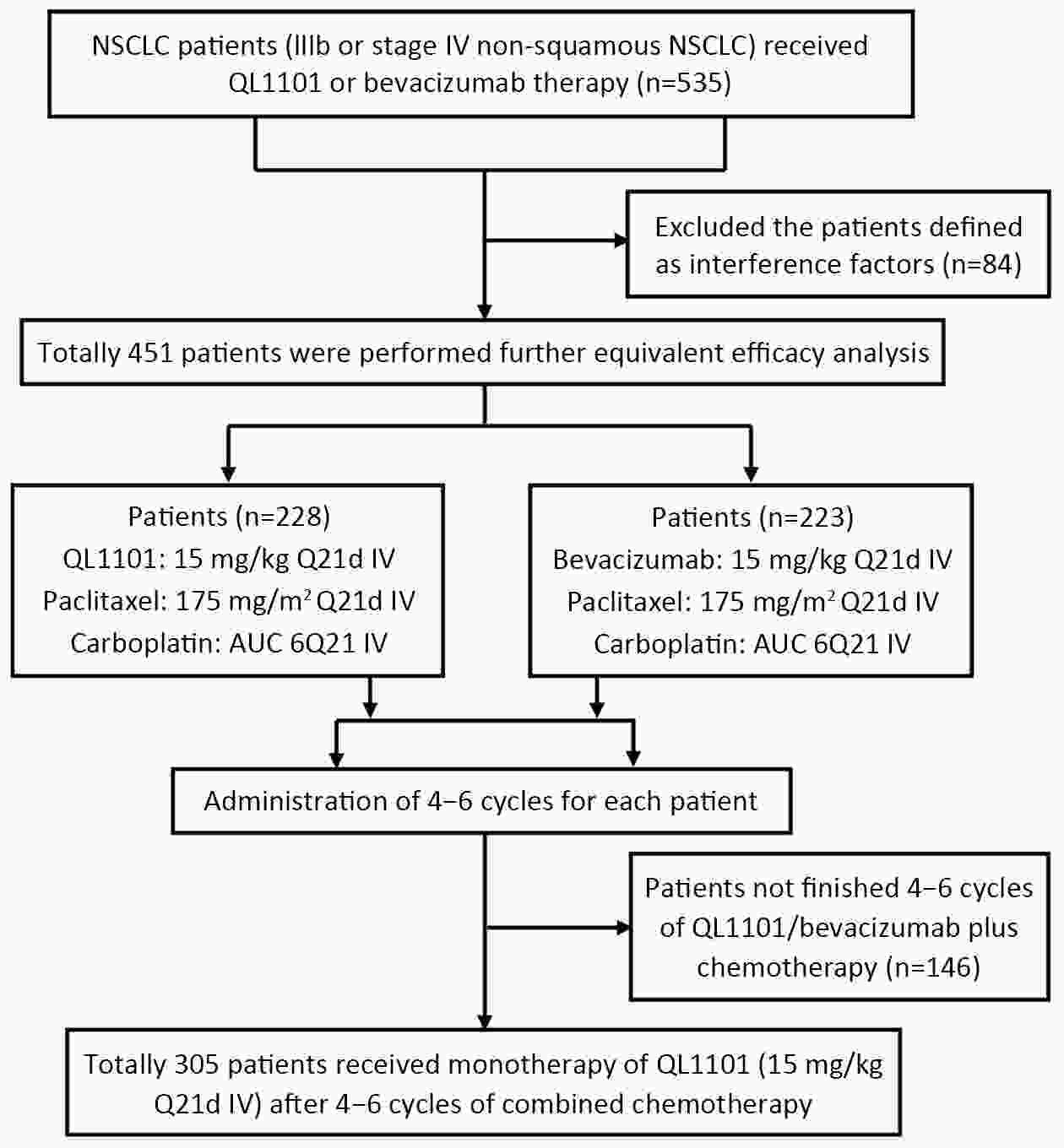
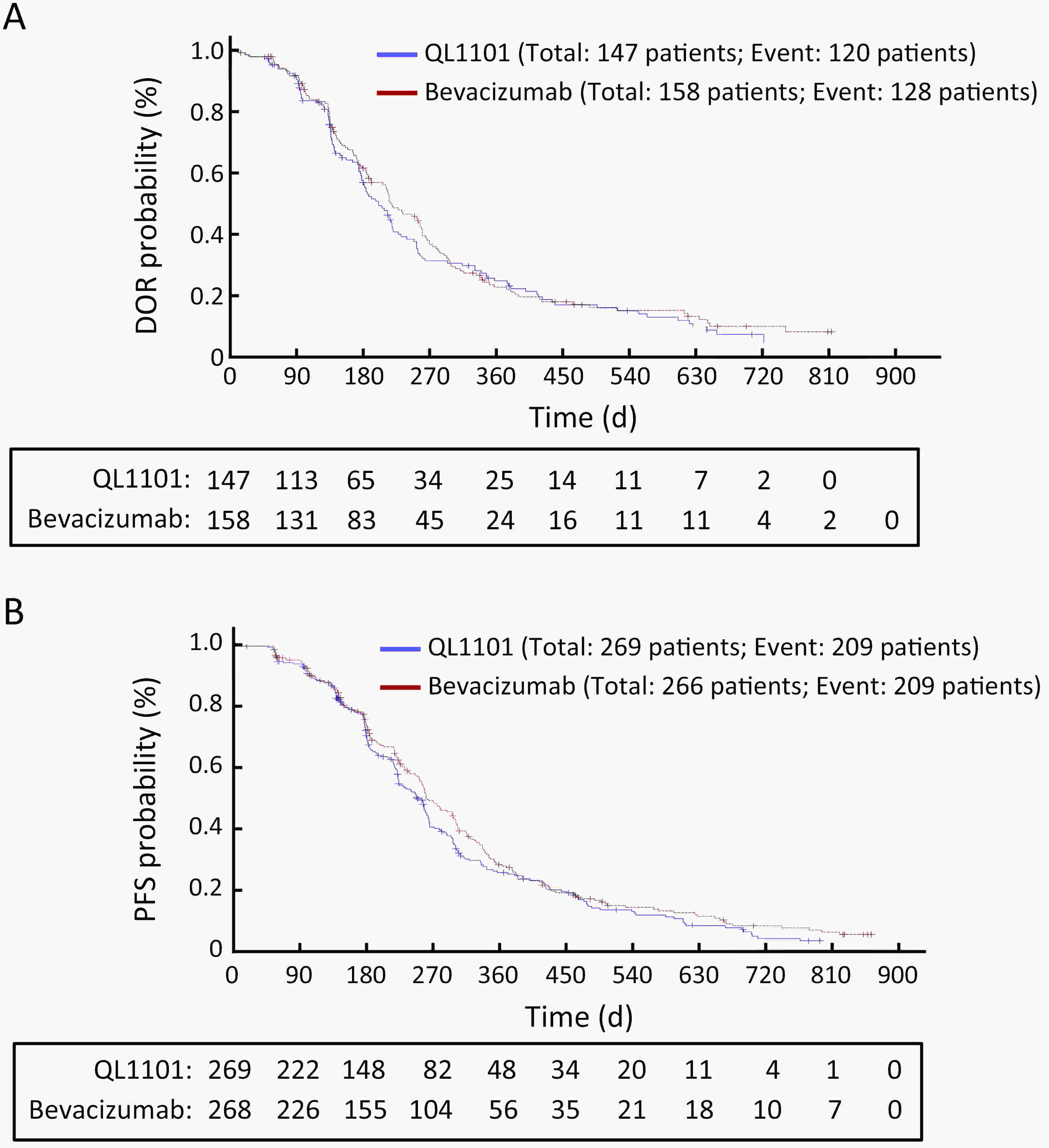
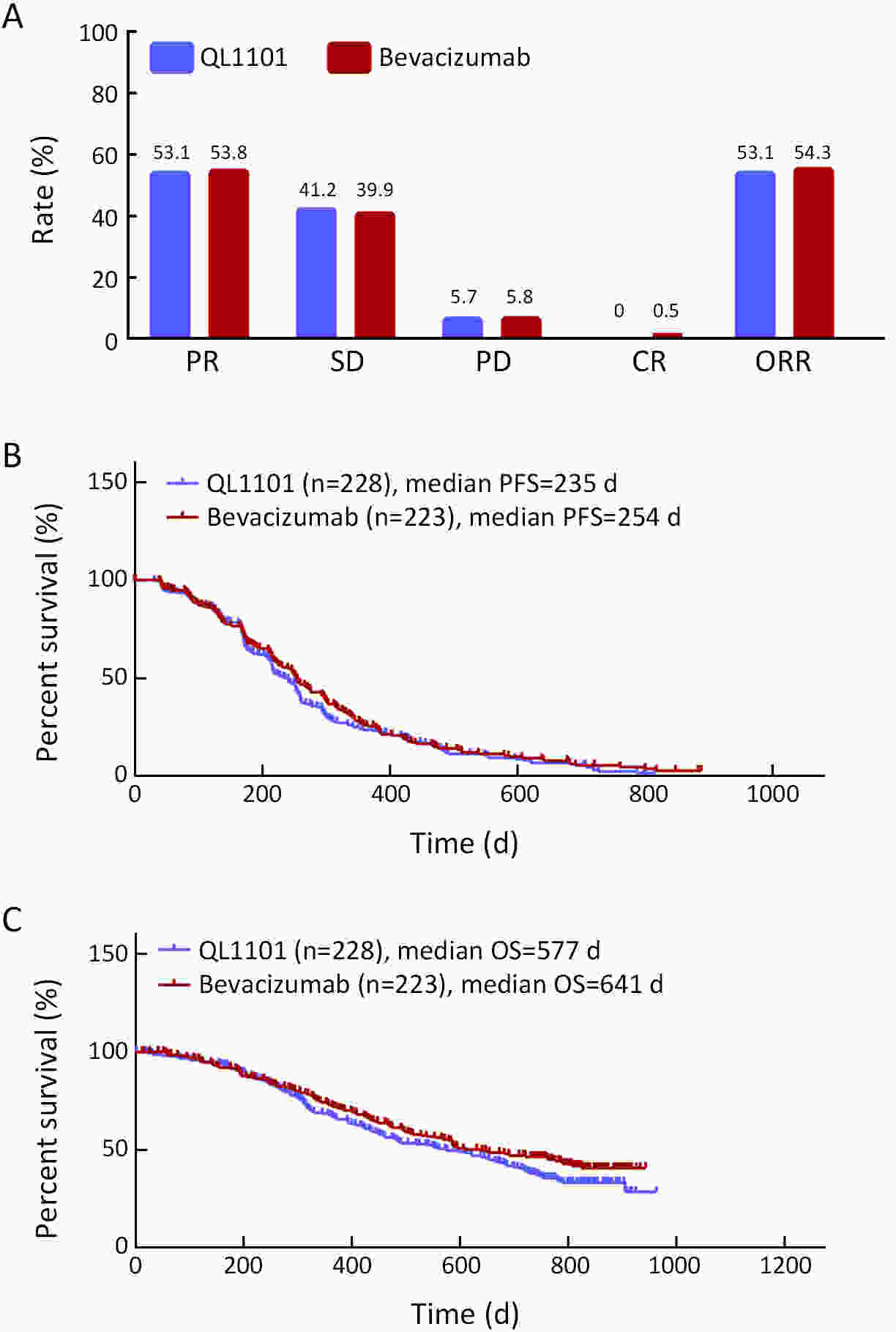
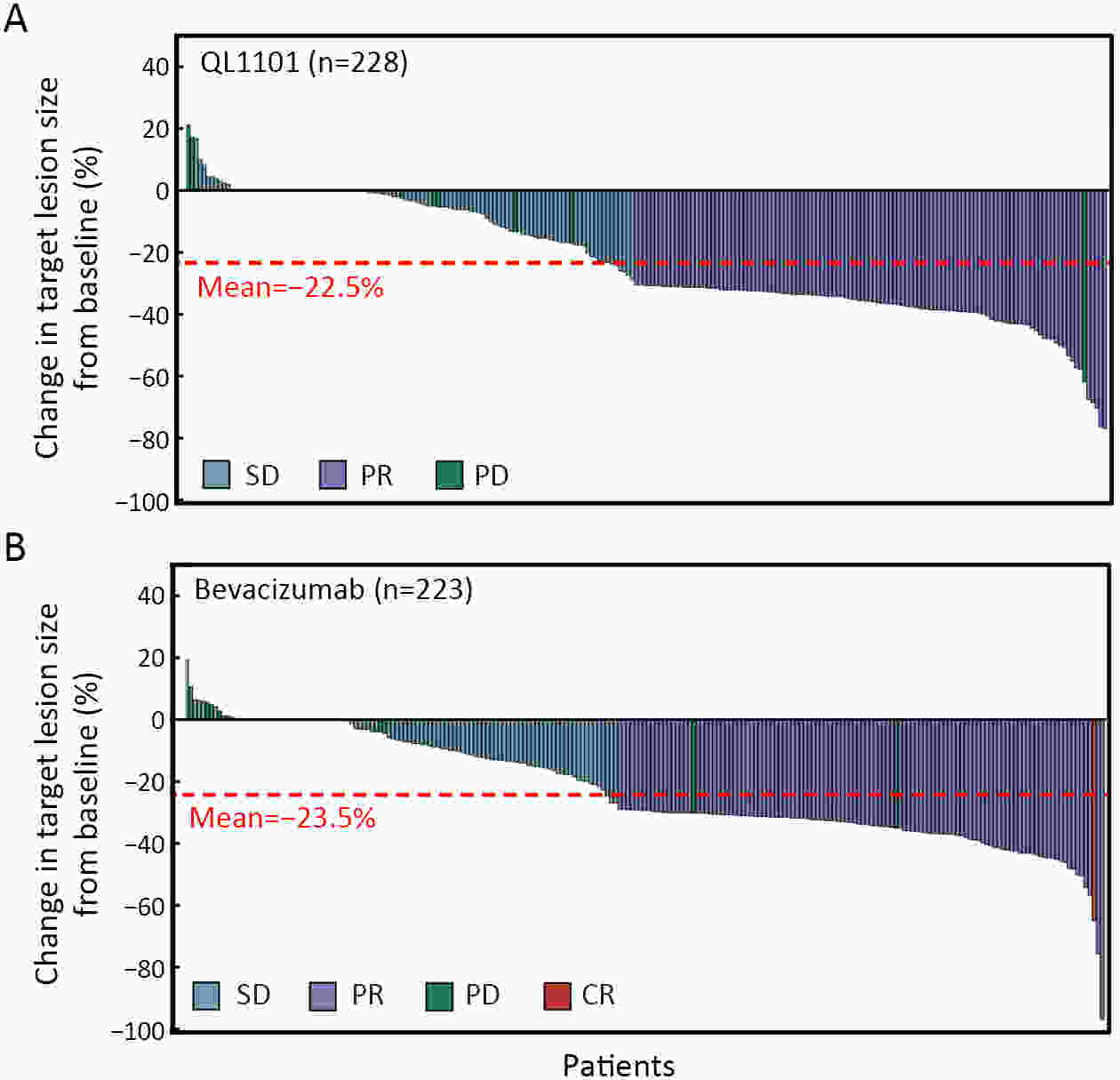
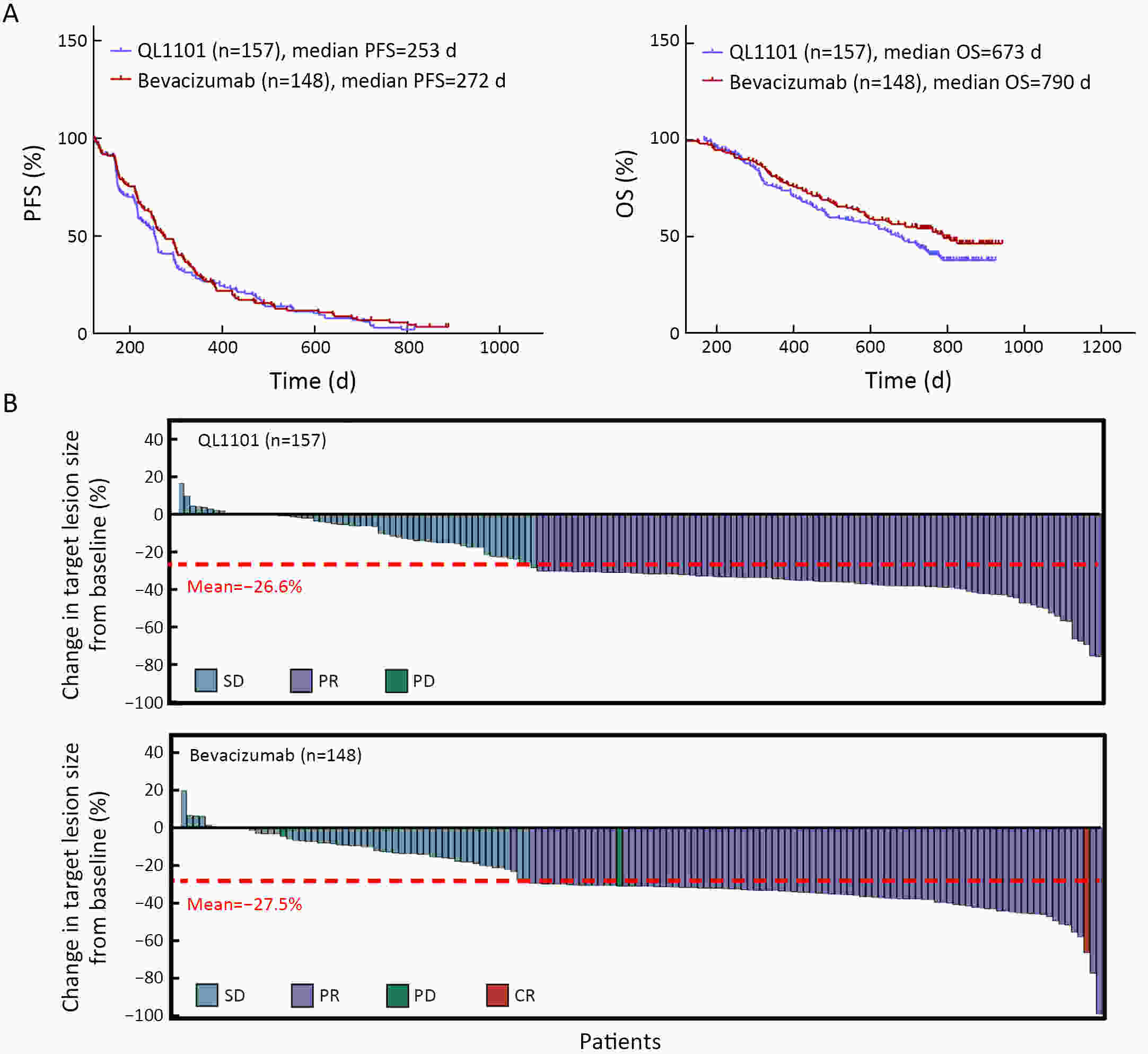
ObjectiveAnti-vascular endothelial growth factor (VEGF) monoclonal antibodies are an effective means of treating non-small cell lung cancer (NSCLC). Here, we aim to update the equivalent efficacy assessment between QL1101 and bevacizumab based on two-year follow-up data. MethodsIn total, 535 eligible NSCLC patients were enrolled in this randomized controlled trial. Patients were randomly assigned 1:1 to the QL1101 group and the bevacizumab group. The full end time of this study was defined as 24 months after the last enrolled patient was