2022 Vol.34(4)
Display Mode: |
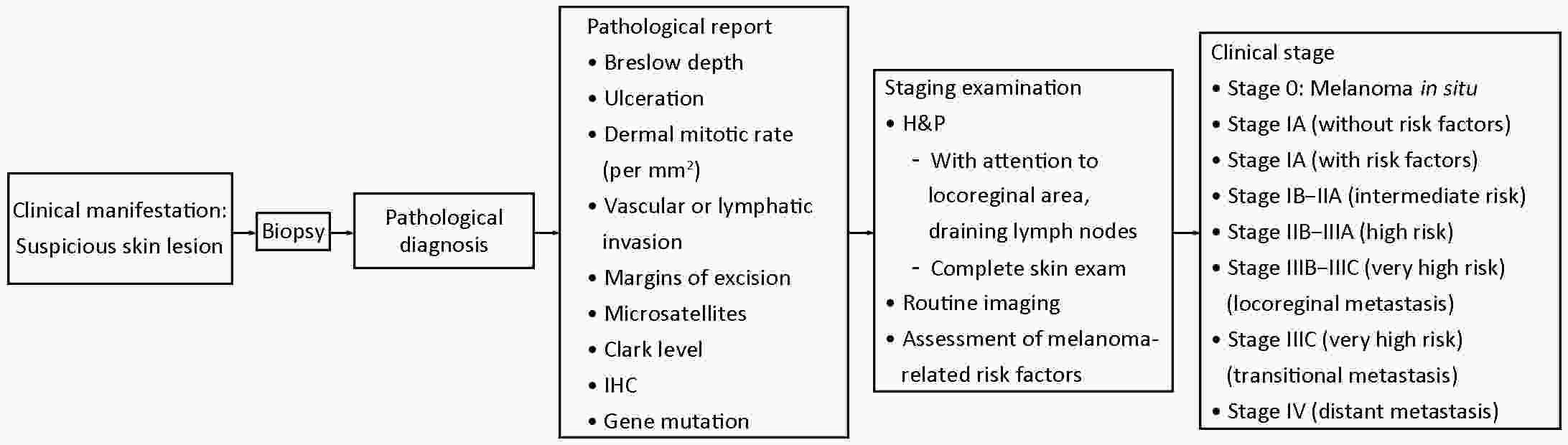
2022, 34(4): 309-334.
doi: 10.21147/j.issn.1000-9604.2022.04.01
Abstract:
2022, 34(4): 335-342.
doi: 10.21147/j.issn.1000-9604.2022.04.02
Abstract:
2022, 34(4): 343-352.
doi: 10.21147/j.issn.1000-9604.2022.04.03
Abstract:
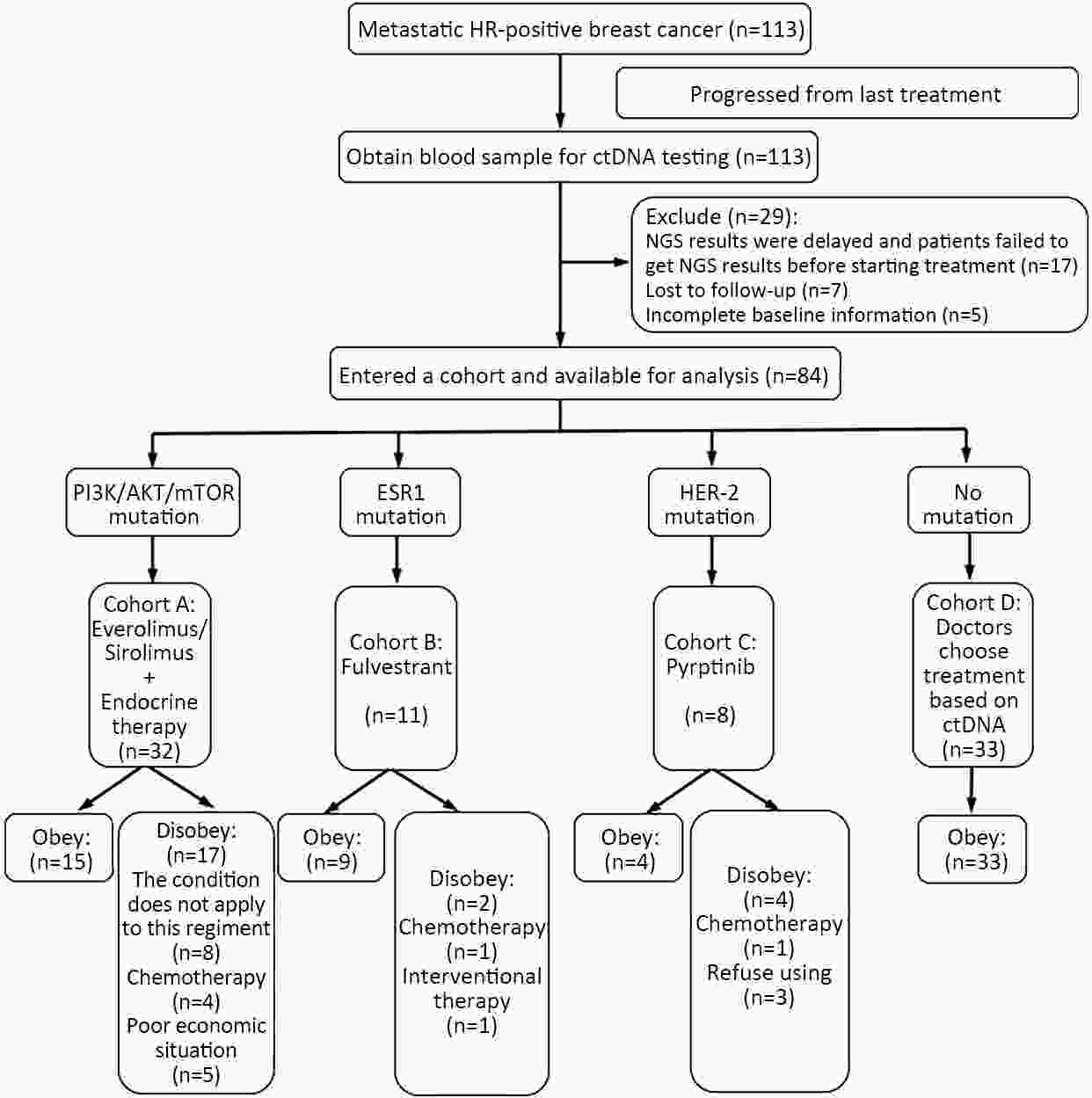
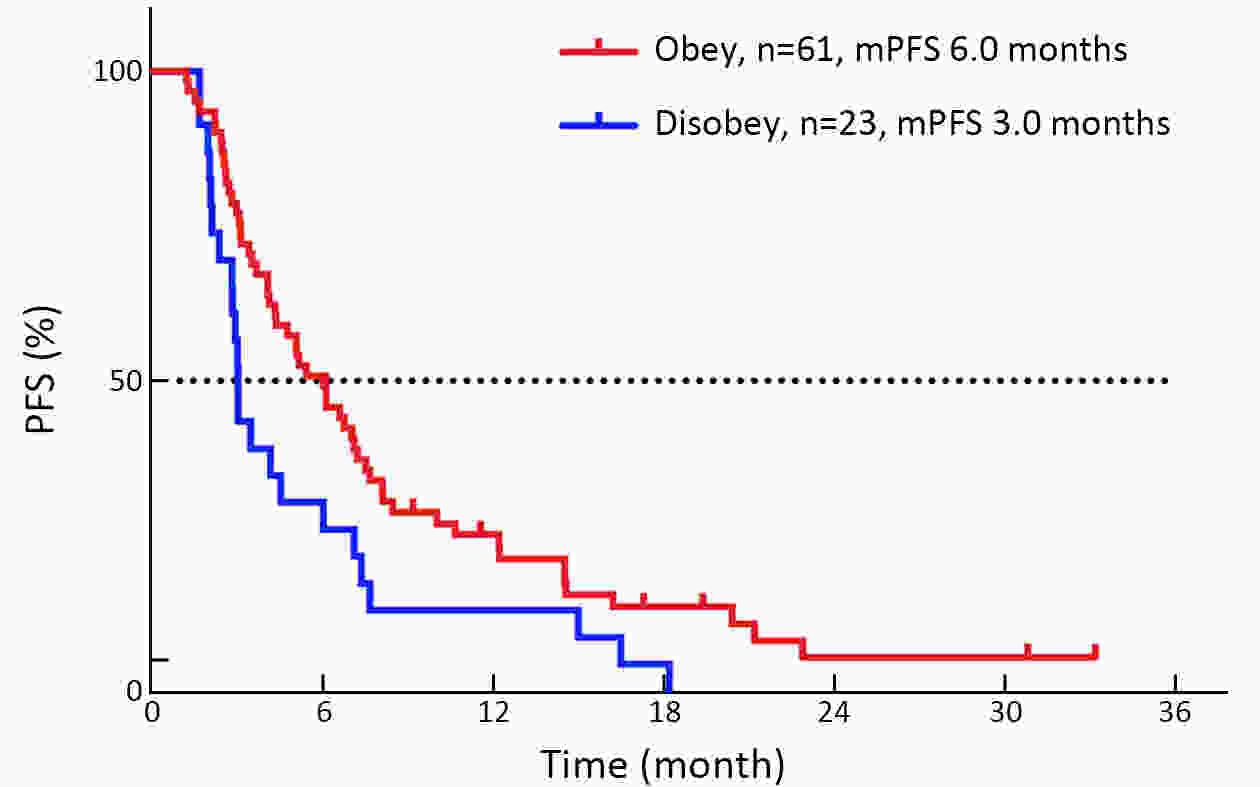
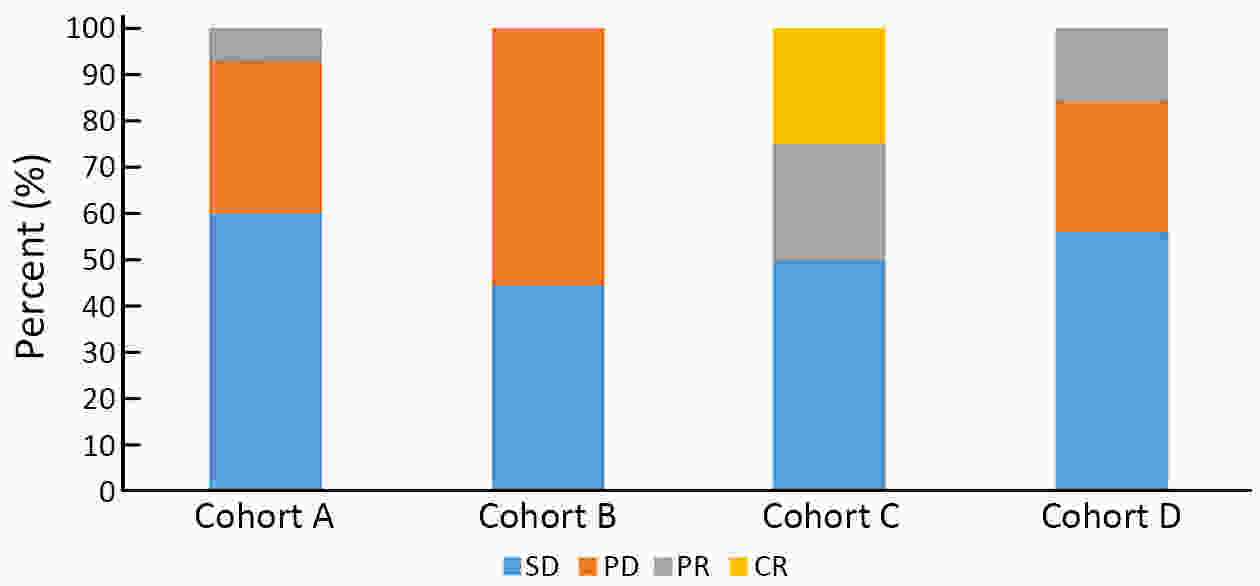
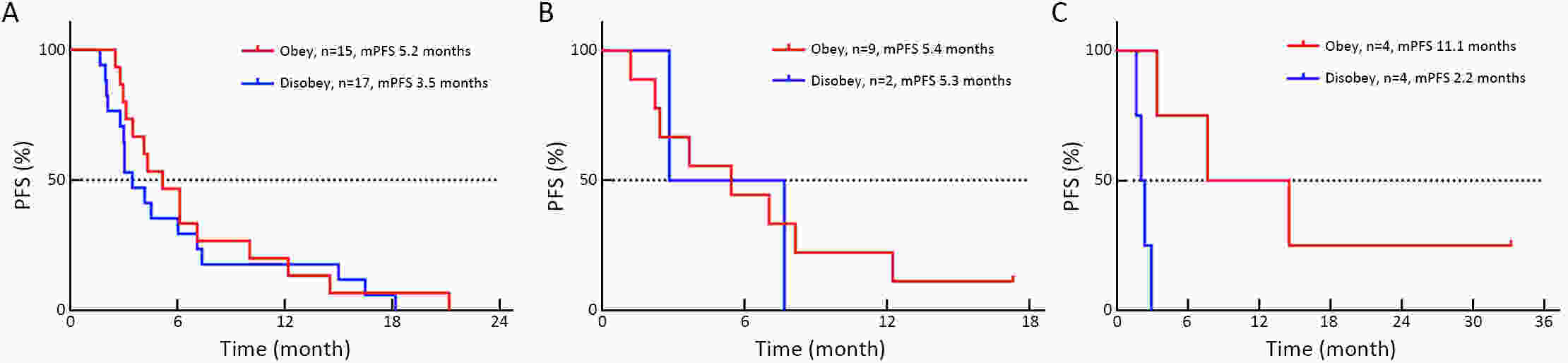
ObjectiveThe mechanism of acquired gene mutation plays a major role in resistance to endocrine therapy in hormone receptor (HR)-positive advanced breast cancer. Circulating tumor DNA (ctDNA) has been allowed for the assessment of the genomic profiles of patients with advanced cancer. We performed this study to search for molecular markers of endocrine therapy efficacy and to explore the clinical value of ctDNA to guide precise endocrine therapy for HR-positive/human epidermal growth factor receptor-2 (HER-2)-negative metastatic breast cancer patients. MethodsIn this open-label, multicohort, prospective study, patients were assigned to four parallel cohorts and matched according to mutations identified in ctDNA: 1) activation of the phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway preferred mTOR inhibitor combined with endocrine therapy; 2) estrogen receptor 1 (ESR1) mutation preferred fulvestrant; 3) HER-2 mutations preferred pyrotinib; and 4) no actionable mutations received treatment according to the clinical situation. In all cohorts, patients were divided into compliance group and violation group. The primary outcome measure was progression-free survival (PFS), and the secondary outcome measure was overall survival (OS). ResultsIn all cohorts, the combined median PFS was 4.9 months, and median PFS for the compliance and violation groups was 6.0 and 3.0 months, respectively [P=0.022, hazard ratio (HR)=0.57]. Multivariate Cox regression model showed the risk of disease progression was lower in compliance group than in violation group (P=0.023, HR=0.55). Among the patients with HER-2 mutations, the median PFS was 11.1 months in the compliance group and 2.2 months in the violation group (P=0.011, HR=0.20). There was no significant difference in the median PFS between patients who did and did not comply with the treatment protocol in patients with activation of the PI3K/AKT/mTOR or ESR1 mutation. ConclusionsThe results suggest that ctDNA may help to guide the optimal endocrine therapy strategy for metastatic breast cancer patients and to achieve a better PFS. Next-generation sequencing (NGS) detection could aid in distinguishing patients with HER-2 mutation and developing new treatment strategies.
