2022 Vol.34(2)
Display Mode: |
2022, 34(2): 67-70.
doi: 10.21147/j.issn.1000-9604.2022.02.01
Abstract:
2022, 34(2): 71-82.
doi: 10.21147/j.issn.1000-9604.2022.02.02
Abstract:
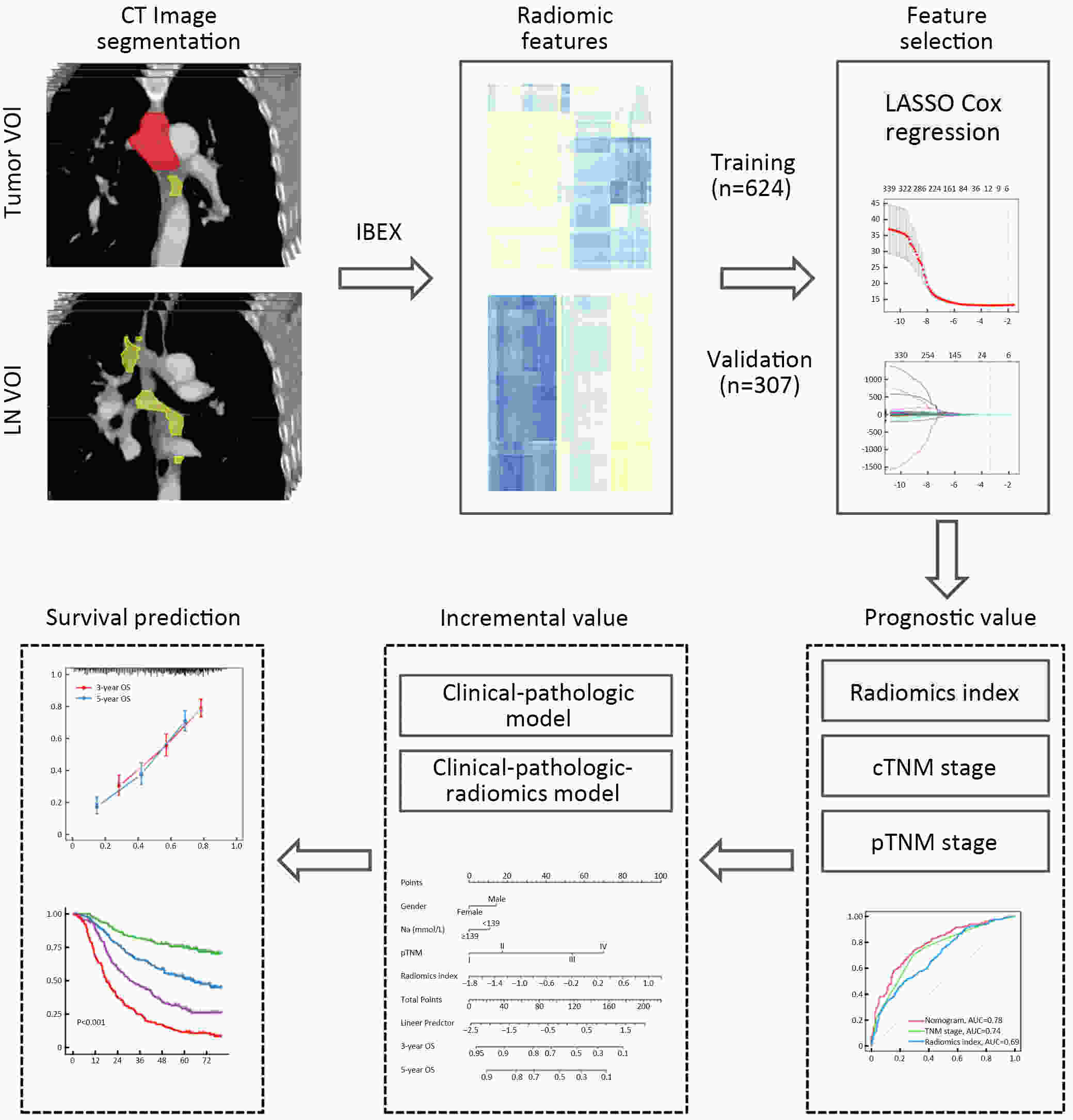
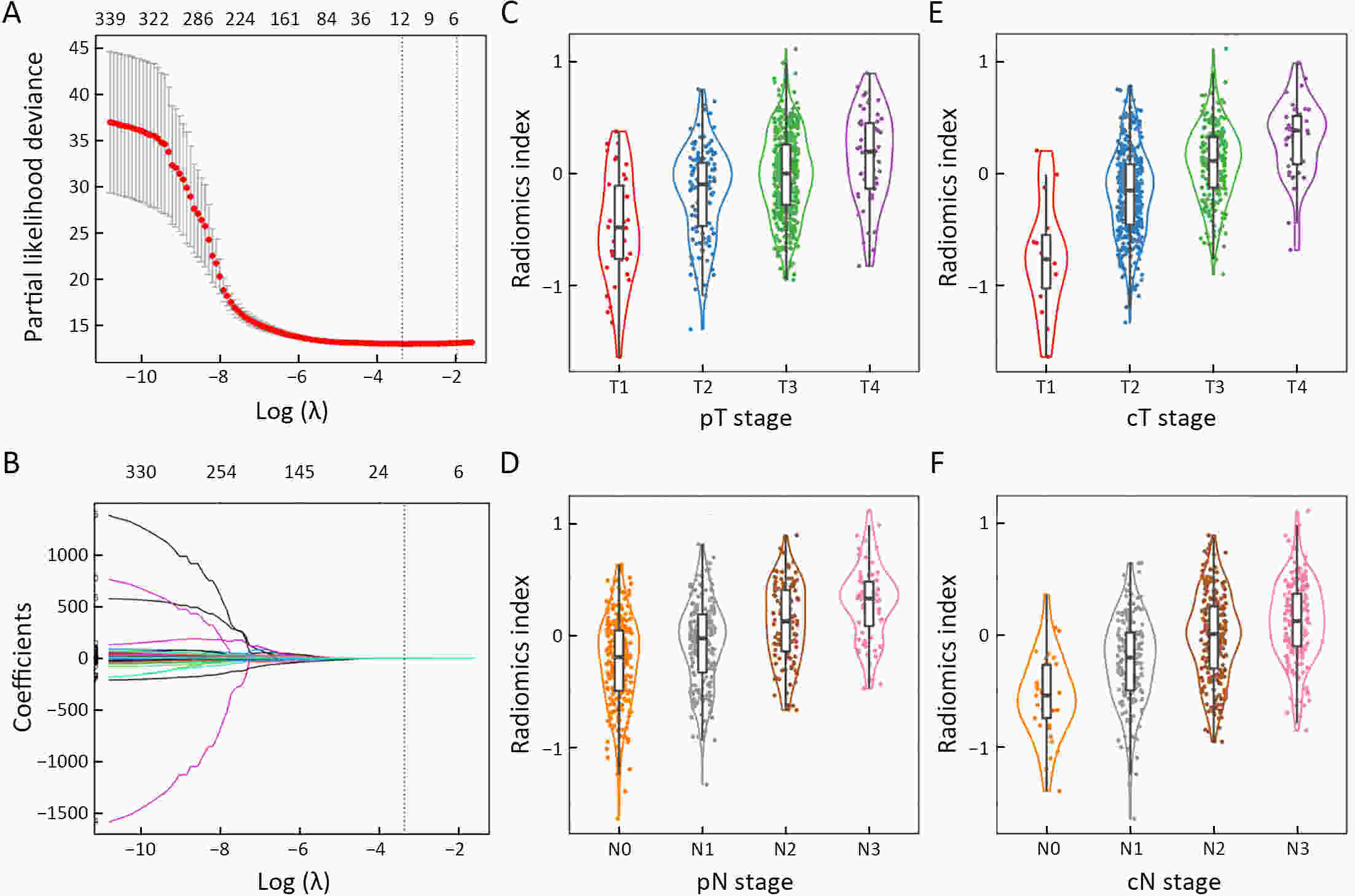
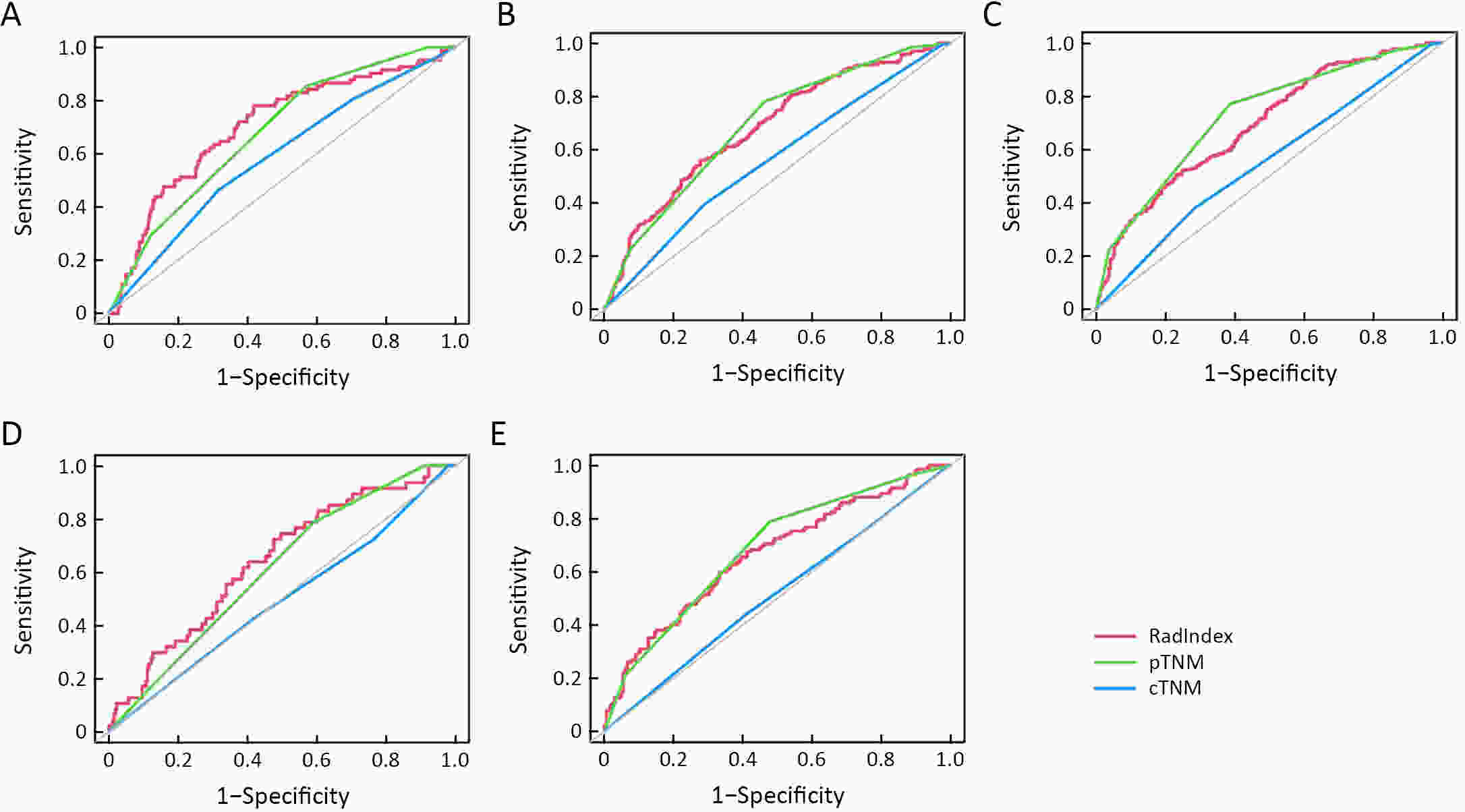
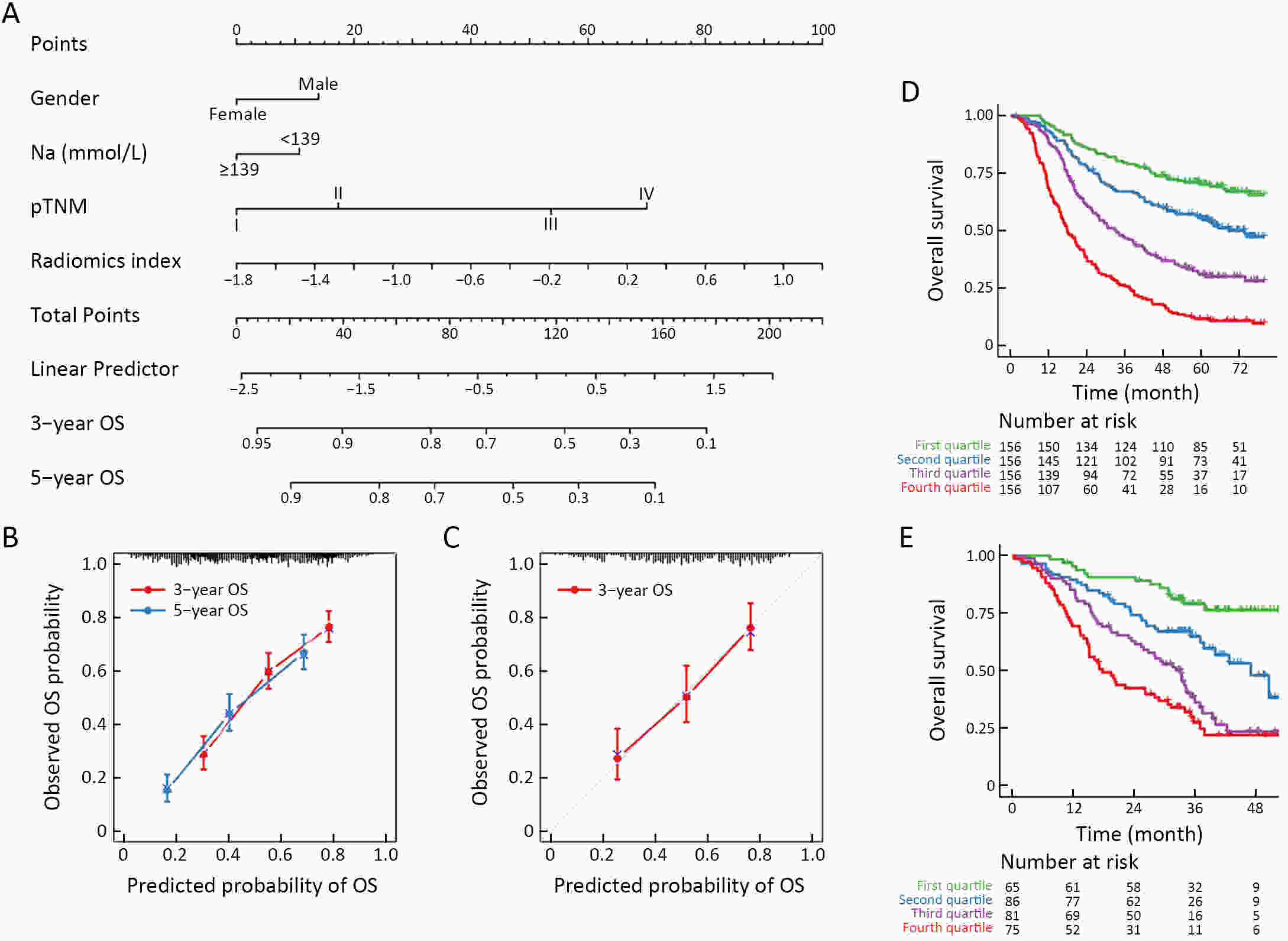
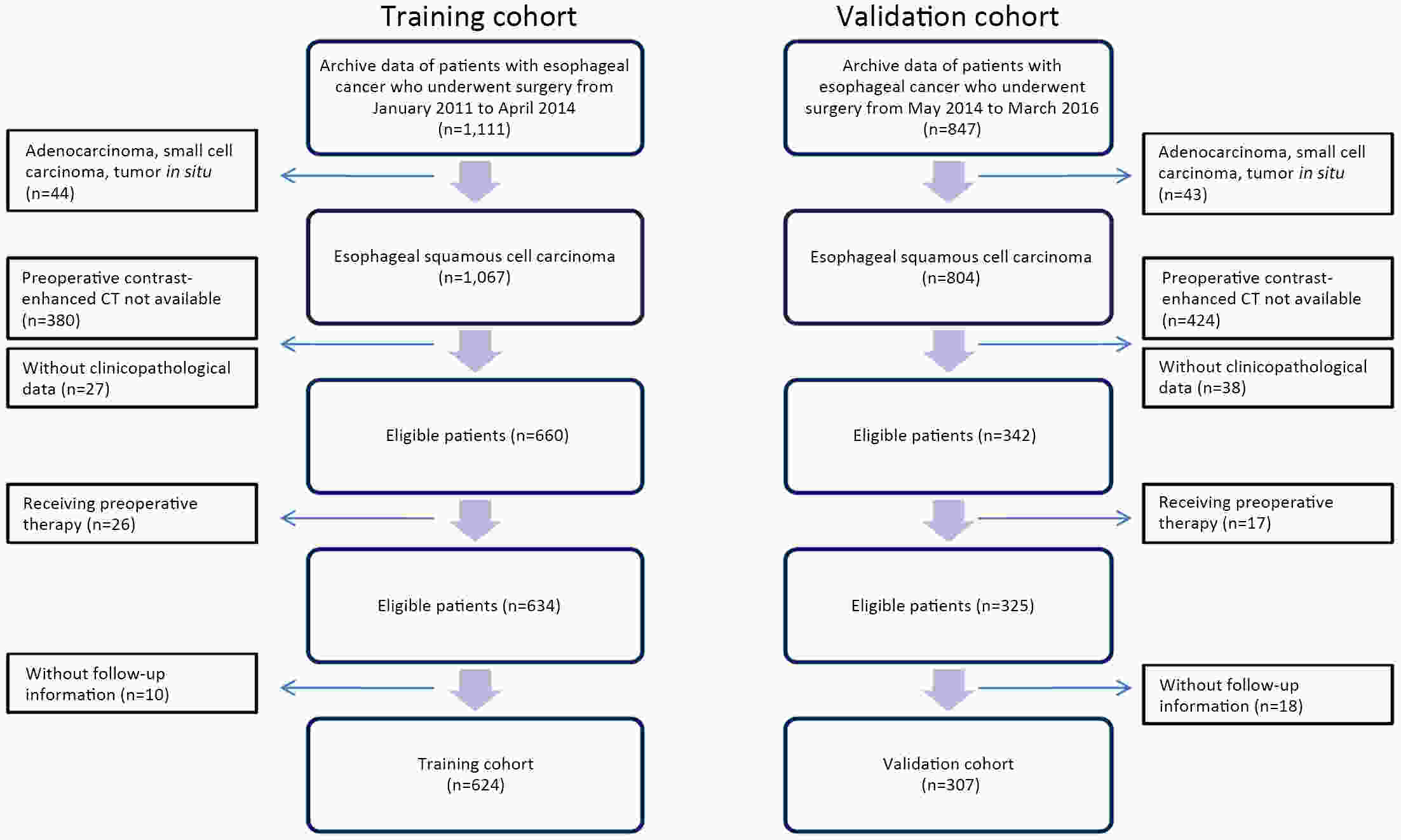
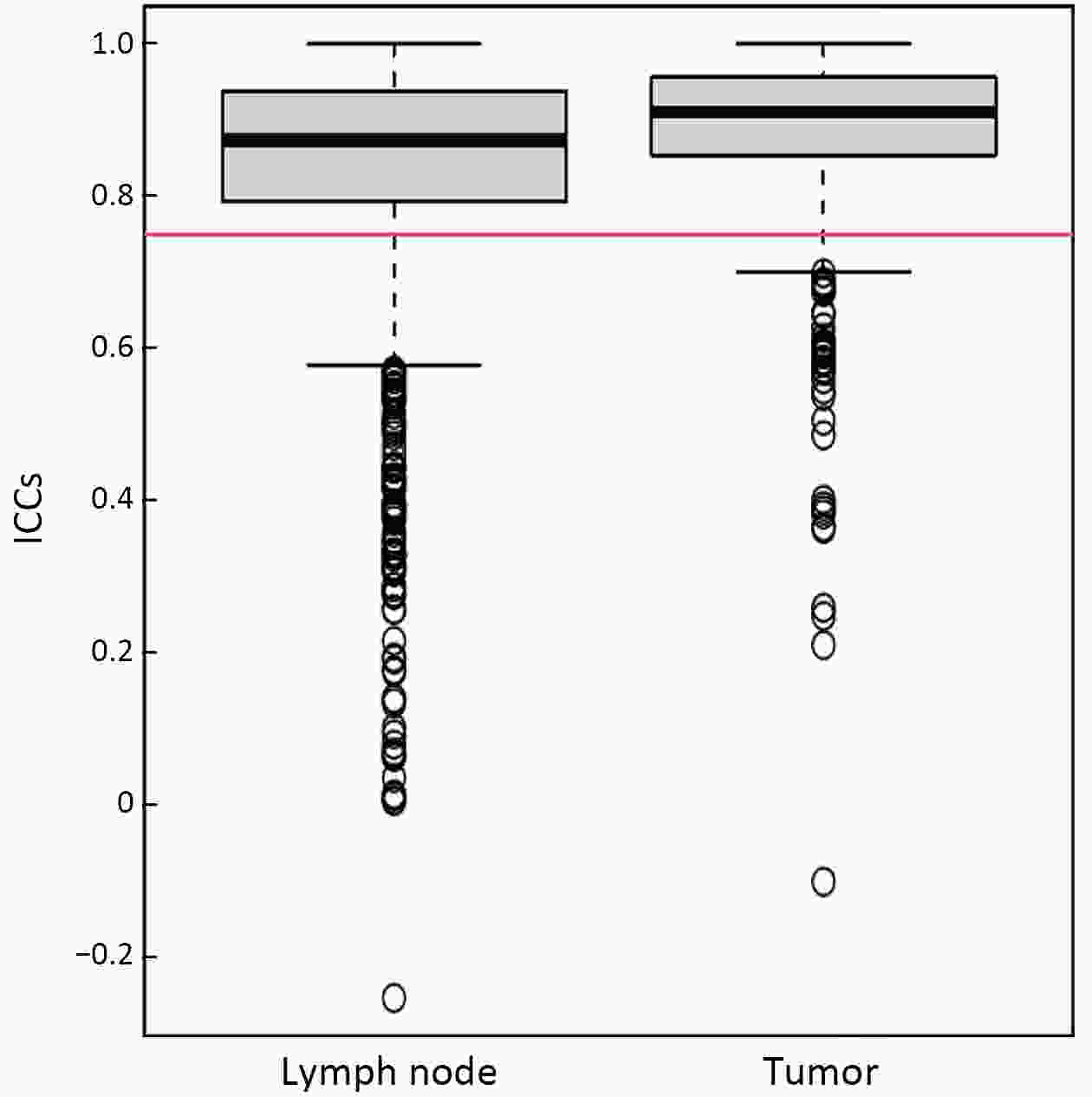
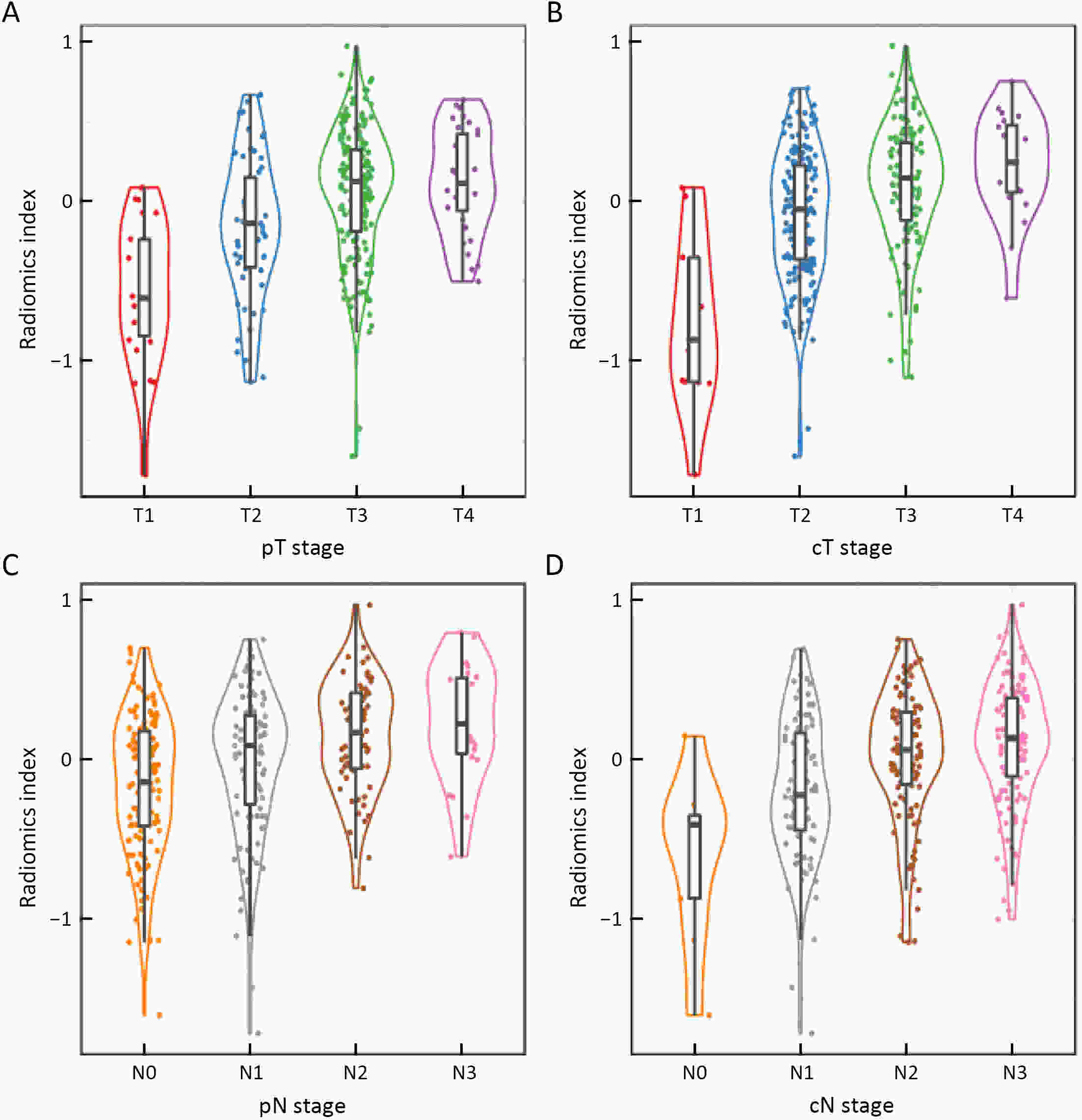
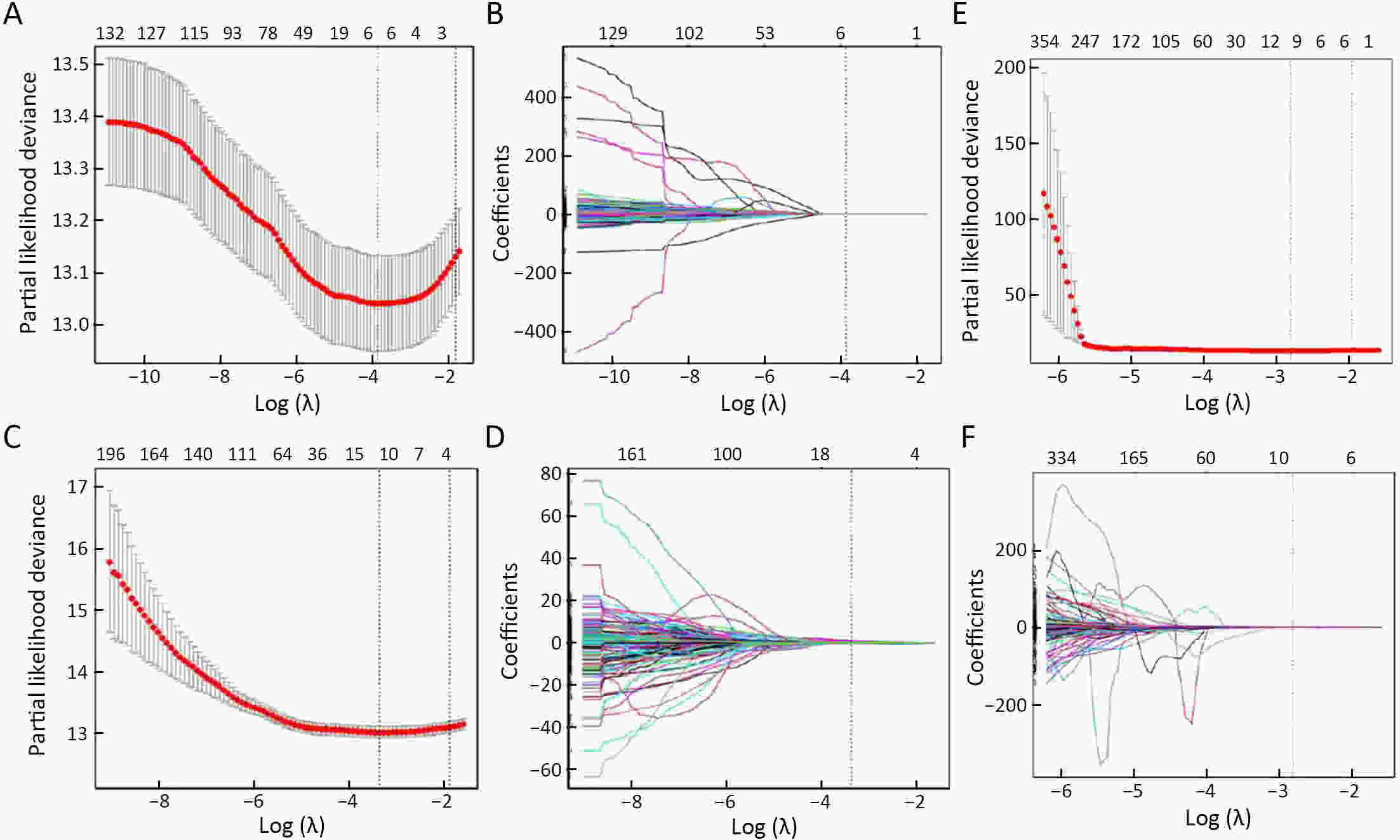
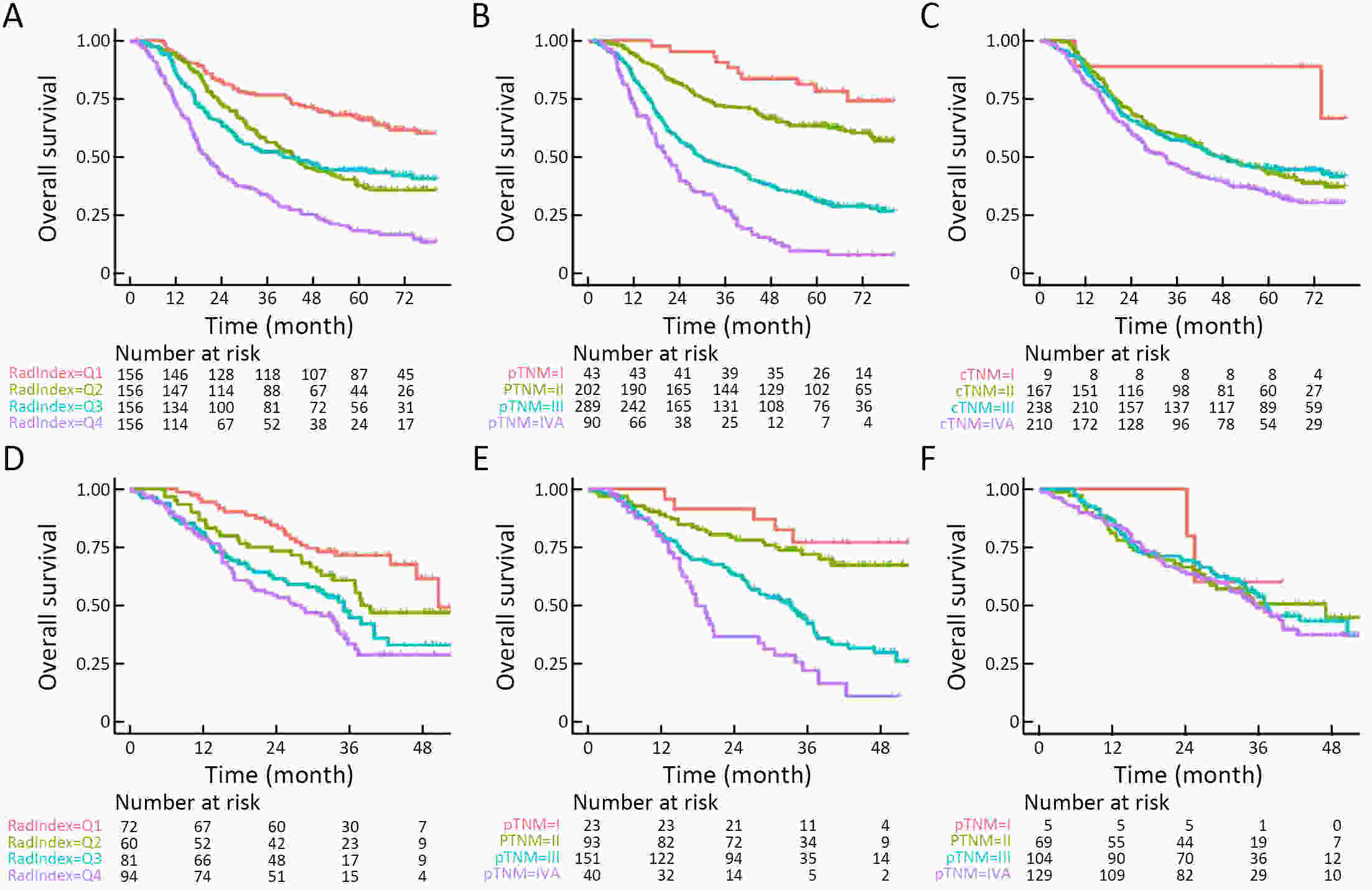
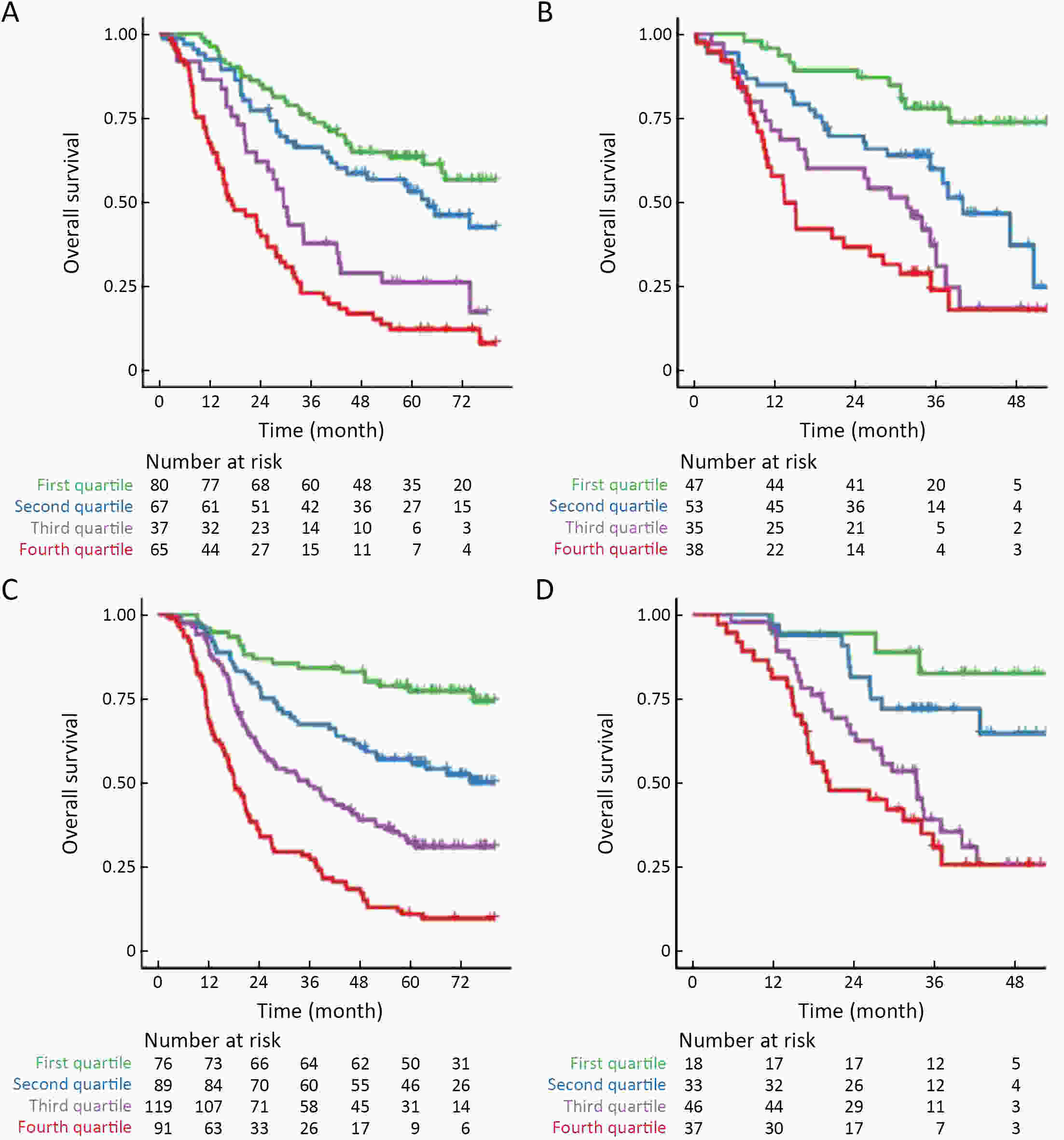
ObjectiveThis study aimed to evaluate the prognostic value of preoperative radiomics and establish an integrated model for esophageal squamous cell cancer (ESCC). MethodsA total of 931 patients were retrospectively enrolled in this study (training