randomized. The primary endpoint was the objective response rate (ORR); equivalence was confirmed if the two-sided 90% confidence interval (90% CI) of the relative risk was within the range of 0.75−1.33. The secondary endpoints were progression-free survival (PFS) and overall survival (OS). ResultsThe two-year updated data showed similar ORR (QL1101 vs. bevacizumab: 53.1% vs. 54.3%; relative risk=0.977; 90% CI: 0.838−1.144), PFS (235 d vs. 254 d, log-rank P=0.311), and OS (577 d vs. 641 d, log-rank P=0.099) results between the QL1101 group and the bevacizumab group. The mean shrinkage ratio of targeted lesions was also similar between the QL1101 group and the bevacizumab group (22.5% vs. 23.5%). For patients who received QL1101 maintenance therapy, similar results were shown between the QL1101 group (n=157) and the bevacizumab group (n=148) (PFS: 253 d vs. 272 d, log-rank P=0.387; OS: 673 d vs. 790 d, log-rank P=0.101; mean tumor shrinkage rate: 26.6% vs. 27.5%). ConclusionsThis study reported that QL1101 had similar efficacy in treating nonsquamous NSCLC in terms of ORR, PFS and OS based on two-year updated data, providing a basis for the clinical application of QL1101.
2022, 34(1): 40-52.
doi: 10.21147/j.issn.1000-9604.2022.01.04
Abstract:
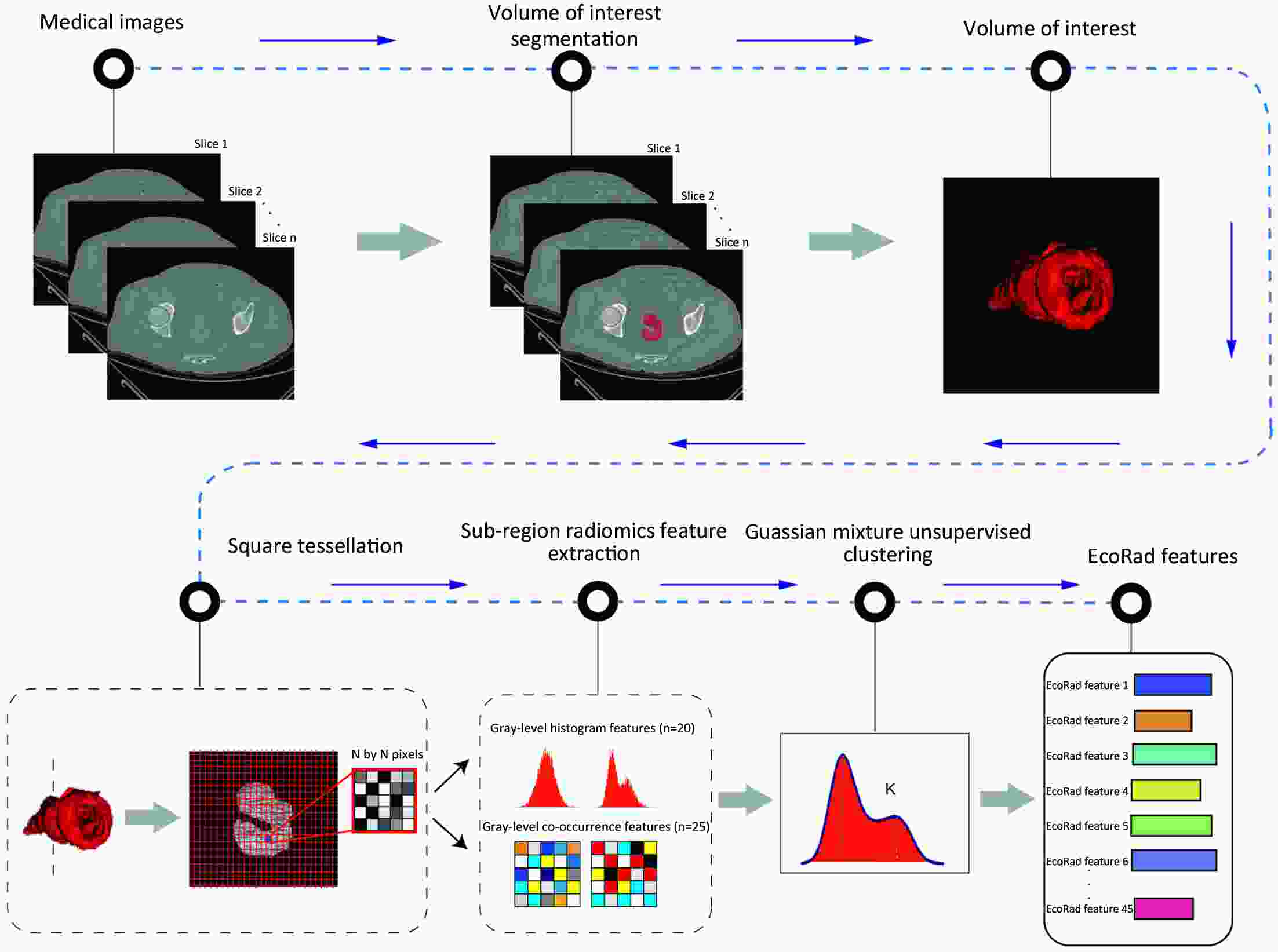
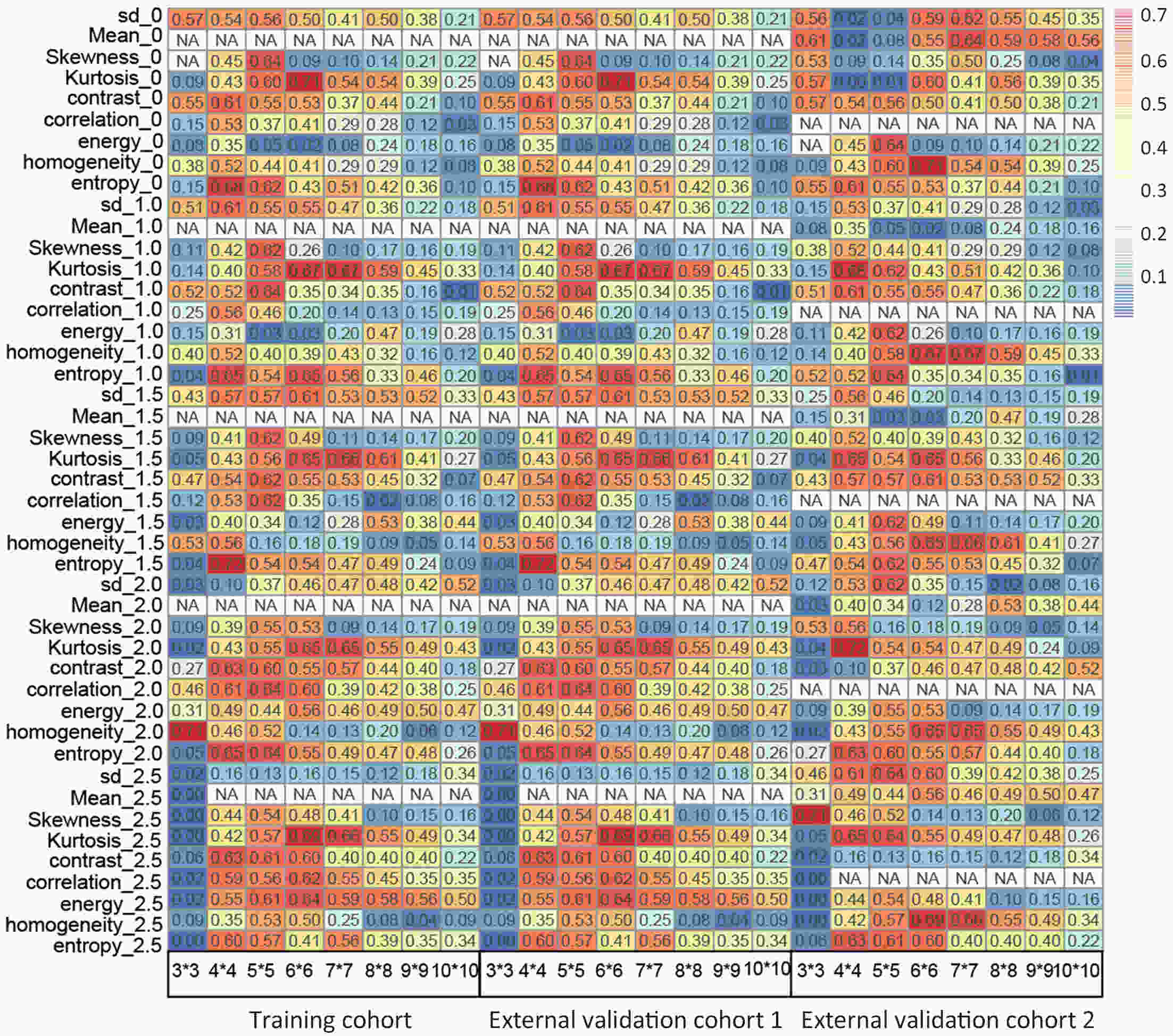
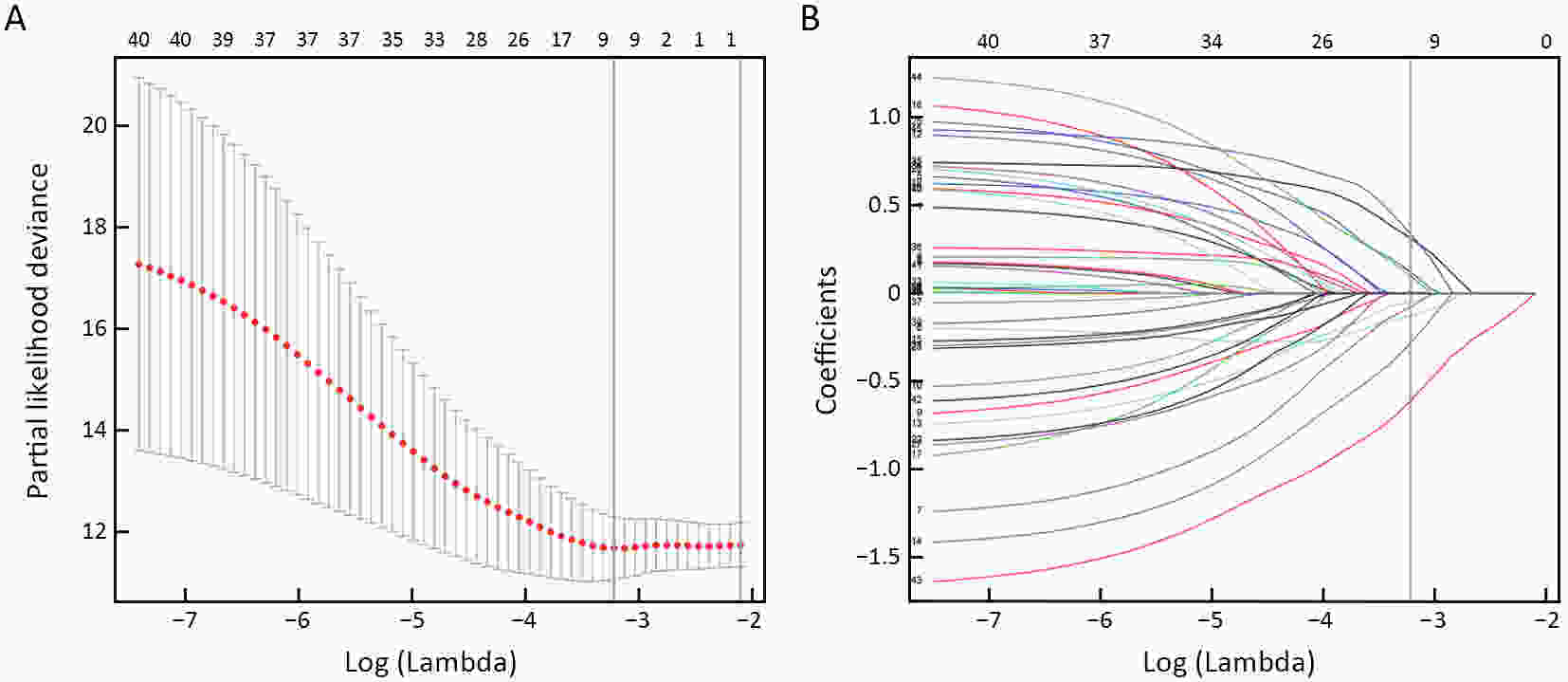
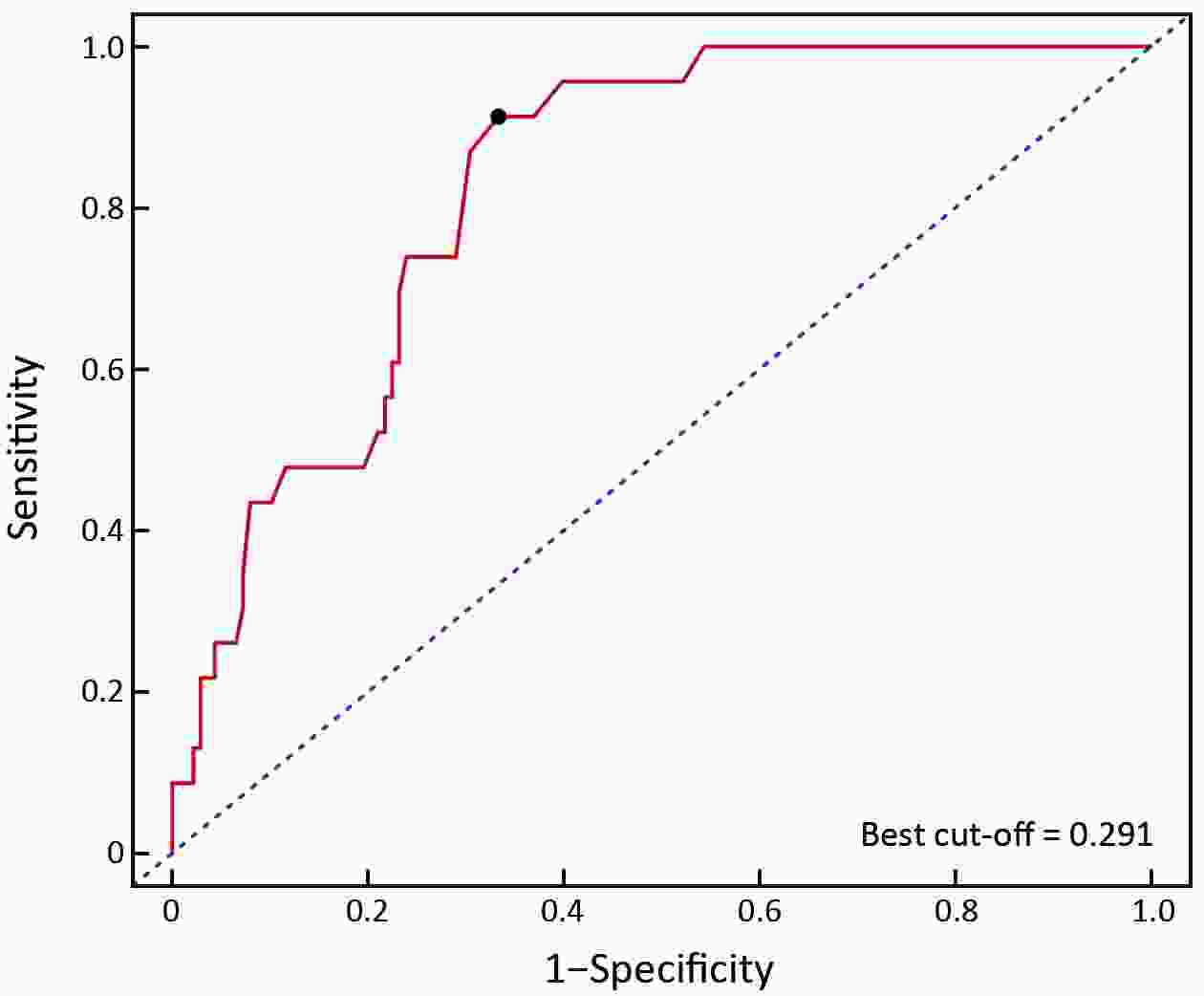
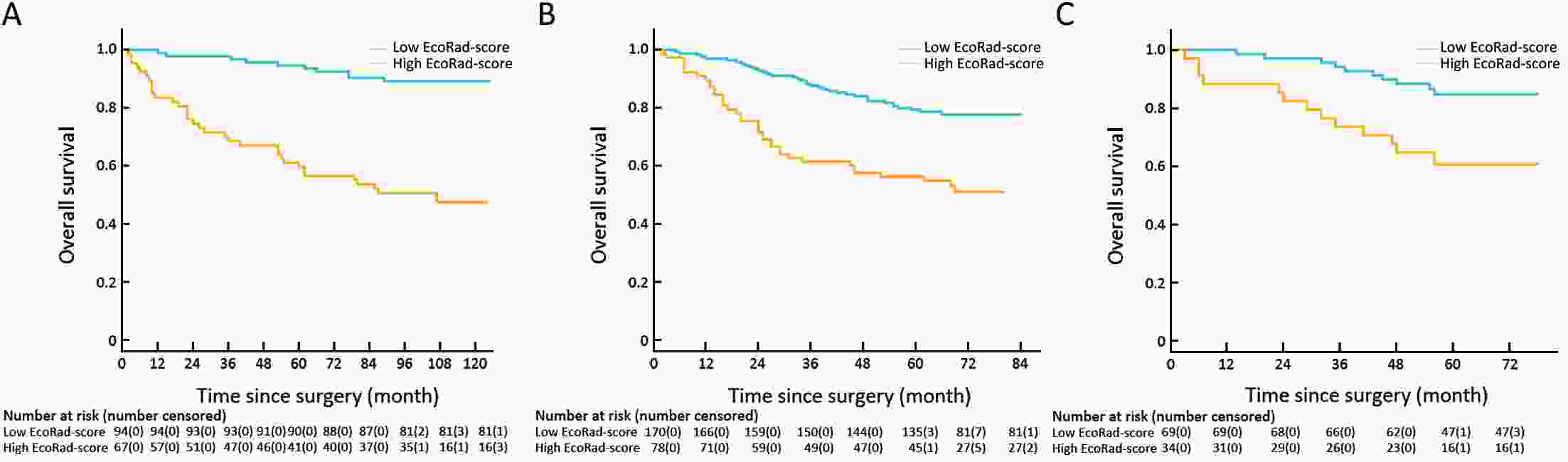
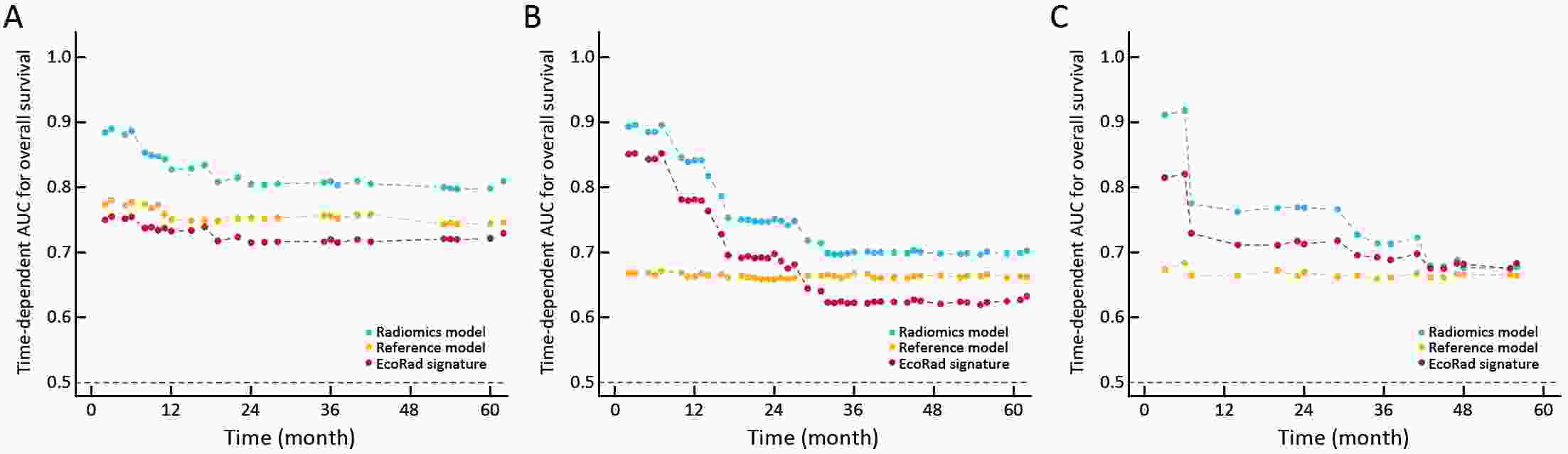