2022, 34(4): 353-364.
doi: 10.21147/j.issn.1000-9604.2022.04.04
Abstract:
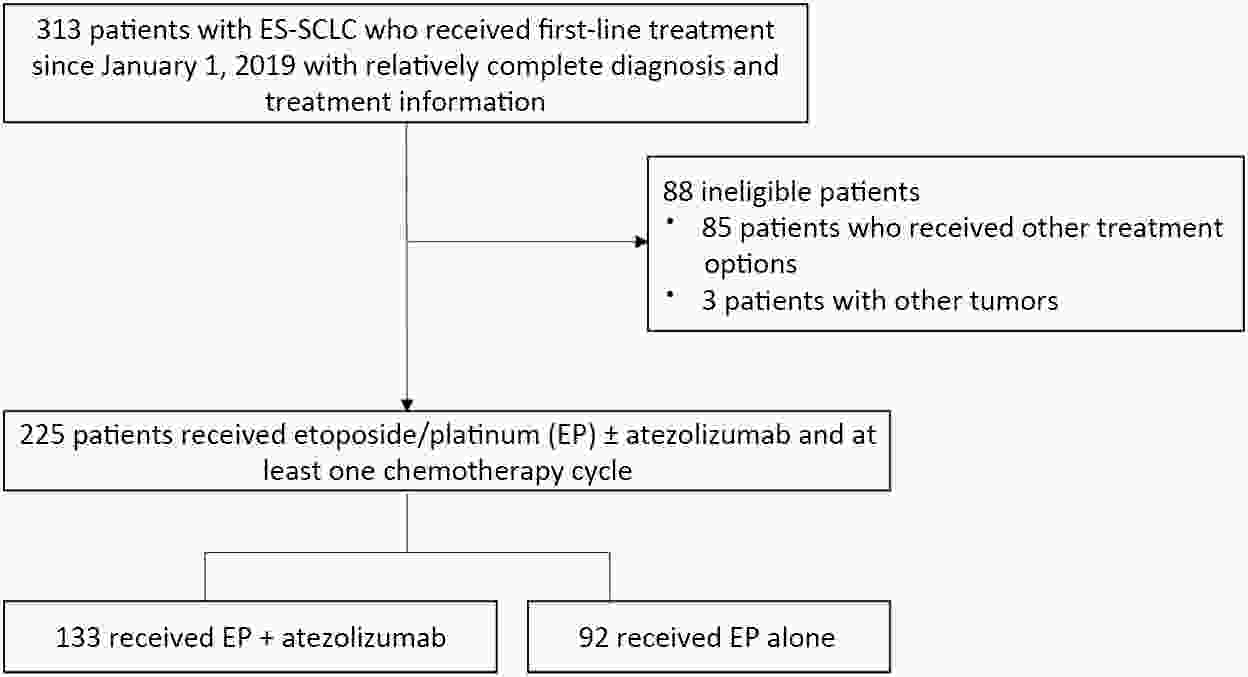
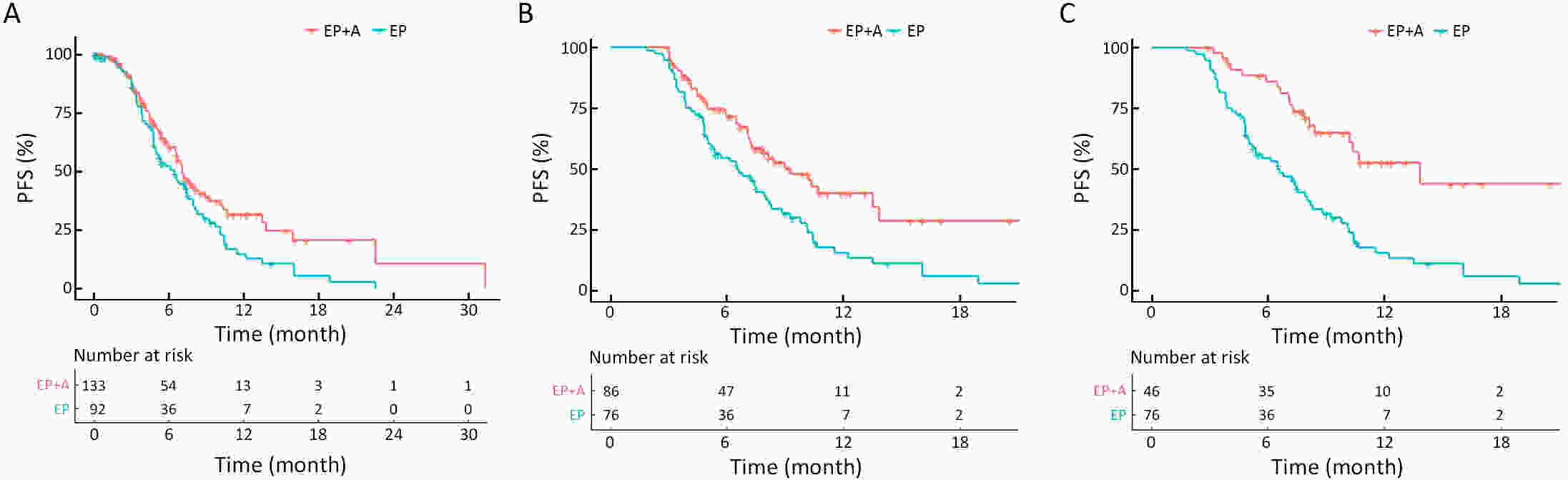
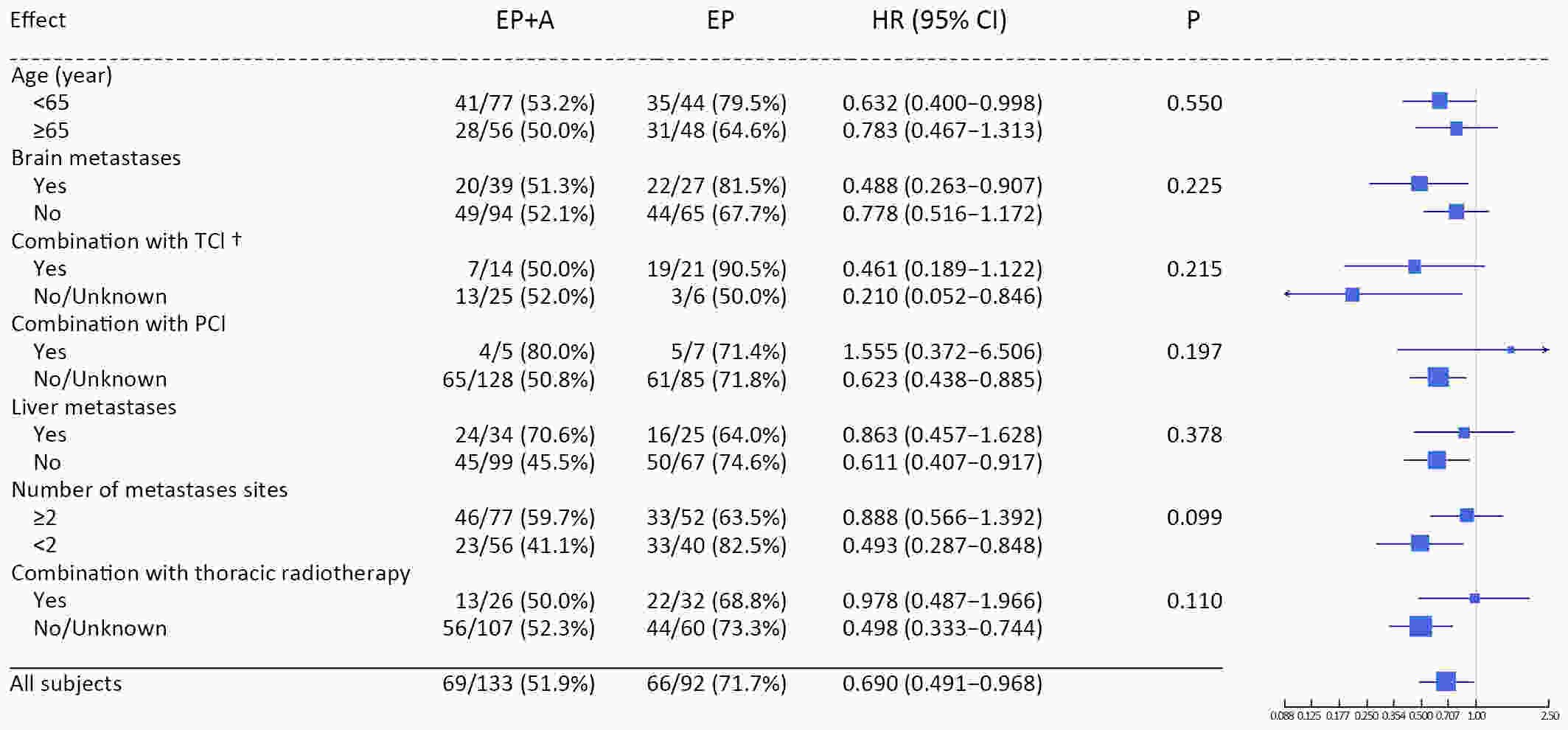
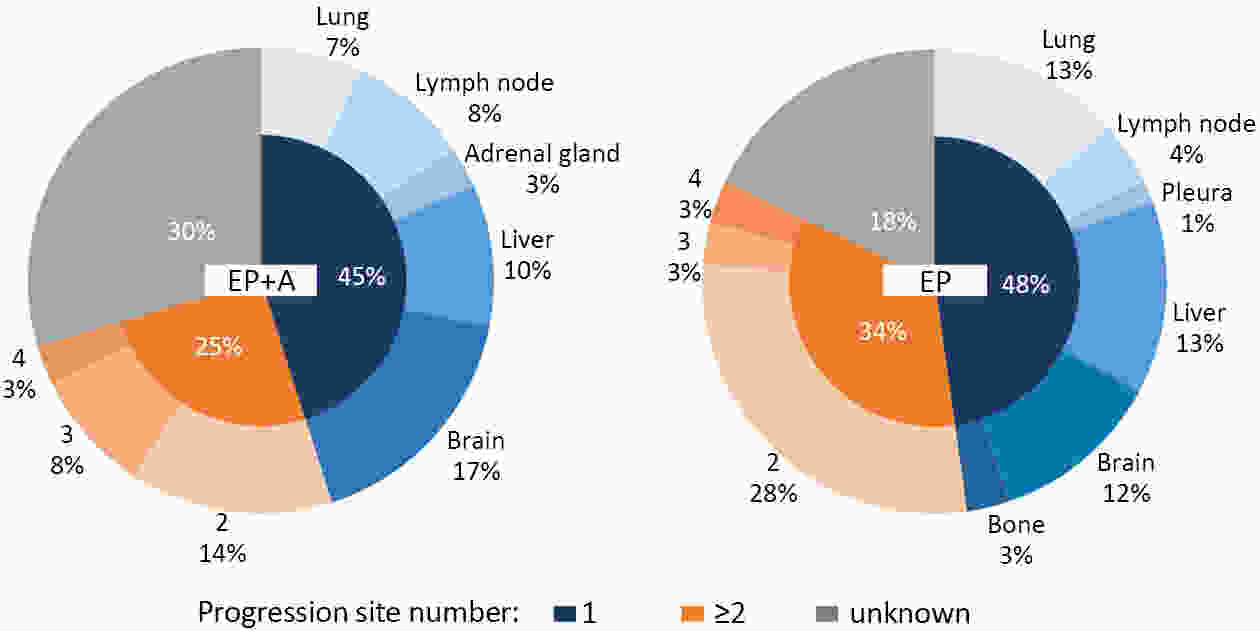
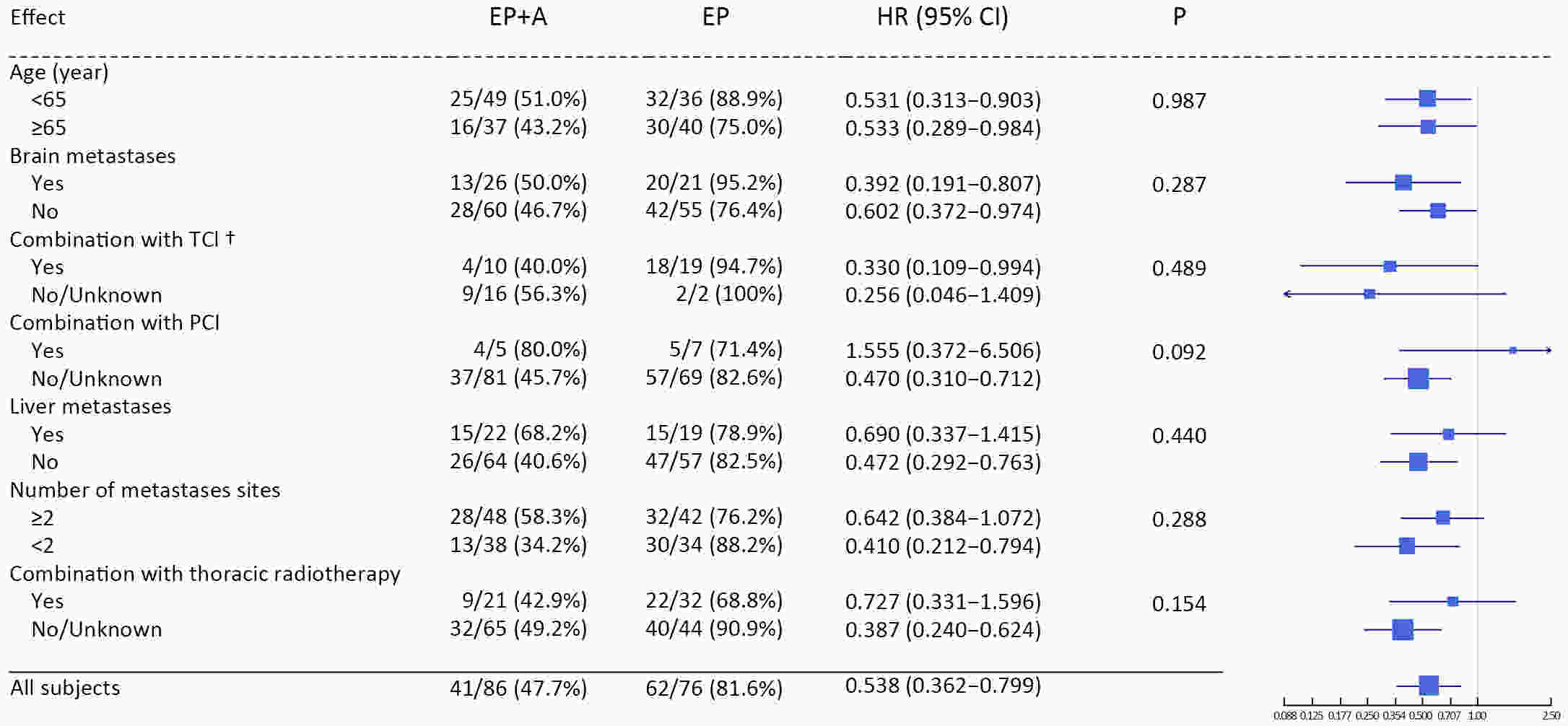
ObjectiveAtezolizumab along with chemotherapy has prolonged the survival of patients with extensive-stage small-cell lung cancer (ES-SCLC) worldwide, although real-world (RW) data are lacking in China. This study was designed to evaluate the efficacy and clinical outcomes of atezolizumab plus etoposide/platinum (EP). MethodsData obtained in this retrospective study were captured from six oncology units of five medical facilities from January 2019 to April 2022. For first-line treatments, atezolizumab combined with EP vs. EP alone, we primarily evaluated progression-free survival (PFS); other efficacy indicators, including overall survival (OS), objective response rate (ORR), and patterns of SCLC progression and adverse events (AEs) were assessed. ResultsThe primary analysis included data from 225 patients, of whom 133 received EP along with atezolizumab (atezolizumab group) and 92 received EP alone (EP group). The PFS duration of the atezolizumab group [7.10 months; 95% confidence interval (95% CI), 6.53−9.00] exceeded that of the EP group (6.50 months; 95% CI, 4.83−7.53). Overall, the hazard ratio (HR) was 0.69 (95% CI, 0.49−0.97) (P=0.029); particularly, the HR was 0.54 (95% CI, 0.36−0.80) among patients undergoing ≥4 chemotherapy cycles and 0.33 (95% CI, 0.20−0.56) among individuals with atezolizumab maintenance. The ORR and disease-control rate (DCR) were similar between the two groups. Because of incomplete OS data, the median OS was not determined for either group. Bone marrow suppression was the most common AE detected (58.6%) in the atezolizumab group. Immune-related AEs occurred in 19 patients in the atezolizumab group (14.3%), with only one case of grade 3 encephalitis. ConclusionsThis RW study in China demonstrated improved clinical outcomes of atezolizumab along with EP for ES-SCLC, particularly in the chemosensitive population. These results align with the results of the IMpower133 study, although the impact of this treatment modality on OS warrants additional follow-up studies.