cohort, n=624; validation cohort, n=307). Radiomics features were obtained by contrast-enhanced computed tomography (CT) before esophagectomy. A radiomics index was set based on features of tumor and reginal lymph nodes by using the least absolute shrinkage and selection operator (LASSO) Cox regression. Prognostic nomogram was built based on radiomics index and other independent risk factors. The prognostic value was assessed by using Harrell’s concordance index, time-dependent receiver operating characteristics and Kaplan-Meier curves. ResultsTwelve radiomic features from tumor and lymph node regions were identified to build a radiomics index, which was significantly associated with overall survival (OS) in both training cohort and validation cohort. The radiomics index was highly correlated with clinical tumor-node-metastasis (cTNM) and pathologic TNM (pTNM) stages, but it demonstrated a better prognostic value compared with cTNM stage and was almost comparable with pTNM stage. Multivariable Cox regression showed that the radiomics index was an independent prognostic factor. An integrated model was constructed based on gender, preoperative serum sodium concentration, pTNM and the radiomics index for clinical usefulness. The integrated model demonstrated discriminatory ability better compared with the traditional clinical-pathologic model and pTNM alone, indicating incremental value for prognosis. ConclusionsCT-based radiomics for primary tumor and reginal lymph nodes was sufficient in predicting OS for patients with ESCC. The integrated model demonstrated incremental value for prognosis and was robust for clinical applications.