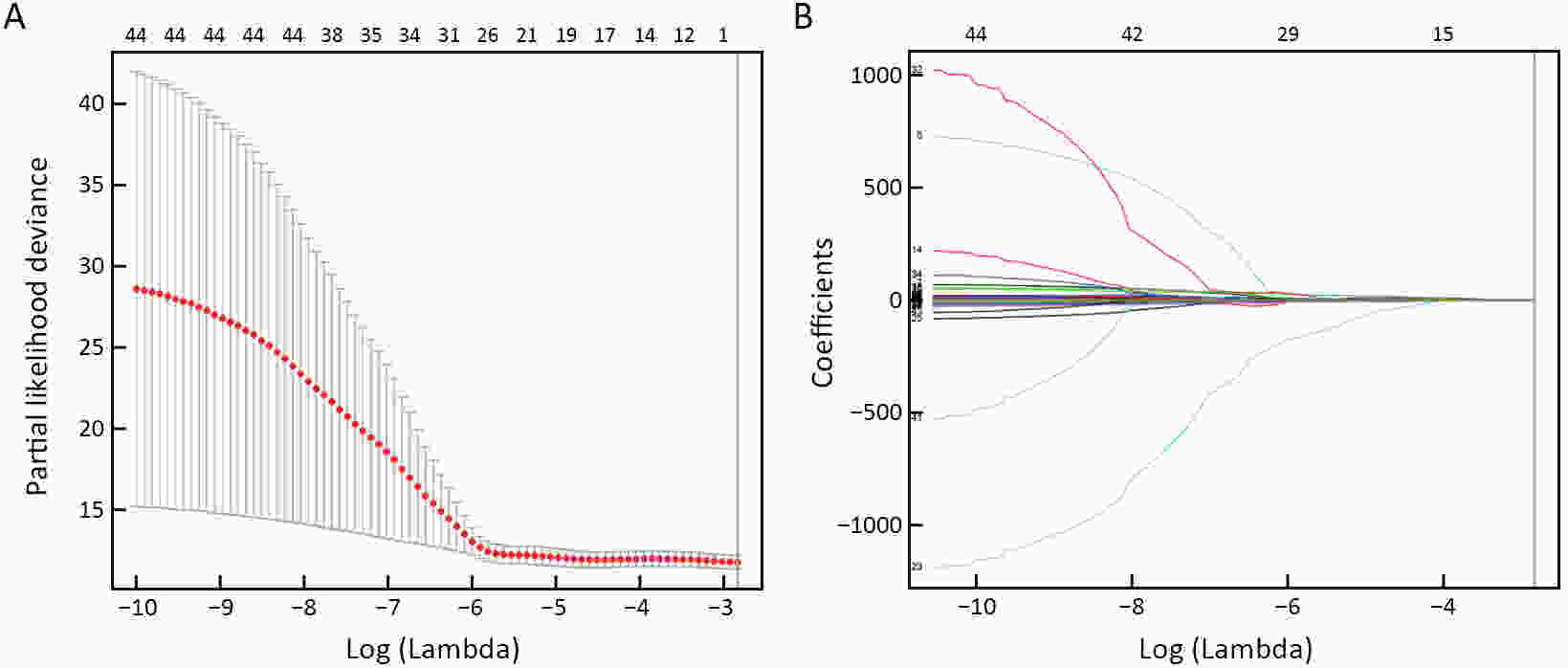
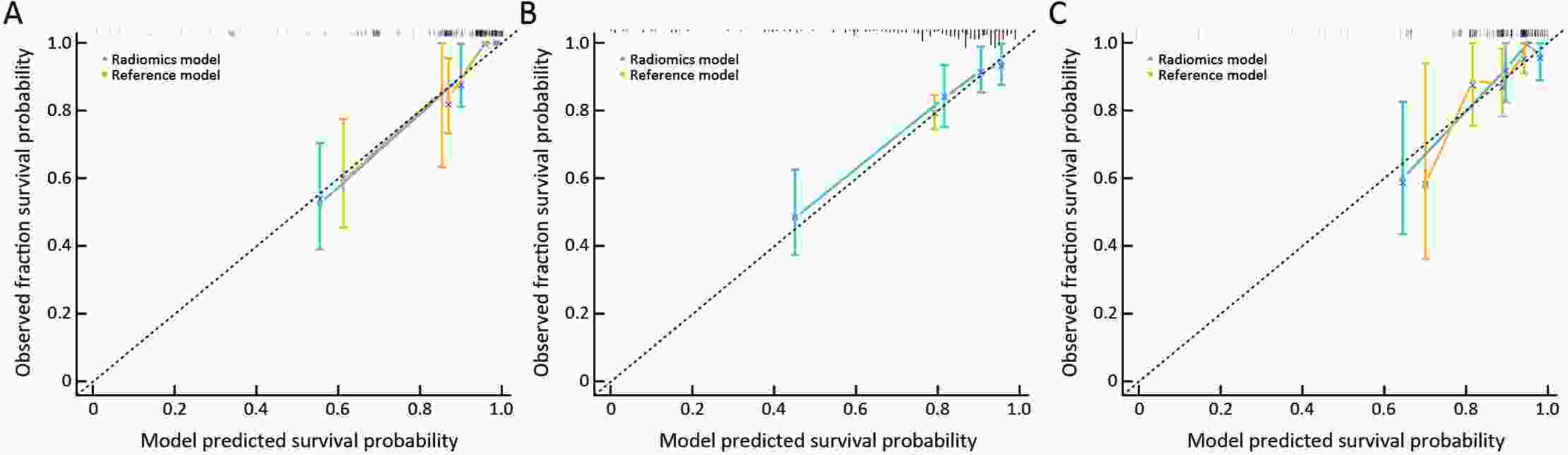
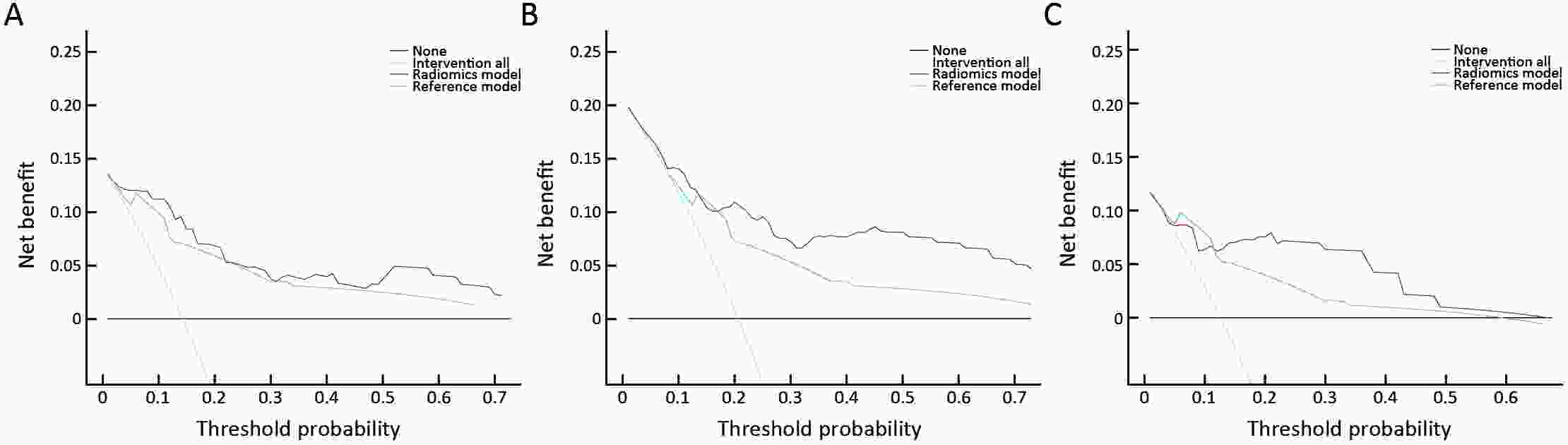
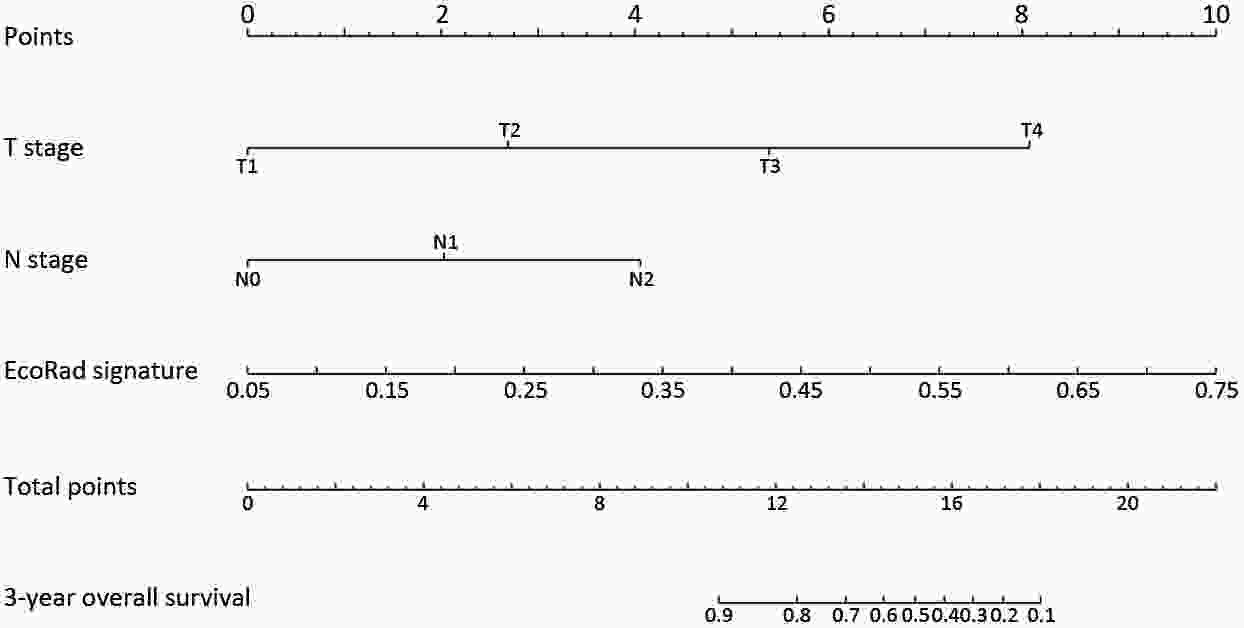
ObjectiveThis study aimed to establish a method to predict the overall survival (OS) of patients with stage I−III colorectal cancer (CRC) through coupling radiomics analysis of CT images with the measurement of tumor ecosystem diversification. MethodsWe retrospectively identified 161 consecutive patients with stage I−III CRC who had underwent radical resection as a training cohort. A total of 248 patients were recruited for temporary independent validation as external validation cohort 1, with 103 patients from an external institute as the external validation cohort 2. CT image features to describe