2022, 34(4): 365-382.
doi: 10.21147/j.issn.1000-9604.2022.04.05
Abstract:
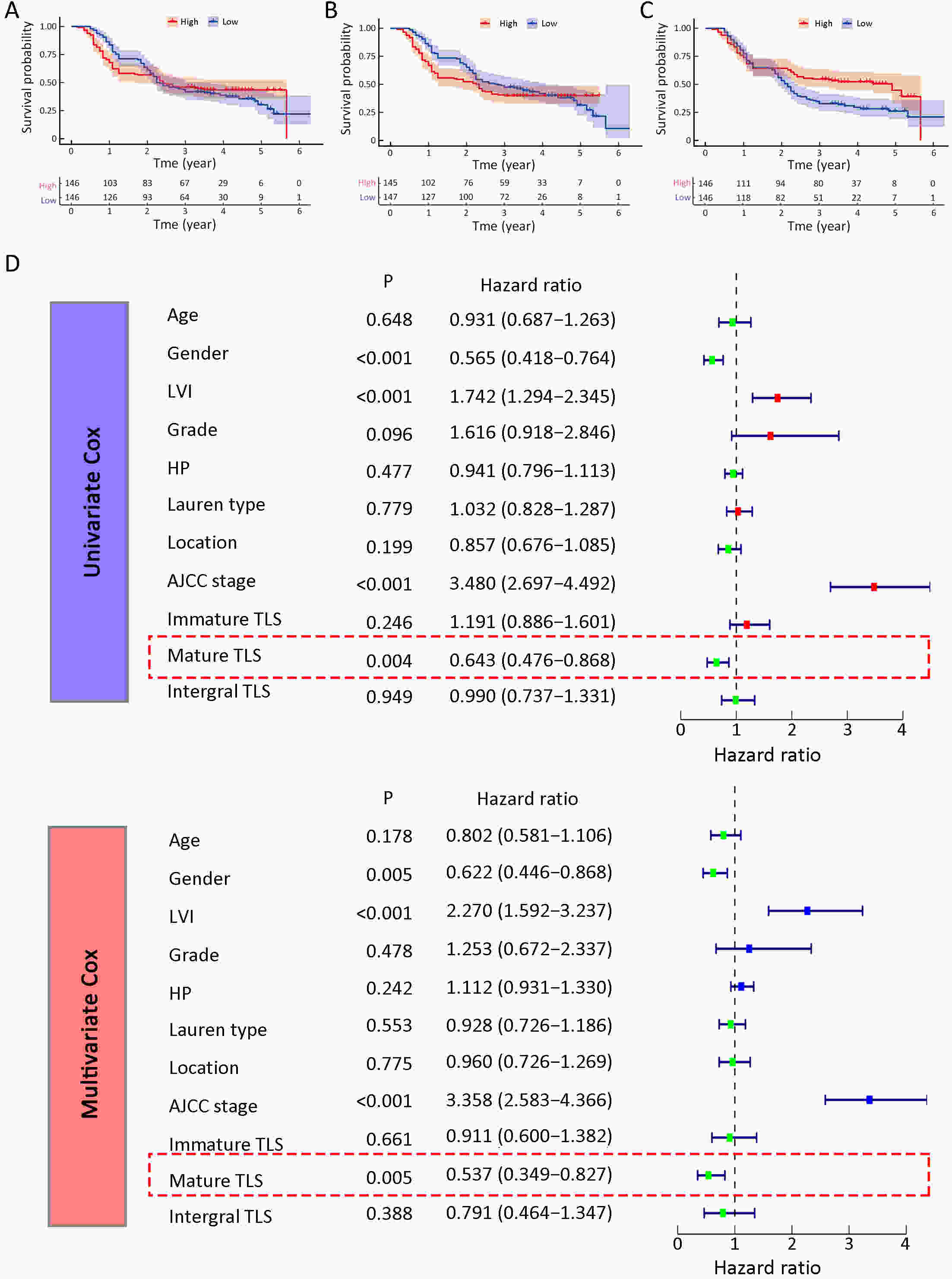