2022, 34(2): 83-94.
doi: 10.21147/j.issn.1000-9604.2022.02.03
Abstract:
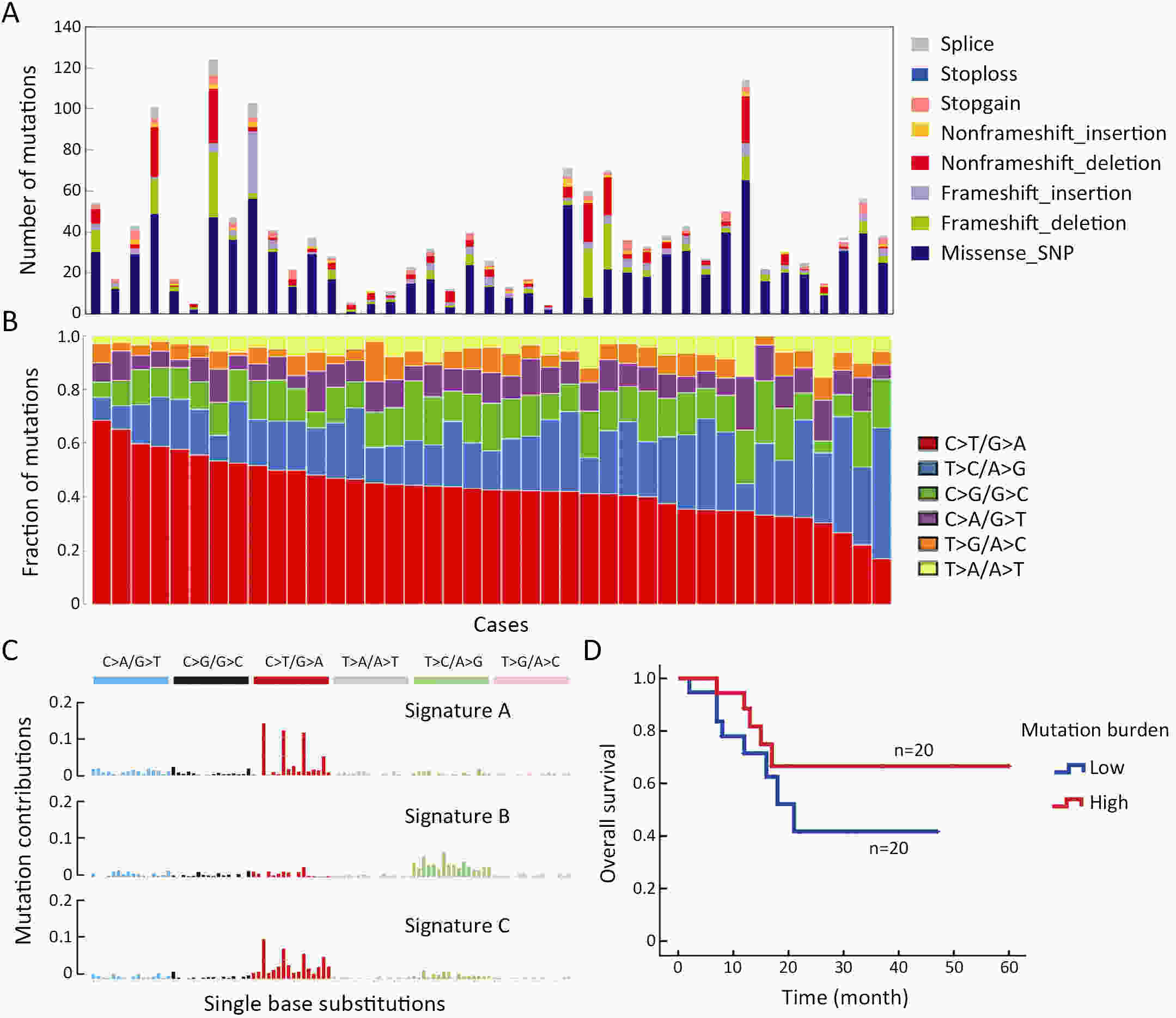
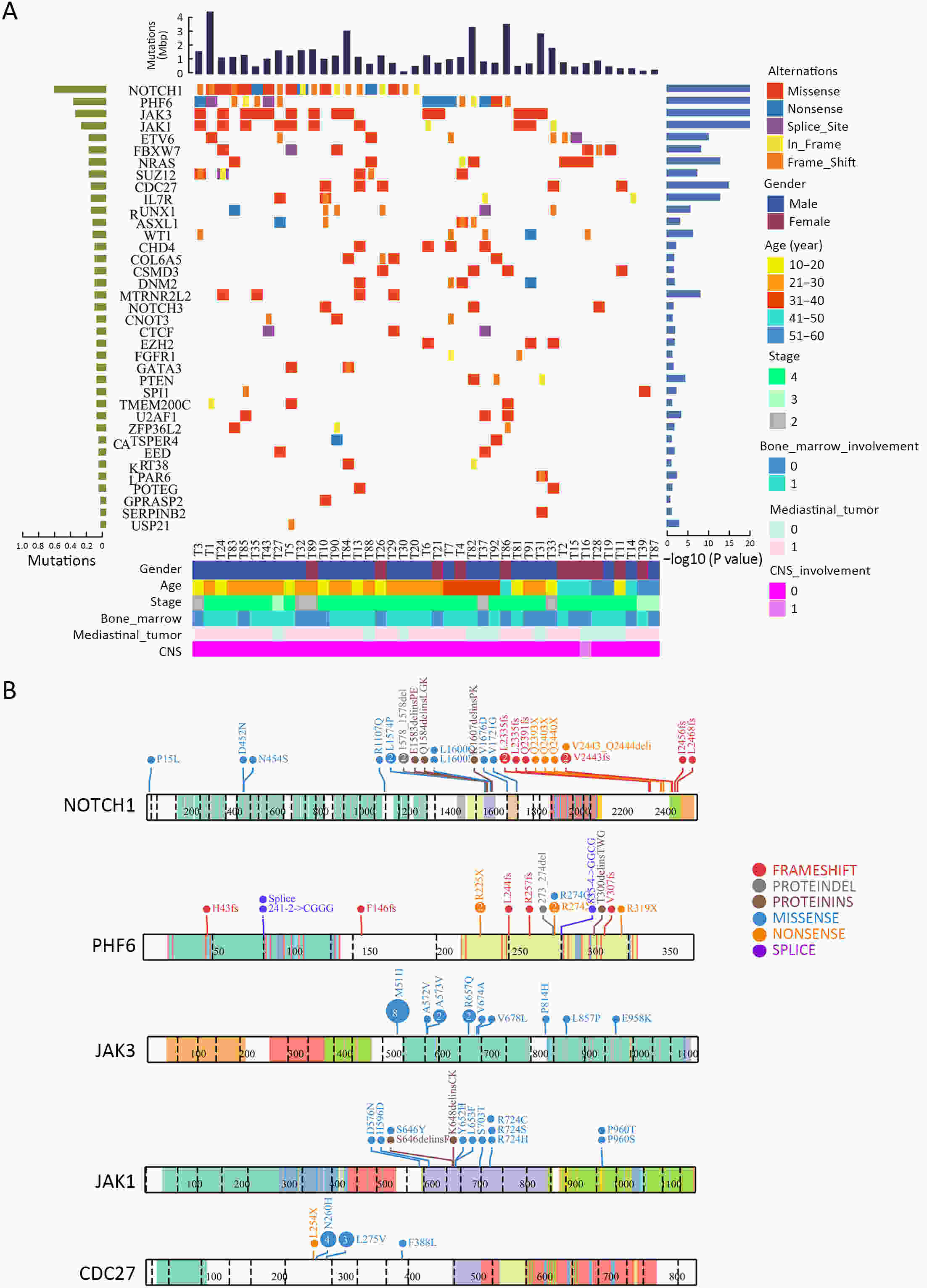
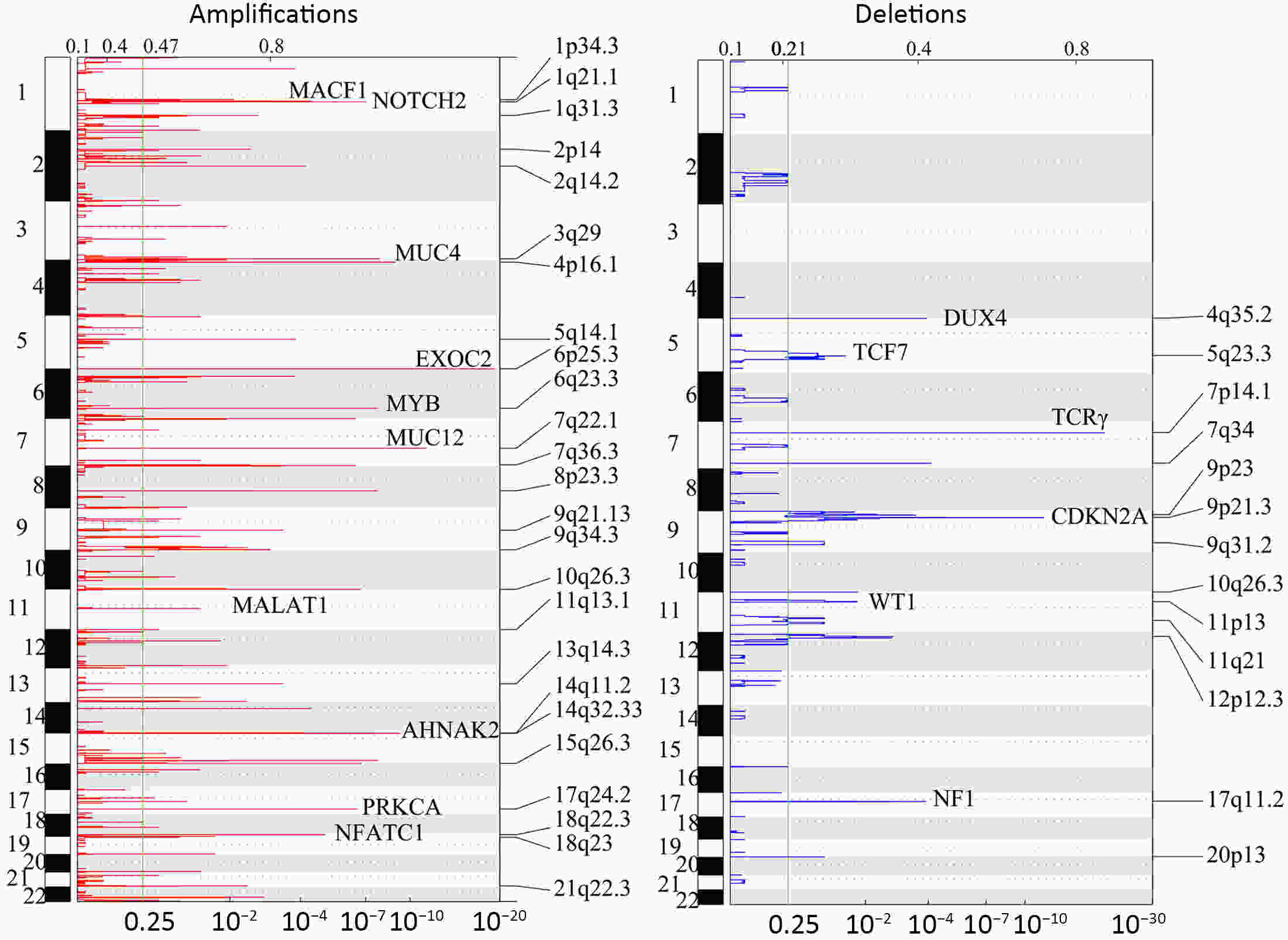
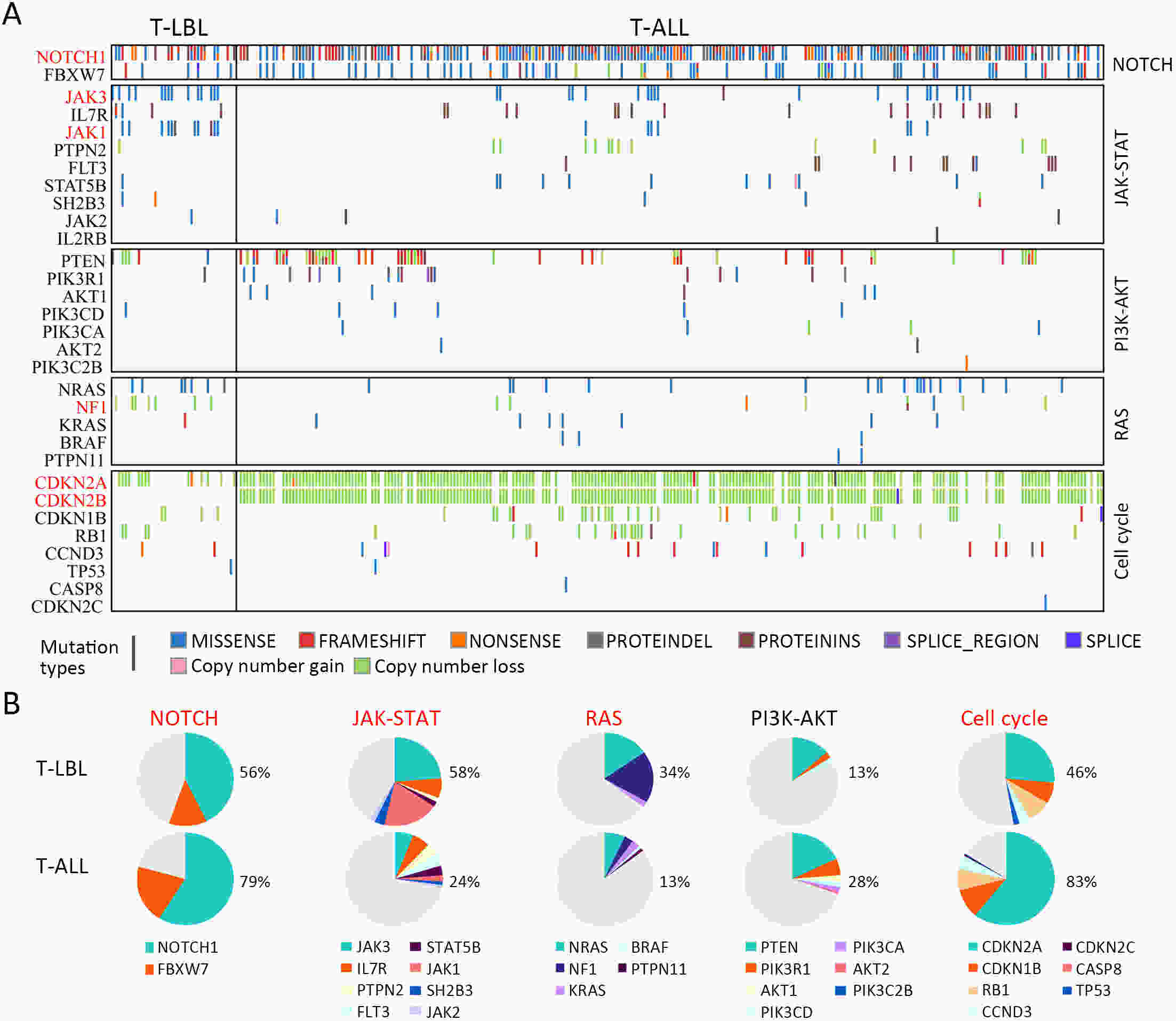
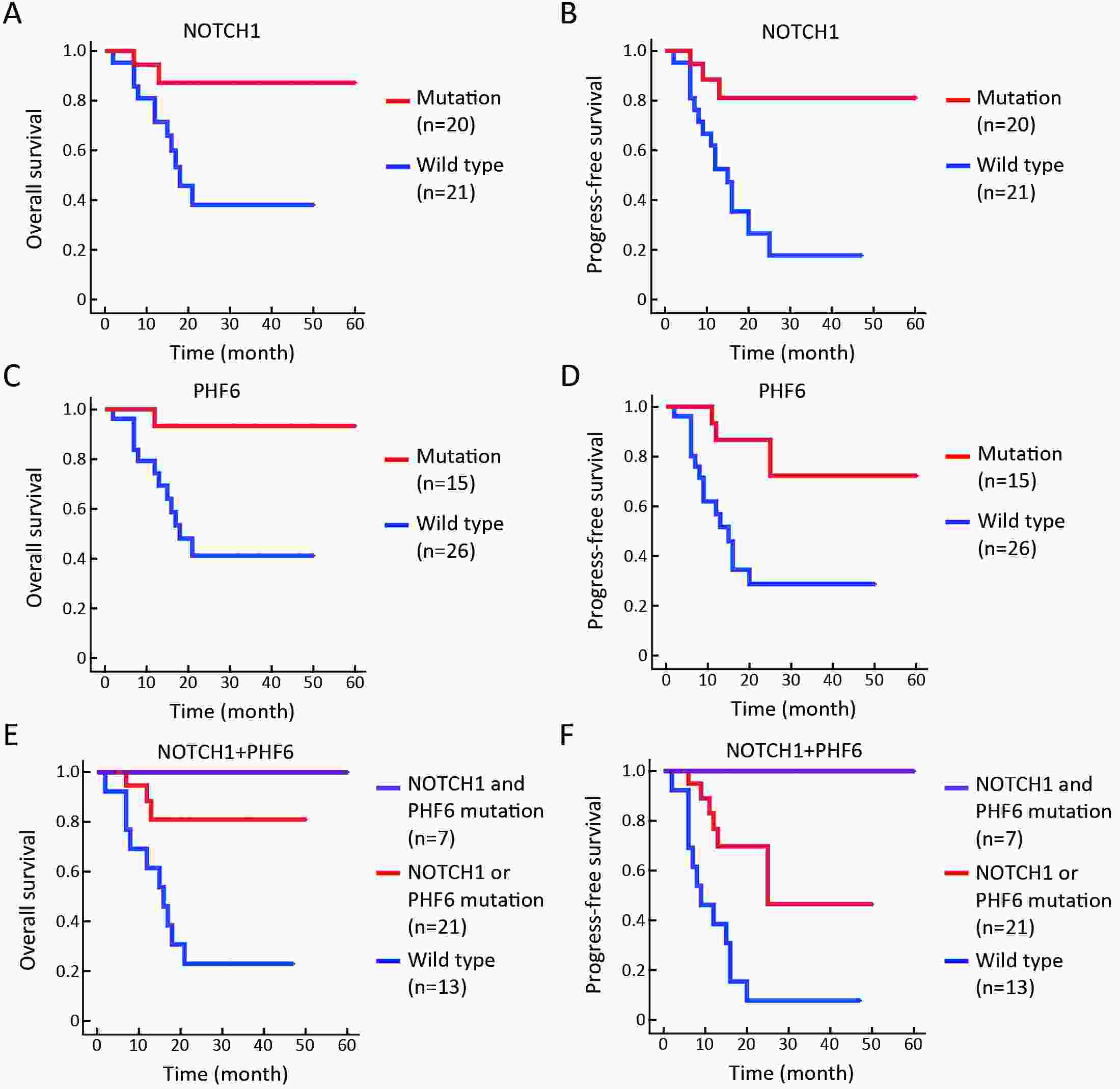
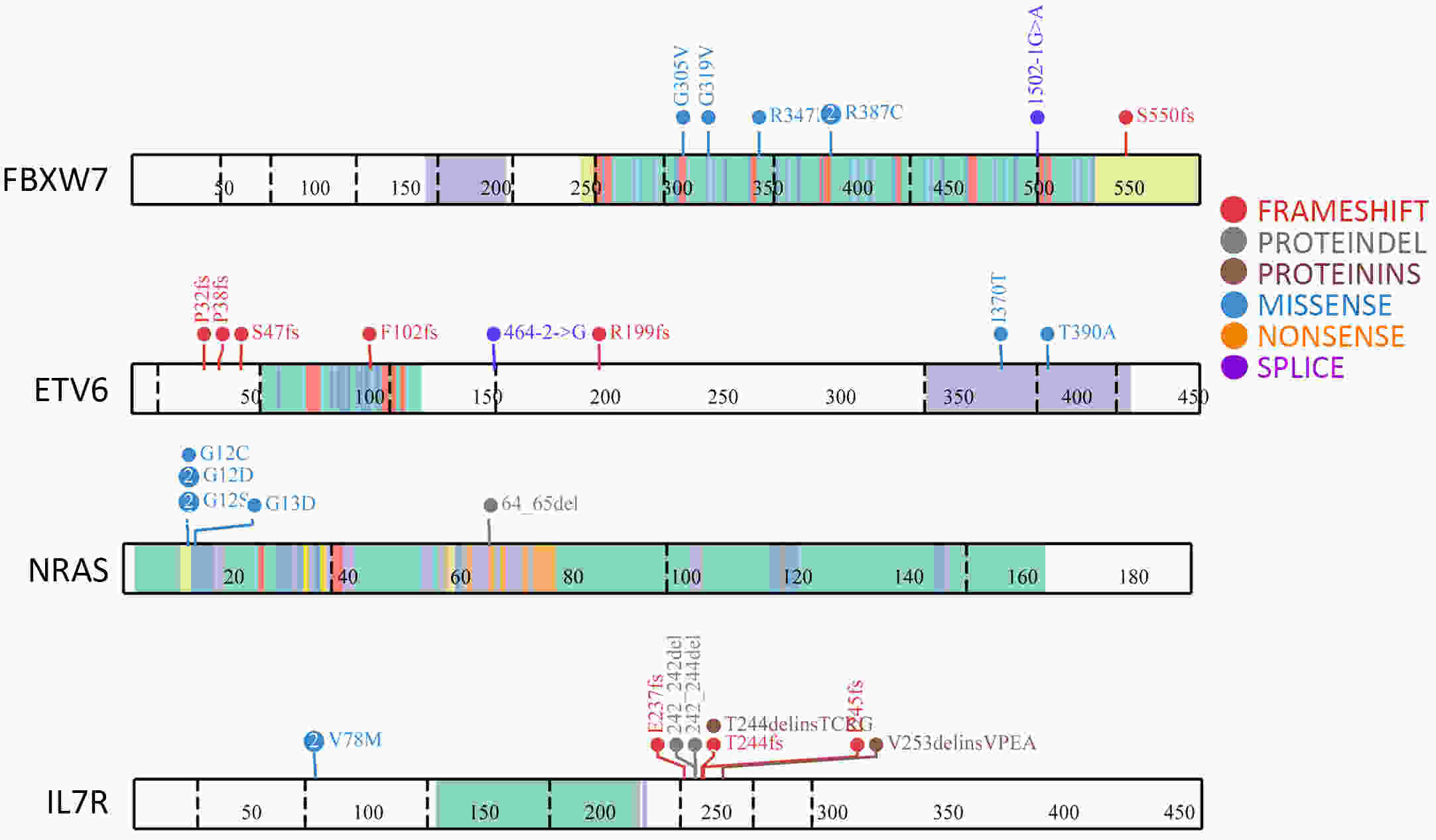
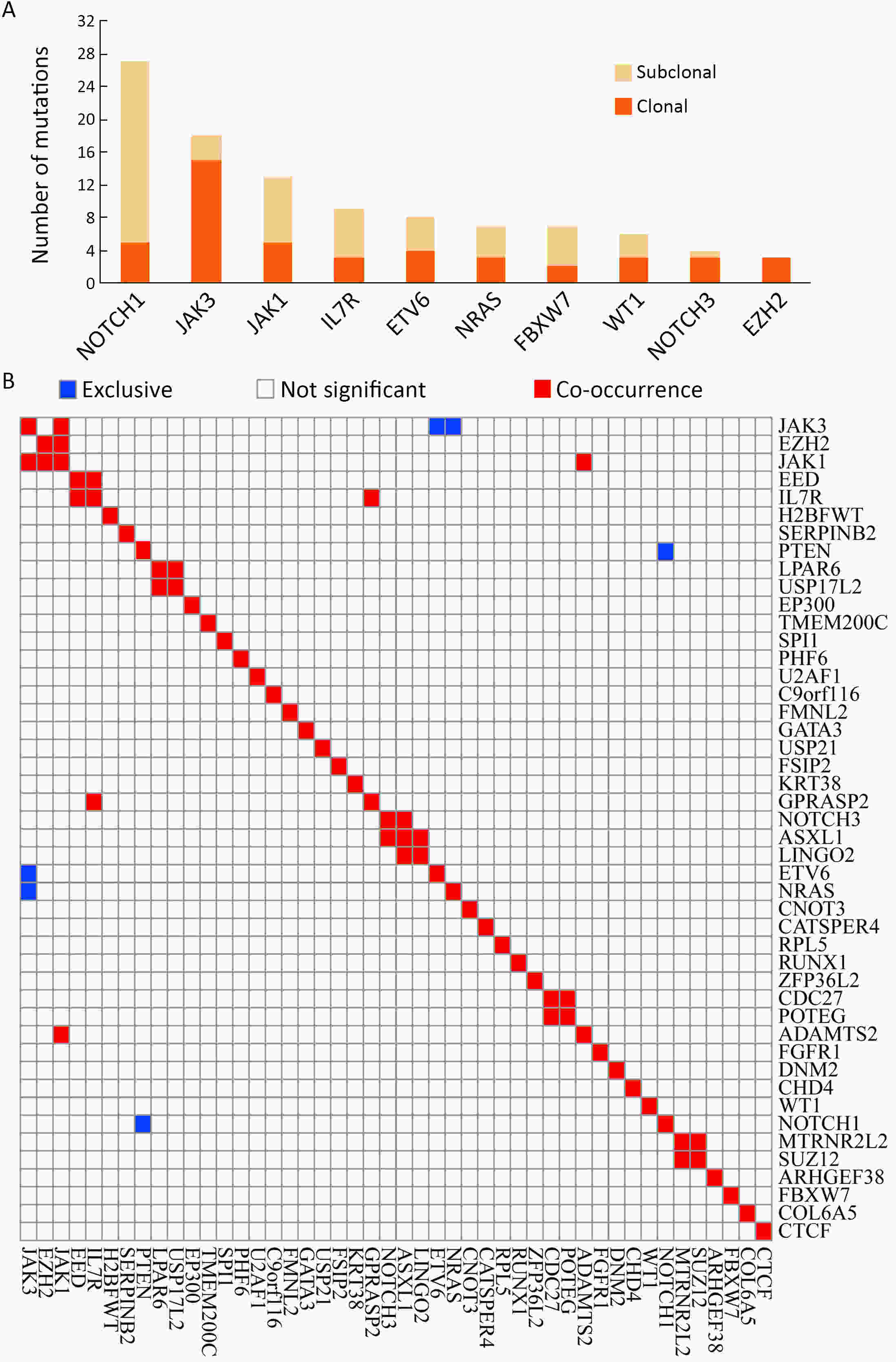
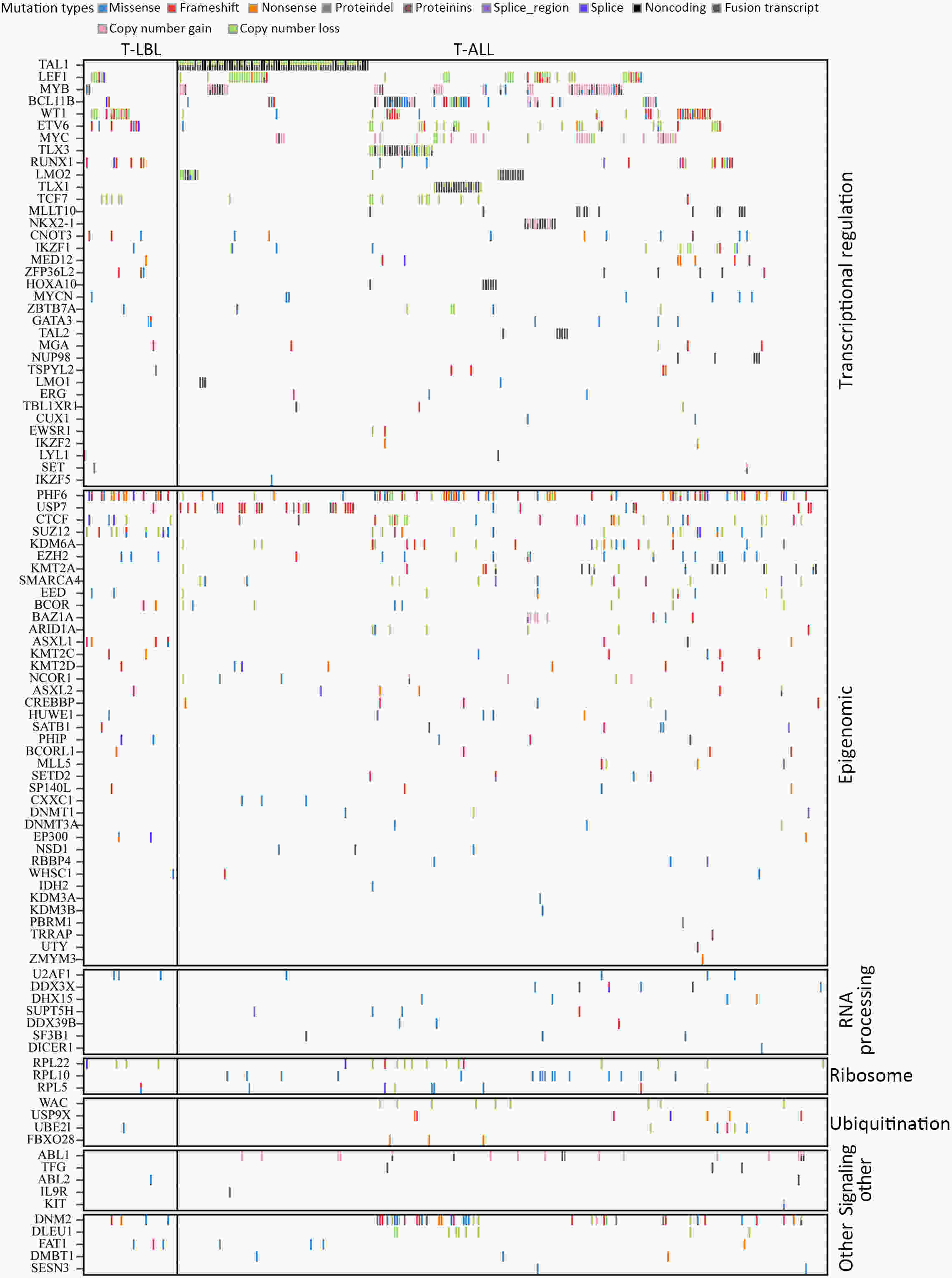
ObjectiveT-cell lymphoblastic lymphoma (T-LBL) is an aggressive neoplasm of precursor T cells, however, detailed genome-wide sequencing of large T-LBL cohorts has not been performed due to its rarity. The purpose of this study was to identify putative driver genes in T-LBL. MethodsTo gain insight into the genetic mechanisms of T-LBL development, we performed whole-exome sequencing on 41 paired tumor-normal DNA samples from patients with T-LBL. ResultsWe identified 32 putative driver genes using whole-exome sequencing in 41 T-LBL cases, many of which have not previously been described in T-LBL, such as Janus kinase 3 (JAK3), Janus kinase 1 (JAK1), Runt-related transcription factor 1 (RUNX1) and Wilms’ tumor suppressor gene 1 (WT1). When comparing the genetic alterations of T-LBL to T-cell acute lymphoblastic leukemia (T-ALL), we found that JAK-STAT and RAS pathway mutations were predominantly observed in T-LBL (58.5% and 34.1%, respectively), whereas Notch and cell cycle signaling pathways mutations were more prevalent in T-ALL. Notably, besides notch receptor 1 (NOTCH1), mutational status of plant homeodomain (PHD)-like finger protein 6 (PHF6) was identified as another independent factor for good prognosis. Of utmost interest is that co-existence of PHF6 and NOTCH1 mutation status might provide an alternative for early therapeutic stratification in T-LBL. ConclusionsTogether, our findings will not only provide new insights into the molecular and genetic mechanisms of T-LBL, but also have tangible implications for clinical practice.