tumor spatial heterogeneity leveraging the measurement of diversification of tumor ecosystem, were extracted to build a marker, termed the EcoRad signature. Multivariate Cox regression was used to assess the EcoRad signature, with a prediction model constructed to demonstrate its incremental value to the traditional staging system for OS prediction. ResultsThe EcoRad signature was significantly associated with OS in the training cohort [hazard ratio (HR)=6.670; 95% confidence interval (95% CI): 3.433−12.956; P<0.001), external validation cohort 1 (HR=2.866; 95% CI: 1.646−4.990; P<0.001) and external validation cohort 2 (HR=3.342; 95% CI: 1.289−8.663; P=0.002). Incorporating the EcoRad signature into the prediction model presented a higher prediction ability (P<0.001) with respect to the C-index (0.813, 95% CI: 0.804−0.822 in the training cohort; 0.758, 95% CI: 0.751−0.765 in the external validation cohort 1; and 0.746, 95% CI: 0.722−0.770 in external validation cohort 2), compared with the reference model that only incorporated tumor, node, metastasis (TNM) system, as well as a better calibration, improved reclassification and superior clinical usefulness. ConclusionsThis study establishes a method to measure the spatial heterogeneity of CRC through coupling radiomics analysis with measurement of diversification of the tumor ecosystem, and suggests that this approach could effectively predict OS and could be used as a supplement for risk stratification among stage I−III CRC patients.