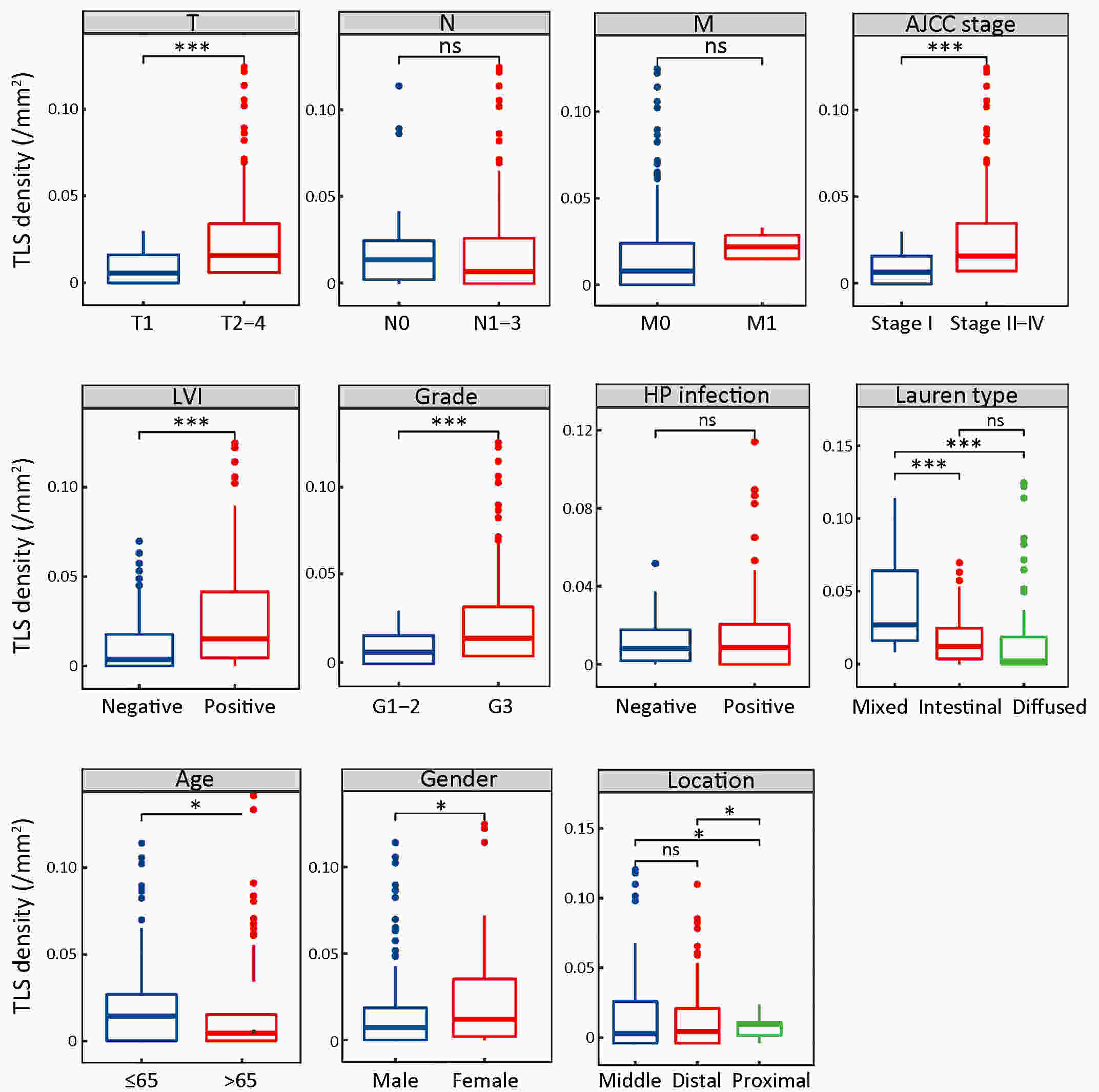
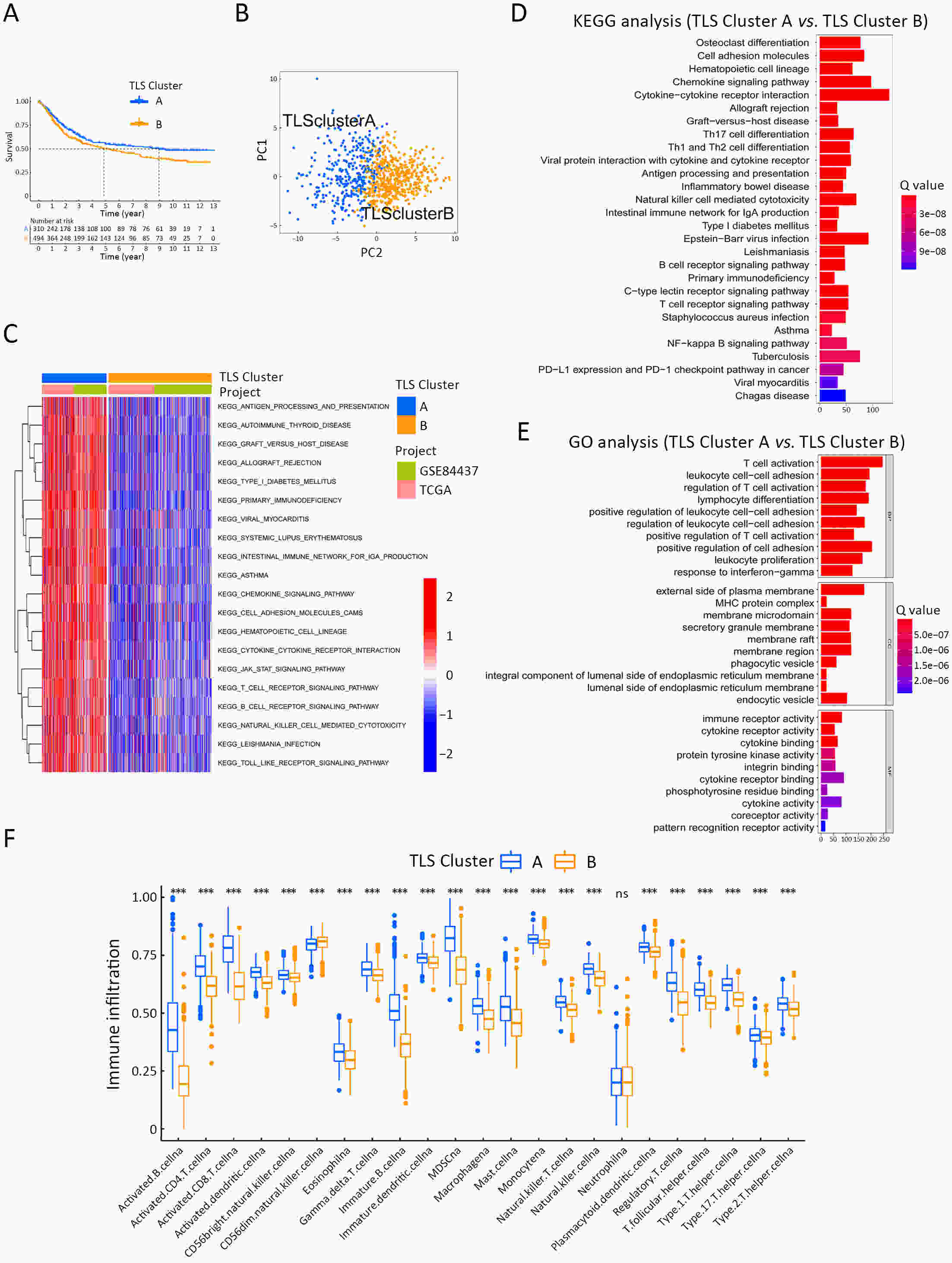
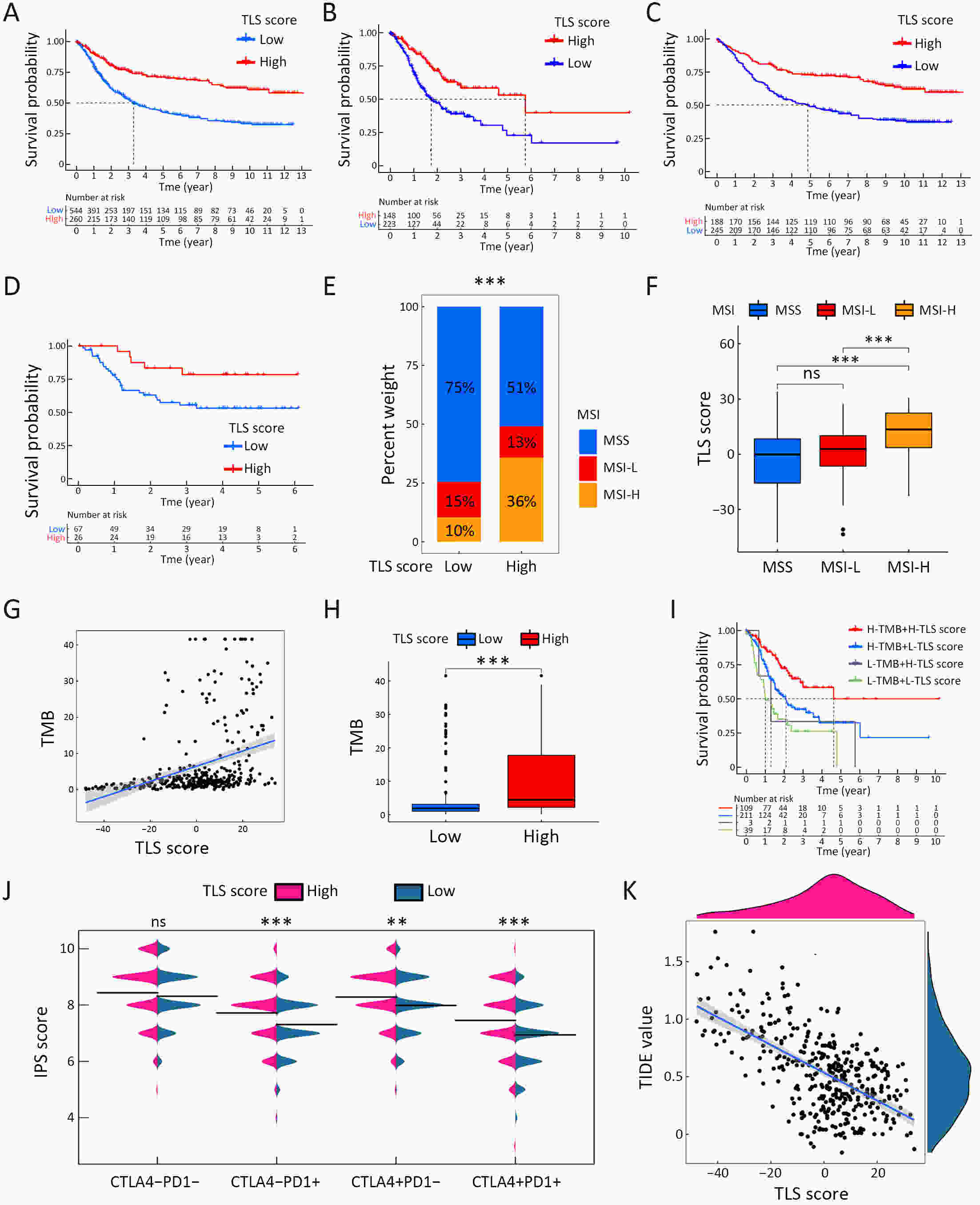