2022, 34(2): 95-108.
doi: 10.21147/j.issn.1000-9604.2022.02.04
Abstract:
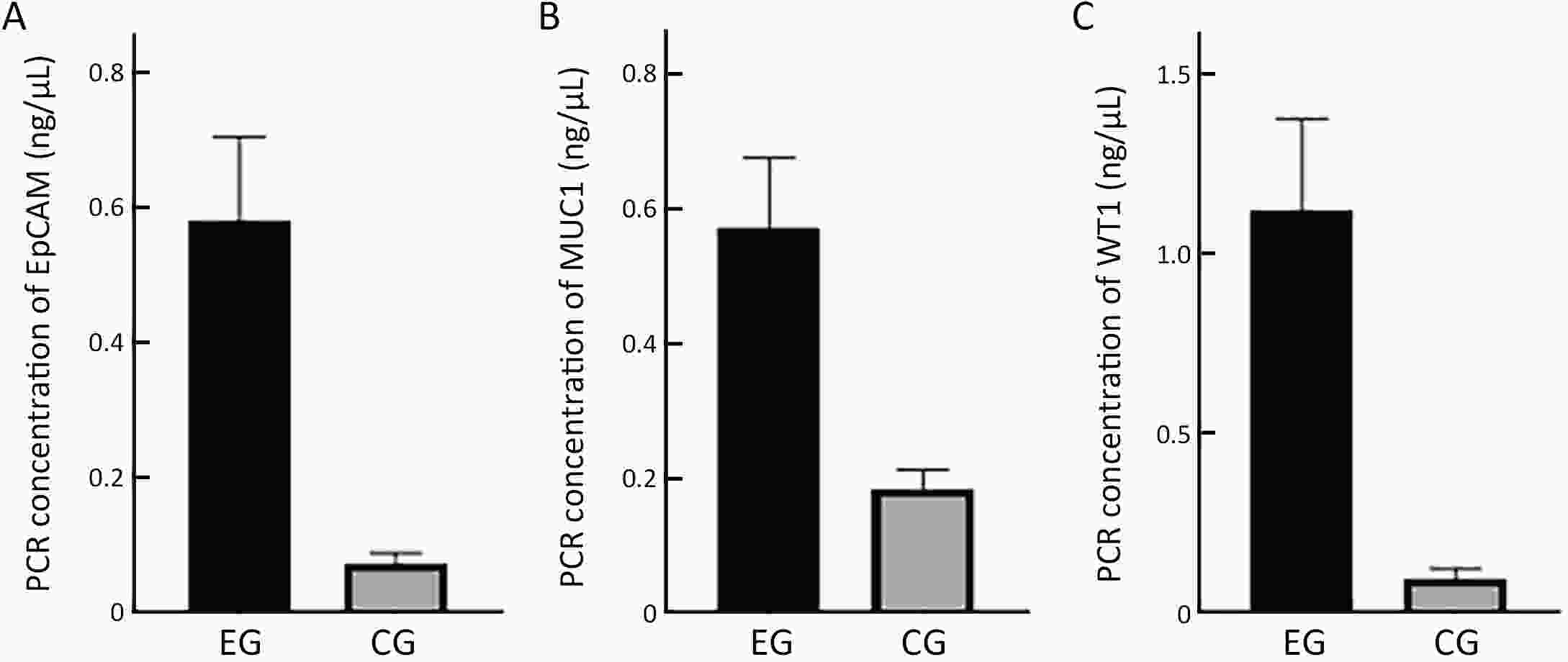
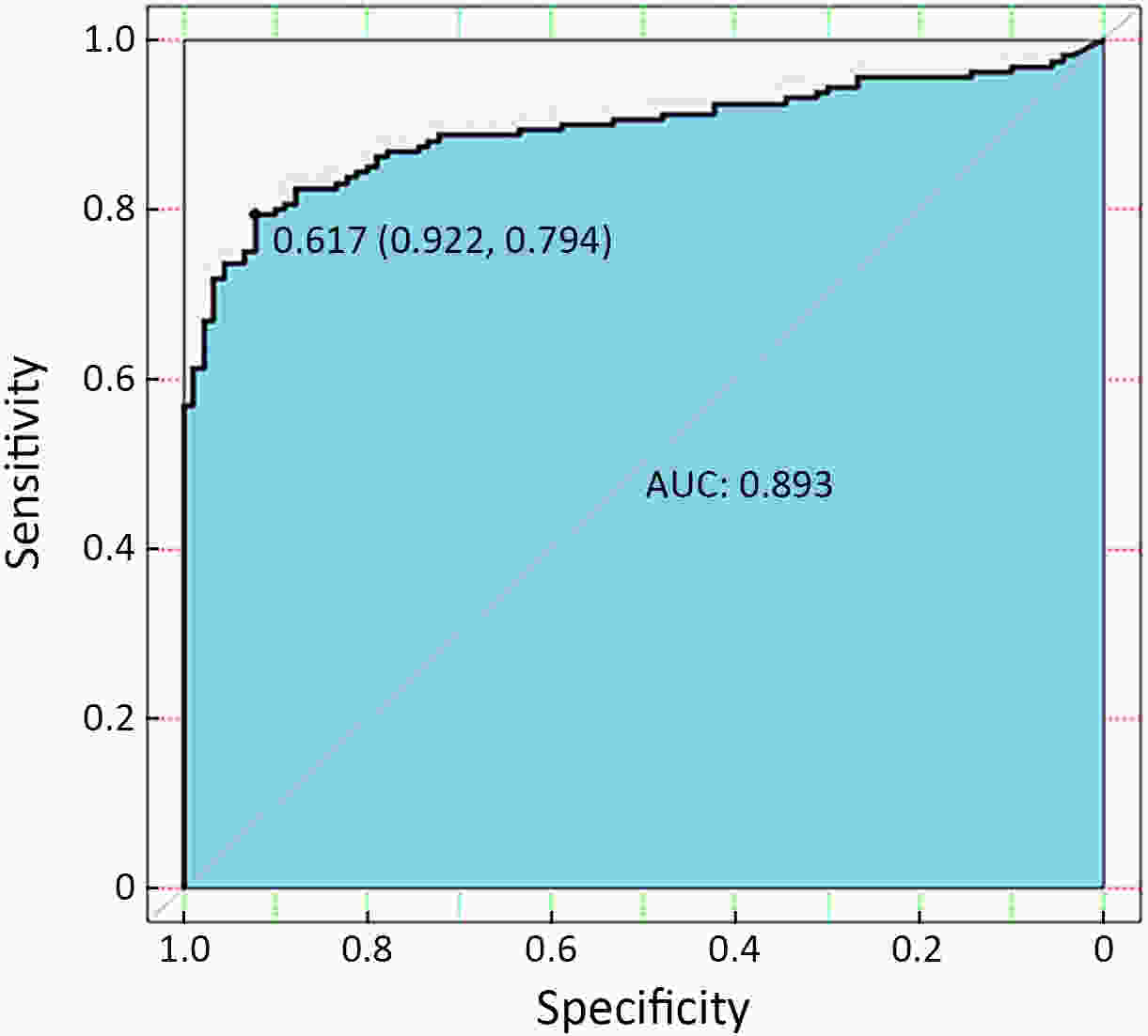
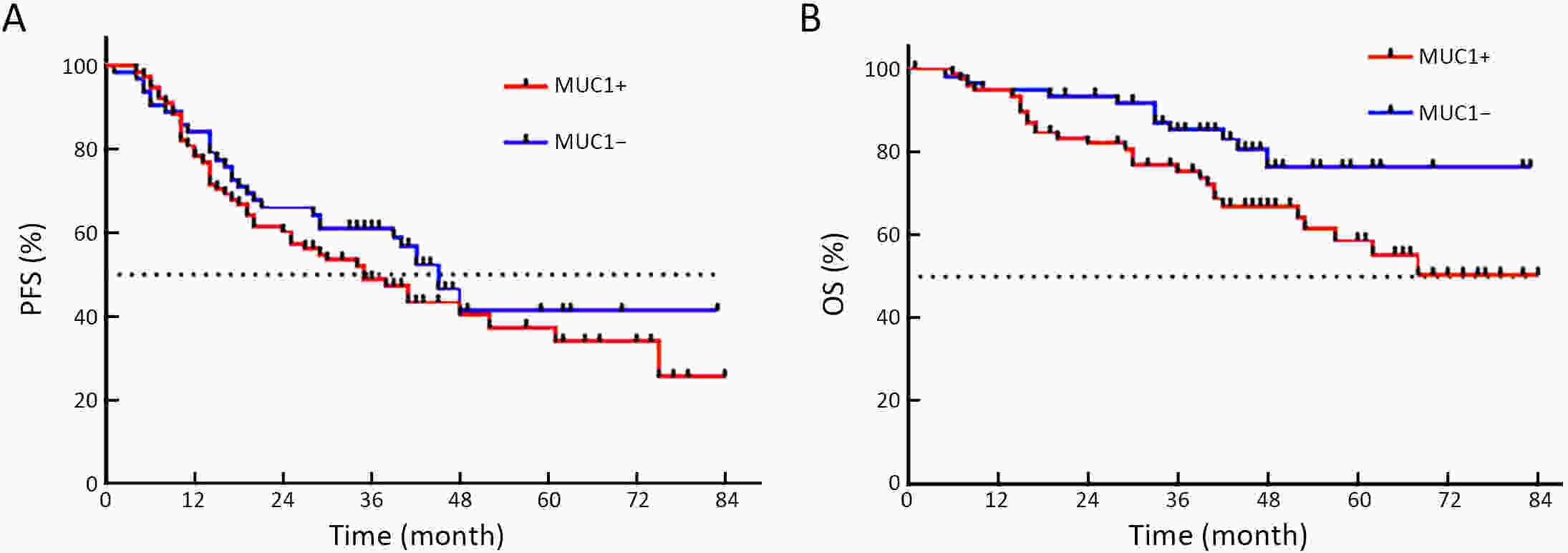
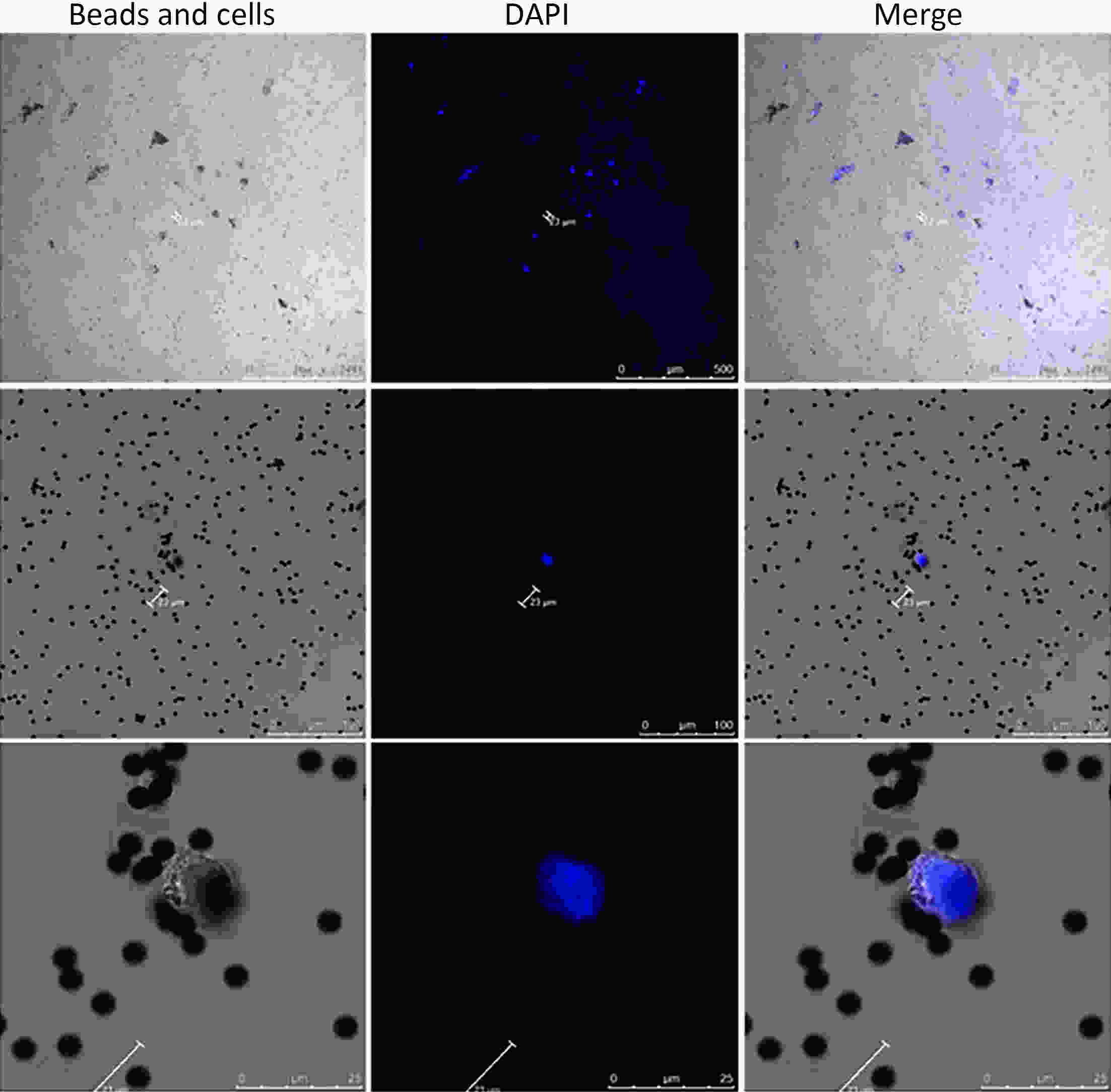
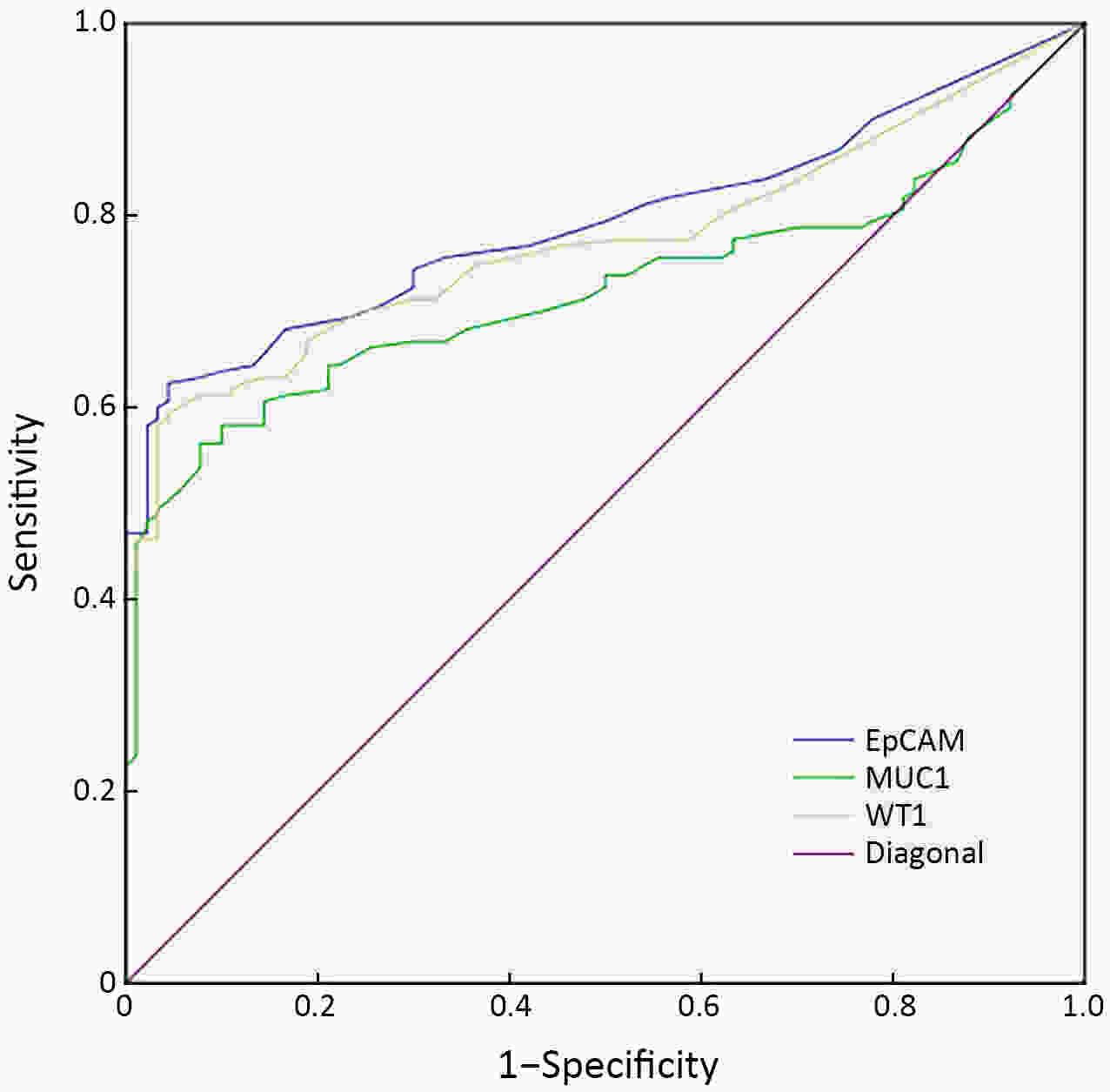
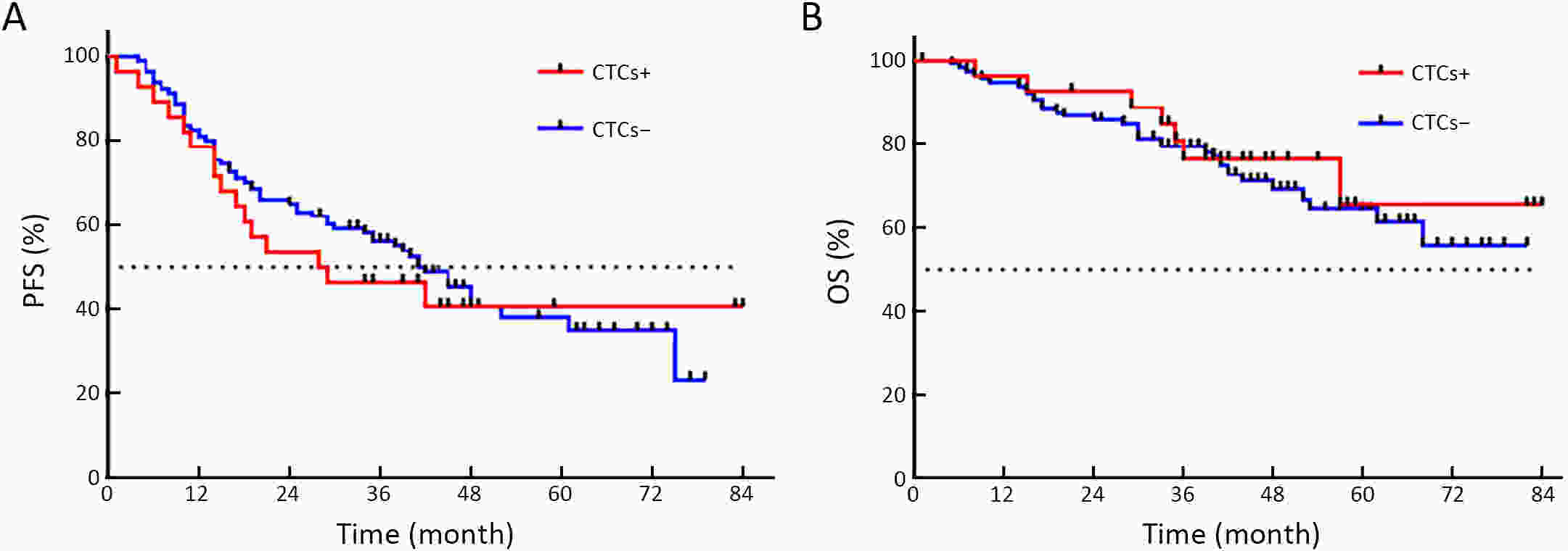
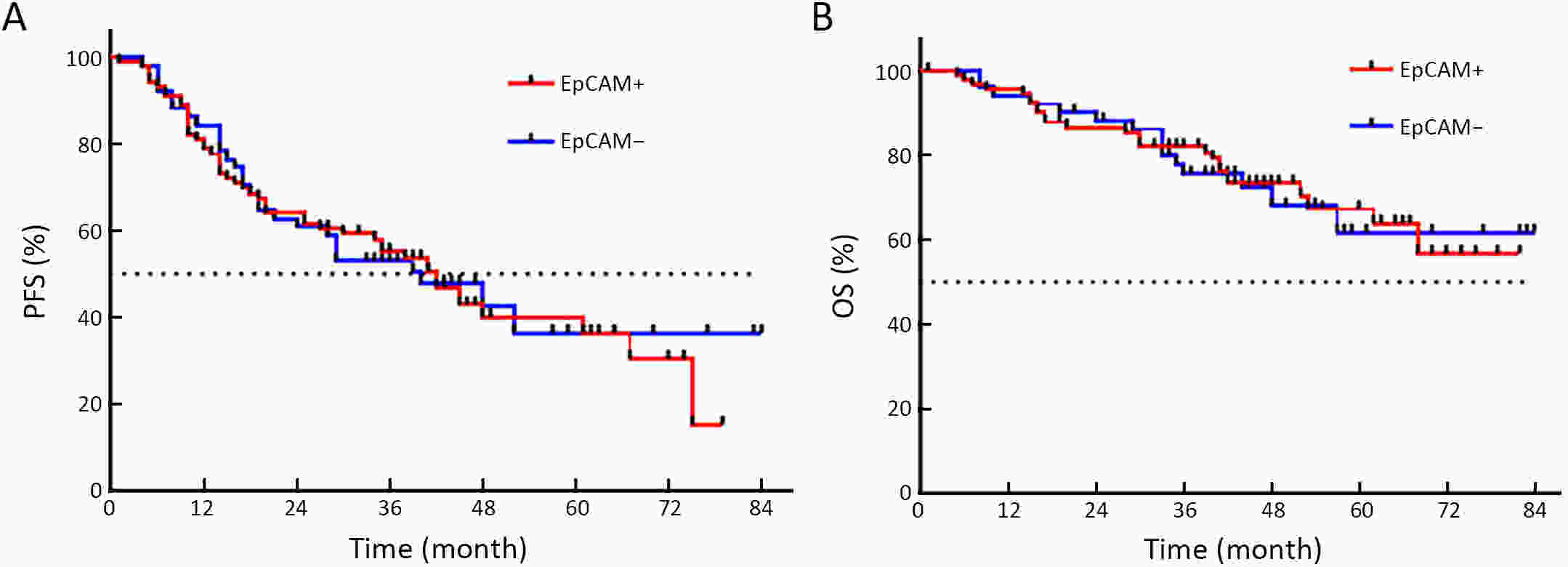
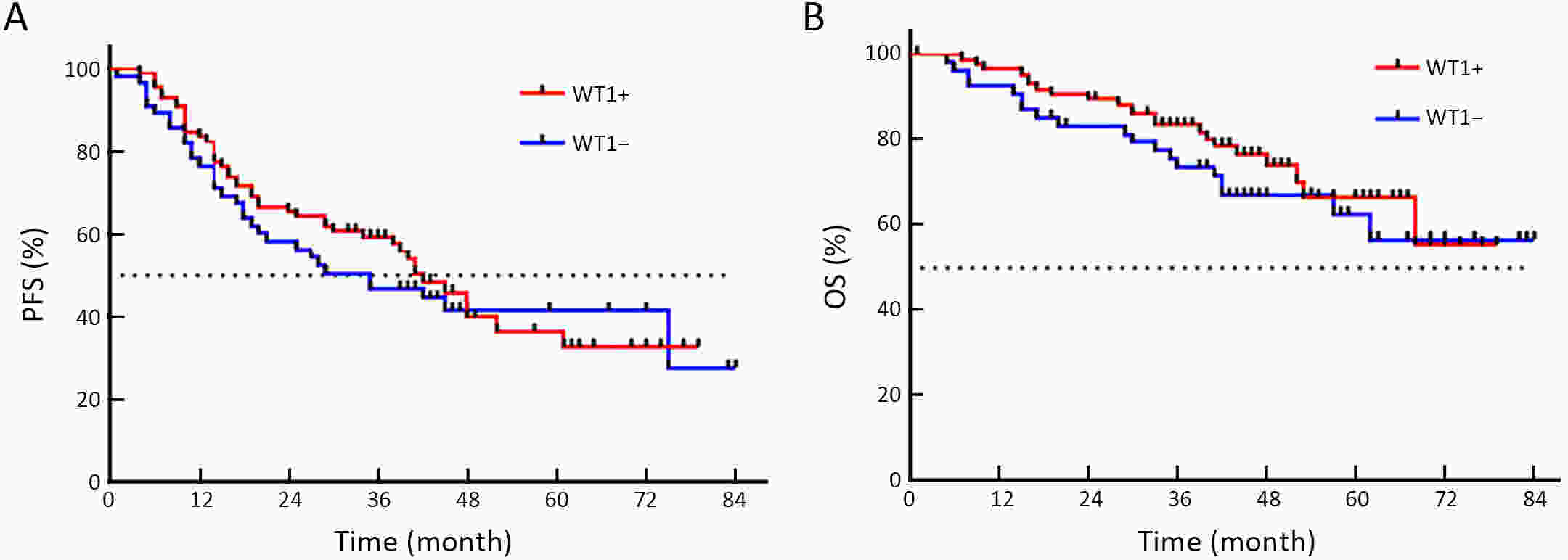
ObjectiveEmerging studies have demonstrated the promising clinical value of circulating tumor cells (CTCs) for diagnosis, disease assessment, treatment monitoring and prognosis in epithelial ovarian cancer. However, the clinical application of CTC remains restricted due to diverse detection techniques with variable sensitivity and specificity and a lack of common standards. MethodsWe enrolled 160 patients with epithelial ovarian cancer as the experimental group, and 90 patients including 50 patients with benign ovarian tumor and 40 healthy females as the control group. We enriched CTCs with immunomagnetic beads targeting two epithelial cell surface antigens (EpCAM and MUC1), and used multiple reverse transcription-polymerase chain reaction (RT-PCR) detecting three markers (EpCAM, MUC1 and WT1) for quantification. And then we used a binary logistic regression analysis and focused on EpCAM, MUC1 and WT1 to establish an optimized CTC detection model. ResultsThe sensitivity and specificity of the optimized model is 79.4% and 92.2%, respectively. The specificity of the CTC detection model is significantly higher than CA125 (92.2% vs. 82.2%, P=0.044), and the detection rate of CTCs was higher than the positive rate of CA125 (74.5% vs. 58.2%, P=0.069) in early-stage patients (stage I and II). The detection rate of CTCs was significantly higher in patients with ascitic volume ≥500 mL, suboptimal cytoreductive surgery and elevated serum CA125 level after 2 courses of chemotherapy (P<0.05). The detection rate of CTCEpCAM+ and CTCMUC1+ was significantly higher in chemo-resistant patients (26.3% vs. 11.9%; 26.4% vs. 13.4%, P<0.05). The median progression-free survival time for CTCMUC1+ patients trended to be longer than CTCMUC1− patients, and overall survival was shorter in CTCMUC1+ patients (P=0.043). ConclusionsOur study presents an optimized detection model for CTCs, which consists of the expression levels of three markers (EpCAM, MUC1 and WT1). In comparison with CA125, our model has high specificity and demonstrates better diagnostic values, especially for early-stage ovarian cancer. Detection of CTCEpCAM+ and CTCMUC1+ had predictive value for chemotherapy resistance, and the detection of CTCMUC1+ suggested poor prognosis.