2022, 34(1): 53-62.
doi: 10.21147/j.issn.1000-9604.2022.01.05
Abstract:
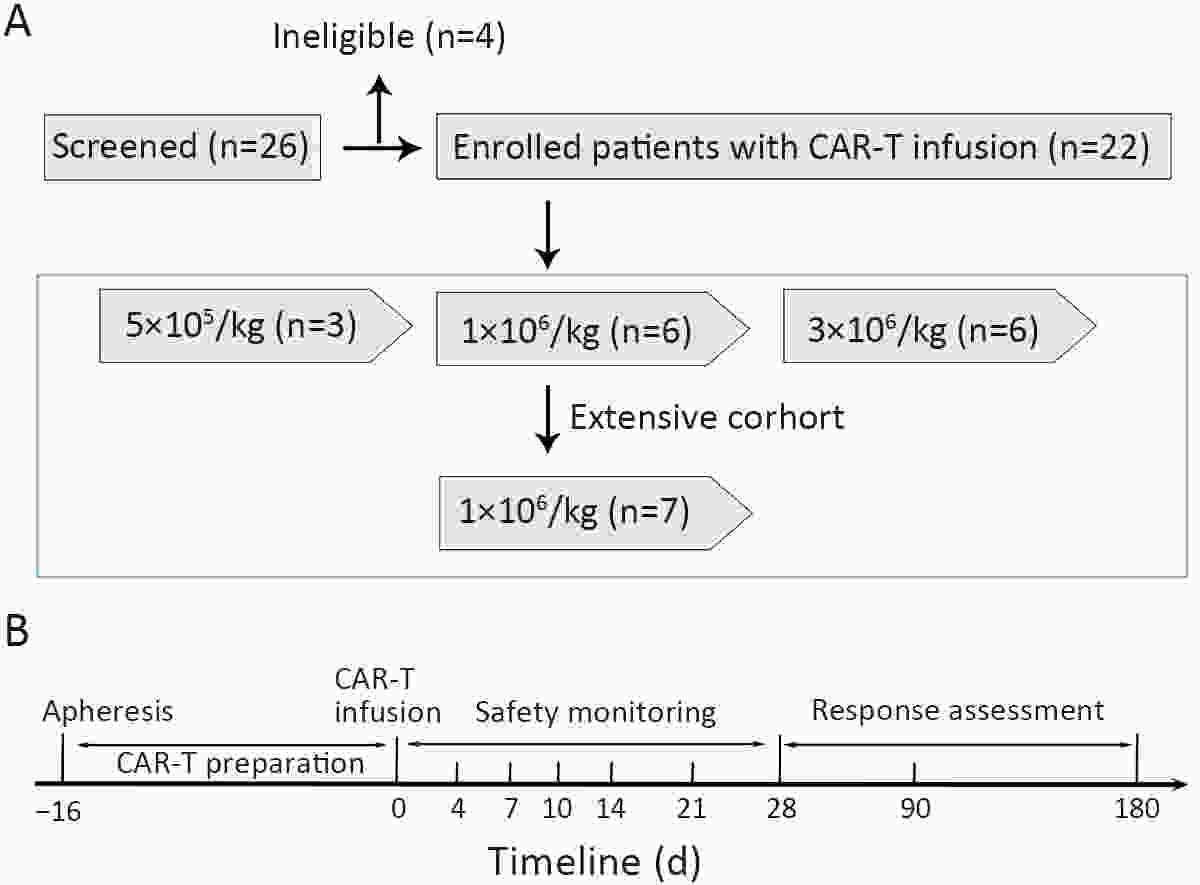
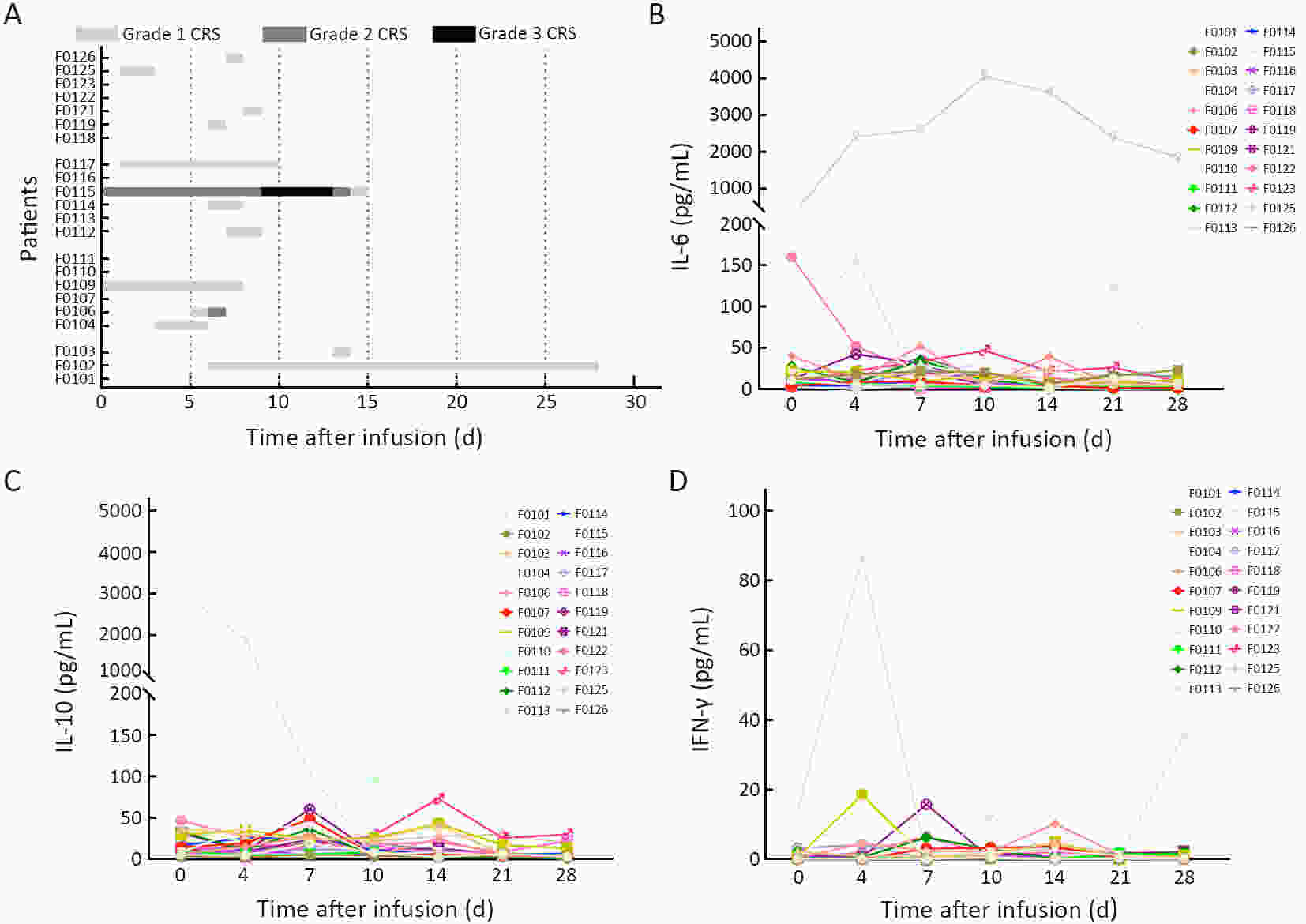
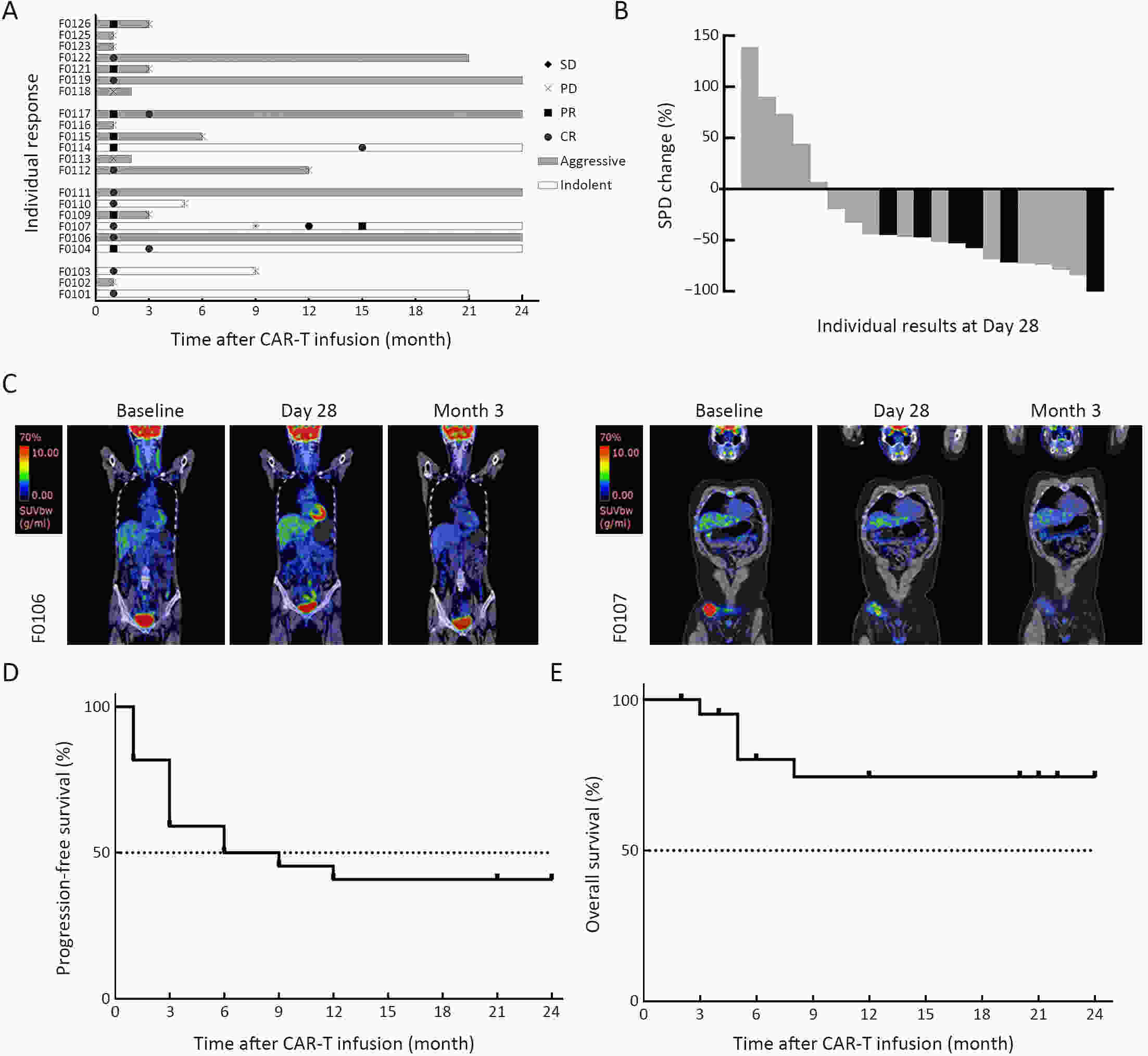
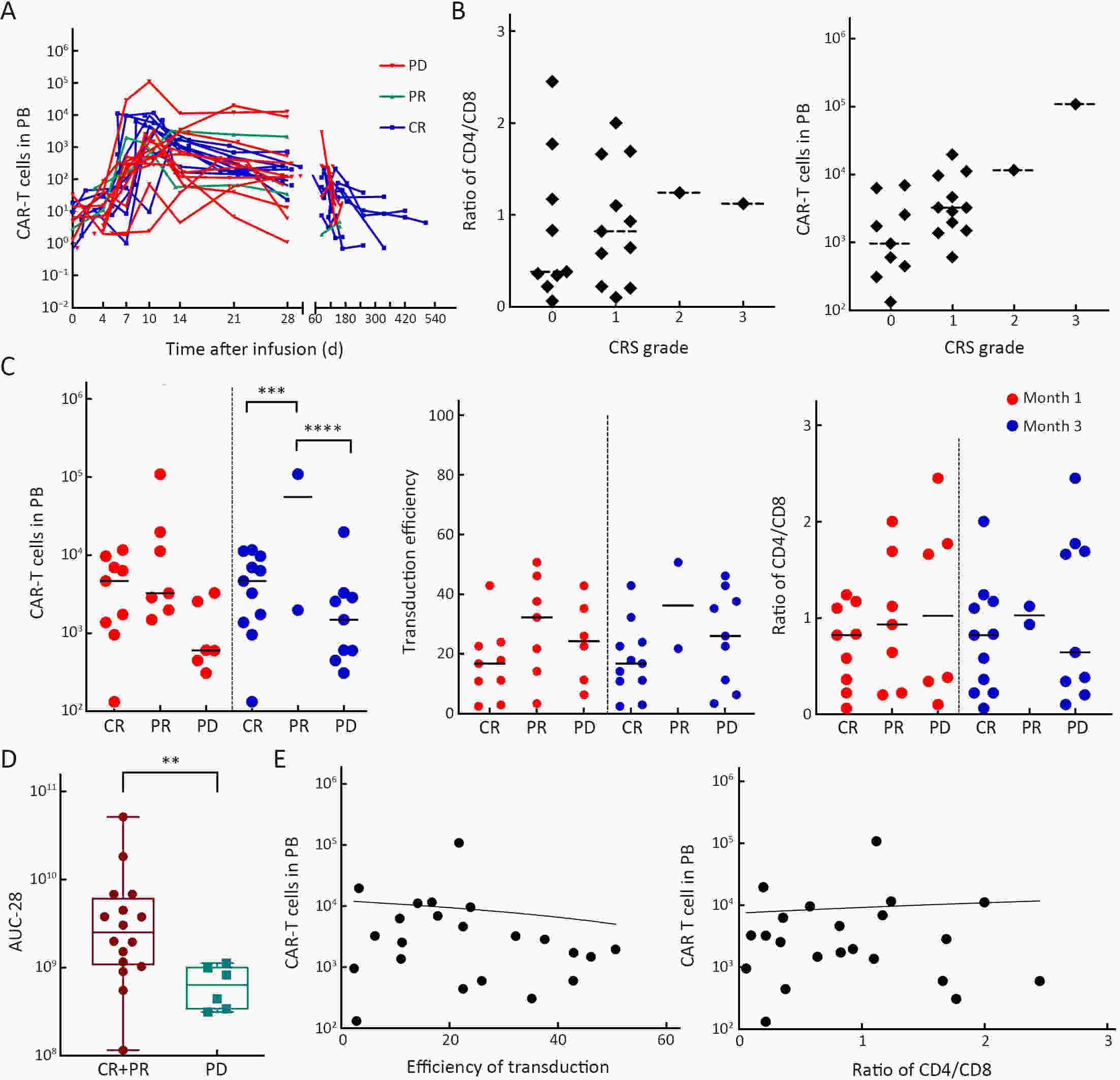
ObjectivePrevious studies reported that 4-1BB-based CD19 chimeric antigen receptor (CAR)-T cells were more beneficial for the clinical outcomes than CD28-based CAR-T cells, especially the lower incidence rate of severe adverse events. However, the median progression-free survival (mPFS) of 4-1BB-based product Kymriah was shorter than that of CD28-based Yescarta (2.9 months vs. 5.9 months), suggesting that Kymriah was limited in the long-term efficacy. Thus, a safe and durable 4-1BB-based CD19 CAR-T needs to be developed. MethodsWe designed a CD19-targeted CAR-T (named as IM19) which consisted of an FMC63 scFv, 4-1BB and CD3ζ intracellular domain and was manufactured into a memory T-enriched formulation. A phase I/II clinical trial was launched to evaluate the clinical outcomes of IM19 in relapsed or refractory (r/r) B cell non-Hodgkin lymphoma (B-NHL). Dose-escalation investigation (at a dose of 5×105/kg, 1×106/kg and 3×106/kg) was performed in 22 r/r B-NHL patients. All patients received a single infusion of IM19 after 3-day conditional regimen. ResultsAt month 3, the overall response rate (ORR) was 59.1%, the complete response rate (CRR) was 50.0%. The mPFS was 6 months and the 1-year overall survival rate was 77.8%. Cytokine release syndrome (CRS) occurred in 13 patients (59.1%), with 54.5% of grade 1−2 CRS. Only one patient (4.5%) experienced grade 3 CRS and grade 3 neurotoxicity. ConclusionsThese results demonstrated the safety and durable efficacy of a 4-1BB-based CD19 CAR-T, IM19, which is promising for further development and clinical investigation.
2022, 34(1): 63-65.
doi: 10.21147/j.issn.1000-9604.2022.01.06
Abstract:
The benefits and popularity of minimally invasive surgery are undeniable around the globe. However, open surgery is necessary and learning open surgery skills is still a necessity. Open surgery allows for better exposure to the surgical field and provides tactile sensation to facilitate the stereo visual assessment to precisely remove the lesion. Open surgery is still the key to surgical training, and the skills learned from open surgeries remain crucial for unforeseen circumstances and certain conditions like emergencies, challenge cases, or patients with compromised status.
The benefits and popularity of minimally invasive surgery are undeniable around the globe. However, open surgery is necessary and learning open surgery skills is still a necessity. Open surgery allows for better exposure to the surgical field and provides tactile sensation to facilitate the stereo visual assessment to precisely remove the lesion. Open surgery is still the key to surgical training, and the skills learned from open surgeries remain crucial for unforeseen circumstances and certain conditions like emergencies, challenge cases, or patients with compromised status.
2022, 34(1): 66-66.
doi: 10.21147/j.issn.1000-9604.2022.01.07
Abstract:

 Abstract
Abstract FullText HTML
FullText HTML PDF 1405KB
PDF 1405KB