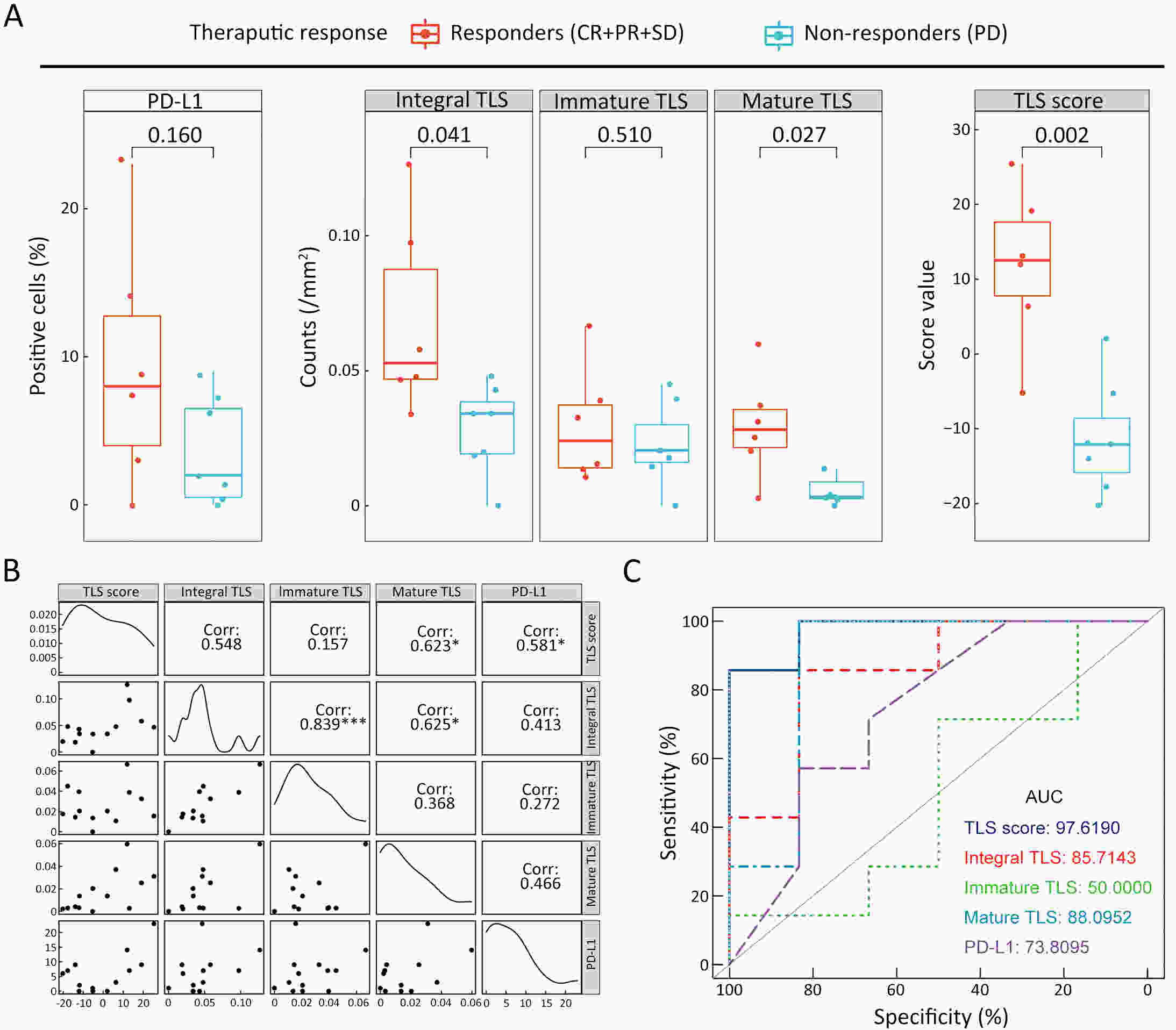
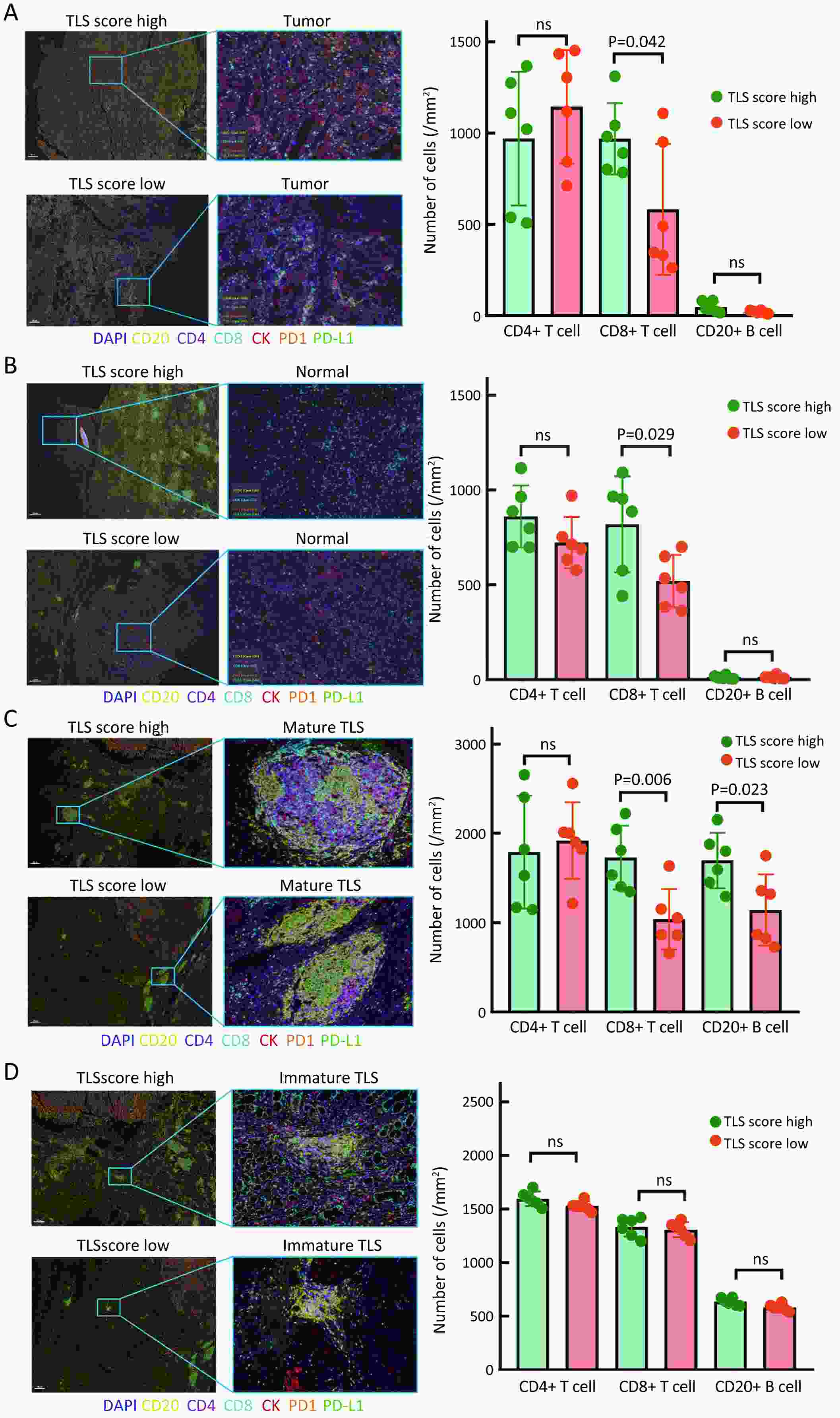