2022, 34(2): 109-114.
doi: 10.21147/j.issn.1000-9604.2022.02.05
Abstract:
The concept and strategy of advanced gastric cancer treatment have gradually undergone profound changes with the in-depth understanding of the biology and heterogeneous characteristics of gastric cancer. Moreover, the development and application of new anticancer drugs, including chemotherapy drugs, molecularly targeted drugs and immunotherapy drugs for advanced gastric cancer are reported. The connotation of conversion therapy refers to the unresectable or borderline resectable tumors for surgical technical and/or oncological reasons, after active and effective chemotherapy and other comprehensive treatment, the primary gastric lesions can be reduced to a lower stage, while the metastatic lesions can be effectively controlled, to achieve R0 resection and improve the long-term survival rate. Current promising research results of conversion therapy are mostly from single-arm phase II clinical studies with small samples or retrospective studies. Conversion therapy still faces many challenges, including limited diagnostic and assessment methods, insufficient evidence of highly effective treatment regimens, difficulty in clarifying surgical indications, etc. Therefore, the integrated conversion therapy for advanced gastric cancer needs to be carried out with the close cooperation of a multidisciplinary team. Prospective, multi-center randomized controlled trial studies should be conducted in the future, and precision medicine such as molecular biology should be combined to provide better anticancer drug regimens and higher-level clinical evidence for conversion therapy of advanced gastric cancer.
The concept and strategy of advanced gastric cancer treatment have gradually undergone profound changes with the in-depth understanding of the biology and heterogeneous characteristics of gastric cancer. Moreover, the development and application of new anticancer drugs, including chemotherapy drugs, molecularly targeted drugs and immunotherapy drugs for advanced gastric cancer are reported. The connotation of conversion therapy refers to the unresectable or borderline resectable tumors for surgical technical and/or oncological reasons, after active and effective chemotherapy and other comprehensive treatment, the primary gastric lesions can be reduced to a lower stage, while the metastatic lesions can be effectively controlled, to achieve R0 resection and improve the long-term survival rate. Current promising research results of conversion therapy are mostly from single-arm phase II clinical studies with small samples or retrospective studies. Conversion therapy still faces many challenges, including limited diagnostic and assessment methods, insufficient evidence of highly effective treatment regimens, difficulty in clarifying surgical indications, etc. Therefore, the integrated conversion therapy for advanced gastric cancer needs to be carried out with the close cooperation of a multidisciplinary team. Prospective, multi-center randomized controlled trial studies should be conducted in the future, and precision medicine such as molecular biology should be combined to provide better anticancer drug regimens and higher-level clinical evidence for conversion therapy of advanced gastric cancer.
2022, 34(2): 115-116.
doi: 10.21147/j.issn.1000-9604.2022.02.06
Abstract:
2022, 34(2): 117-130.
doi: 10.21147/j.issn.1000-9604.2022.02.07
Abstract:
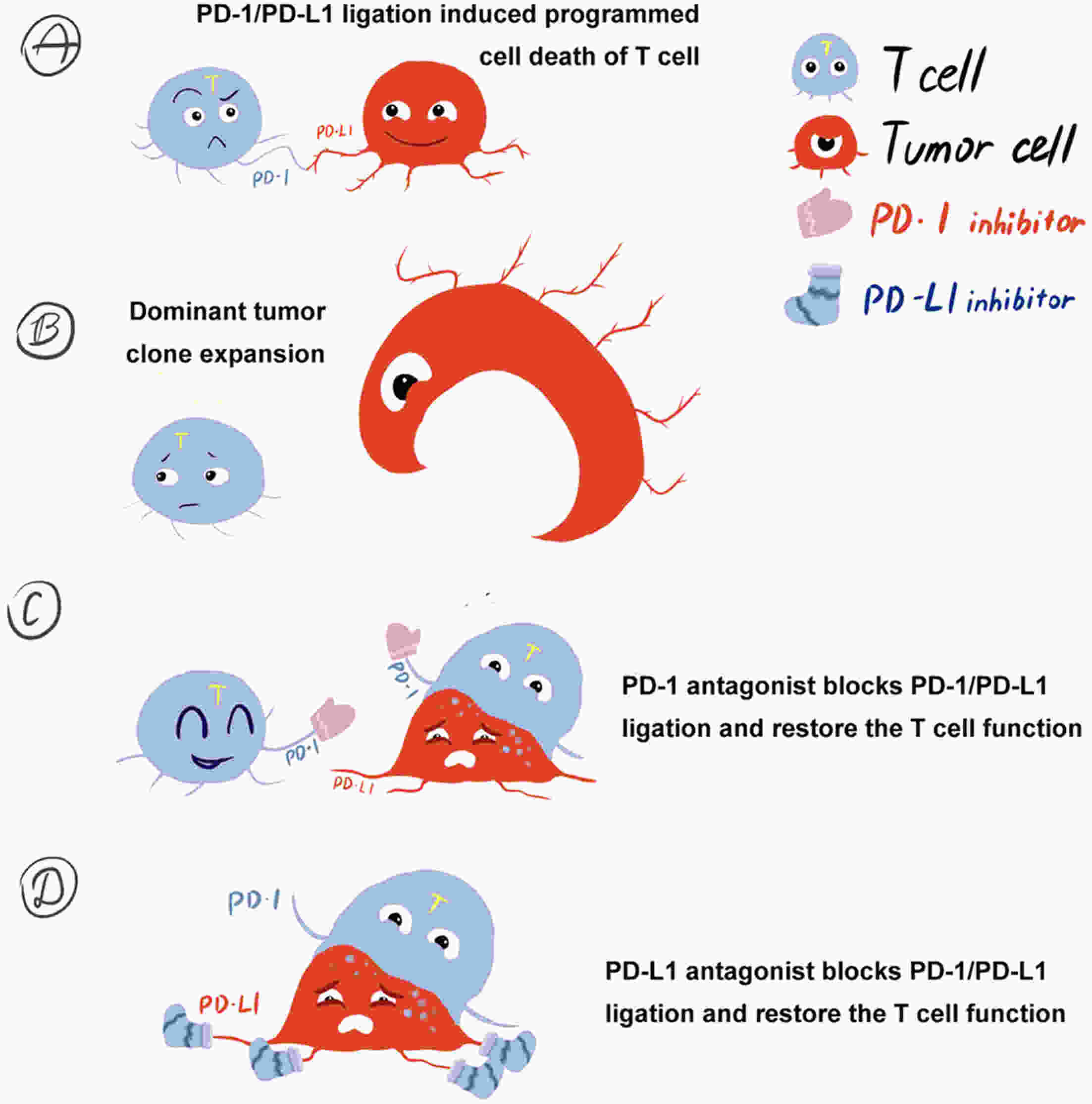
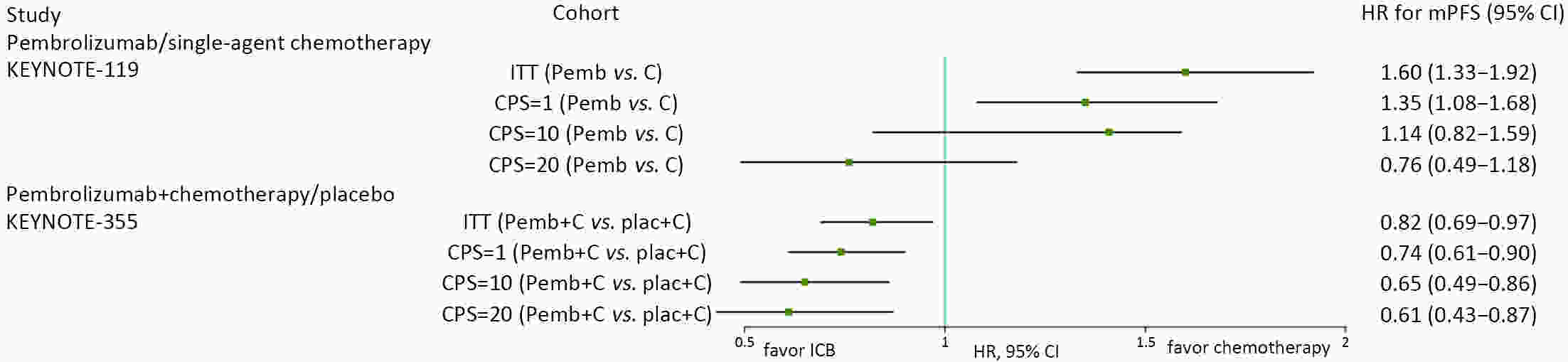
Triple-negative breast cancer (TNBC) has the worst prognosis among all molecular types of breast cancer. Because of the strong immunogenicity of TNBC cells, programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors, two kinds of immune checkpoint blockade agents, might help improve the prognosis of TNBC. However, how to better use PD-1/PD-L1 inhibitors and select patients who may benefit from treatment options remains controversial. This article summarizes published clinical studies in which PD-1/PD-L1 inhibitors were used in patients with advanced TNBC to explore how to maximize effectiveness of these medications.
Triple-negative breast cancer (TNBC) has the worst prognosis among all molecular types of breast cancer. Because of the strong immunogenicity of TNBC cells, programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors, two kinds of immune checkpoint blockade agents, might help improve the prognosis of TNBC. However, how to better use PD-1/PD-L1 inhibitors and select patients who may benefit from treatment options remains controversial. This article summarizes published clinical studies in which PD-1/PD-L1 inhibitors were used in patients with advanced TNBC to explore how to maximize effectiveness of these medications.

 Abstract
Abstract FullText HTML
FullText HTML PDF 410KB
PDF 410KB