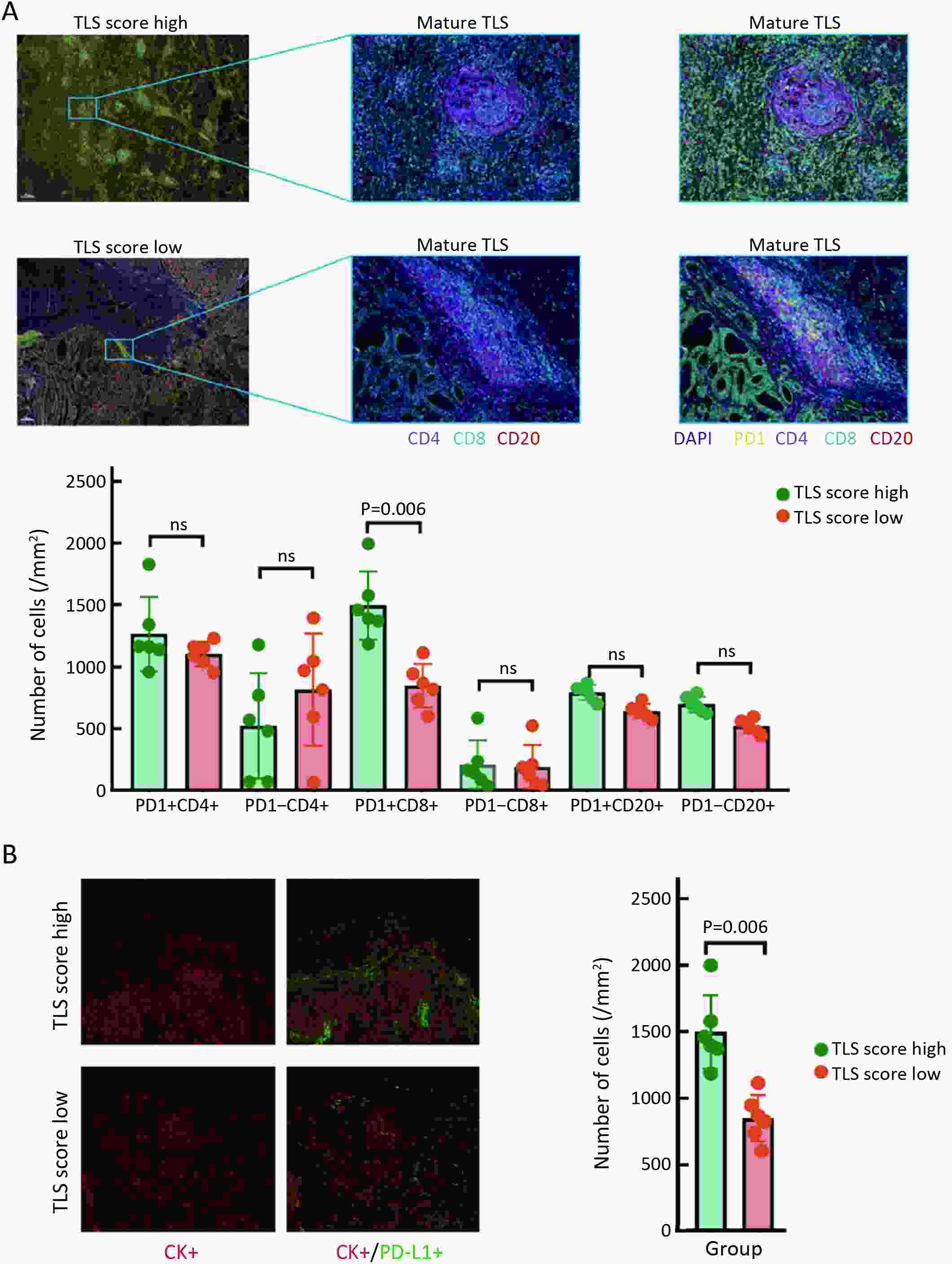
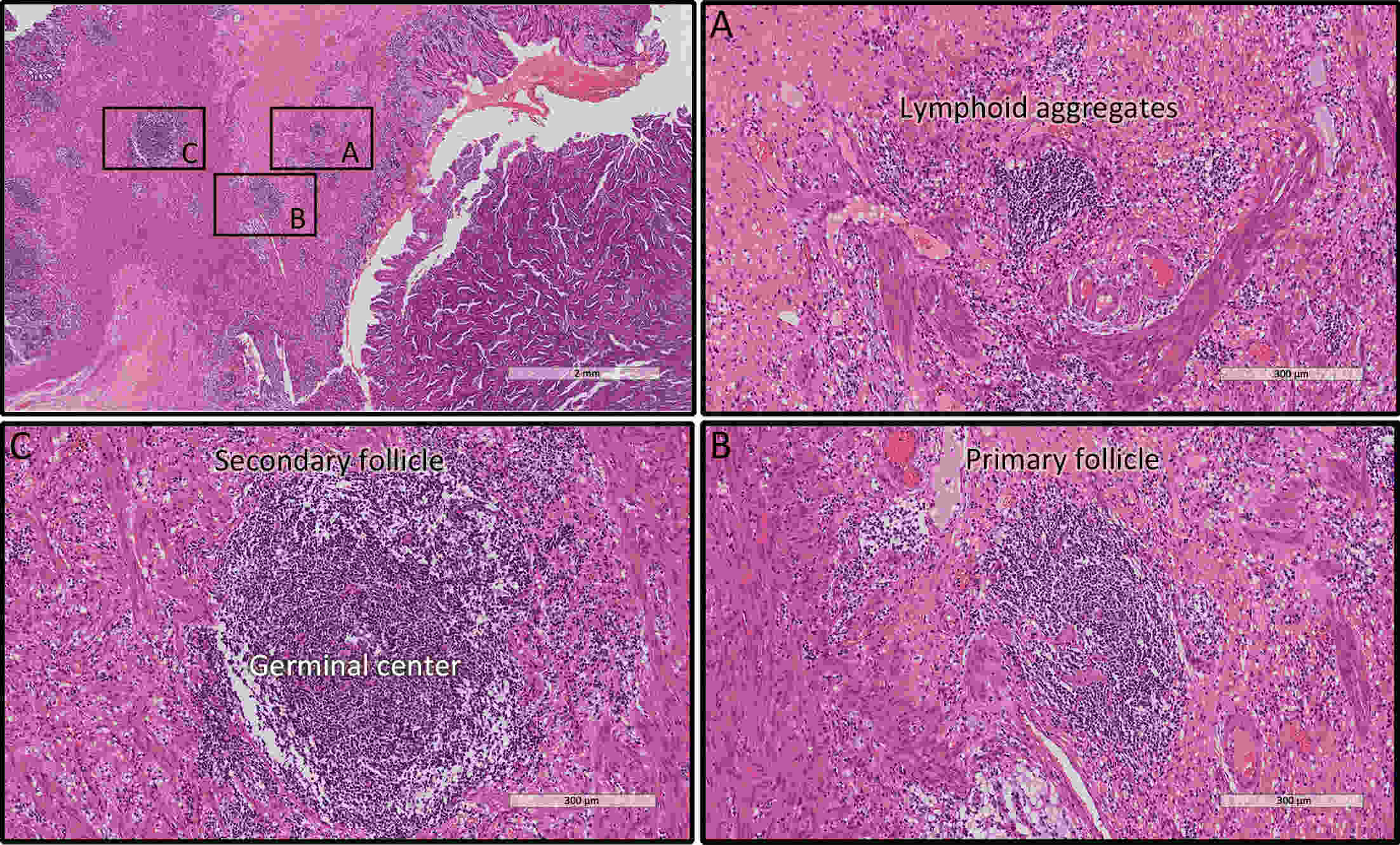
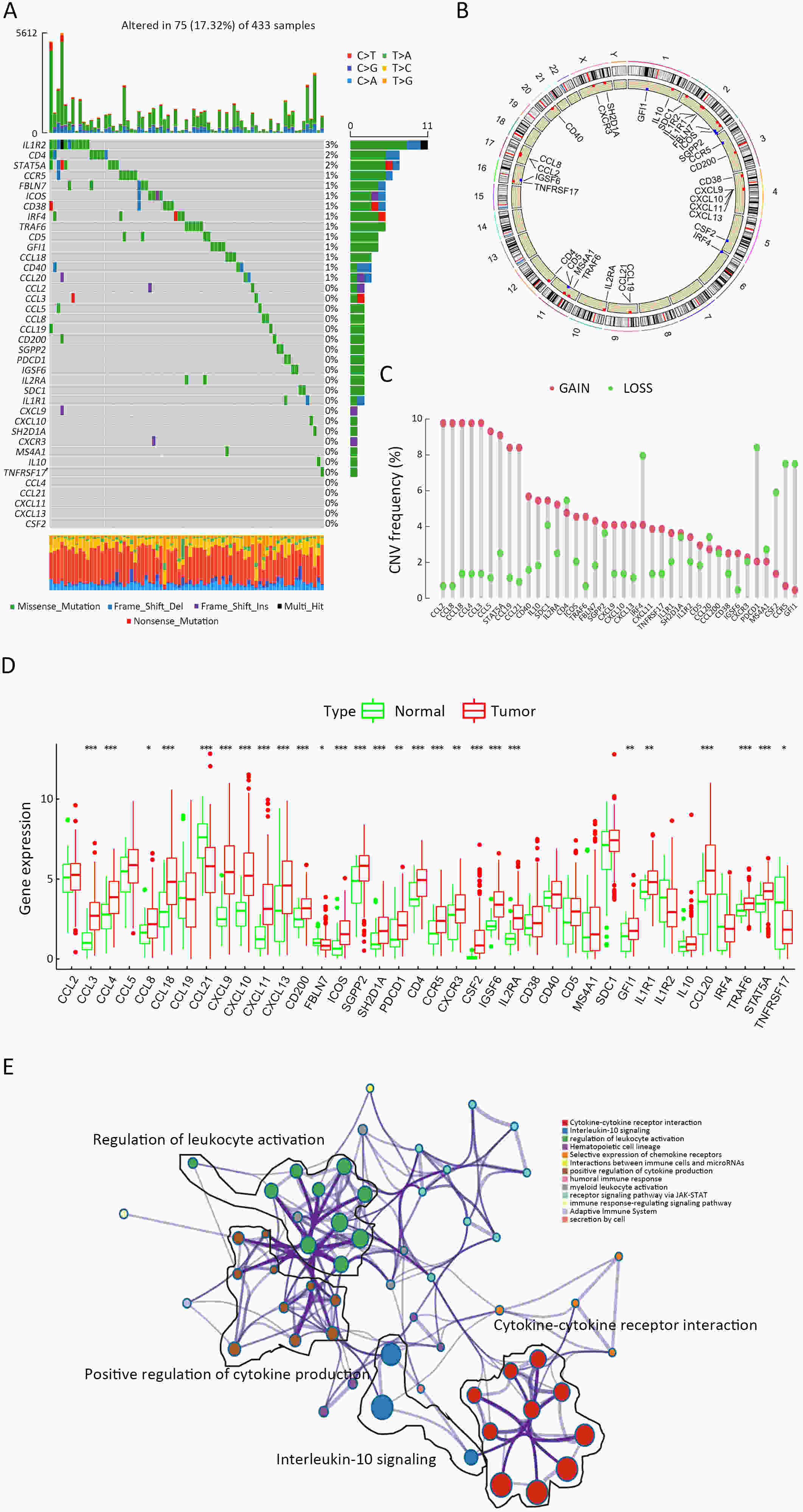
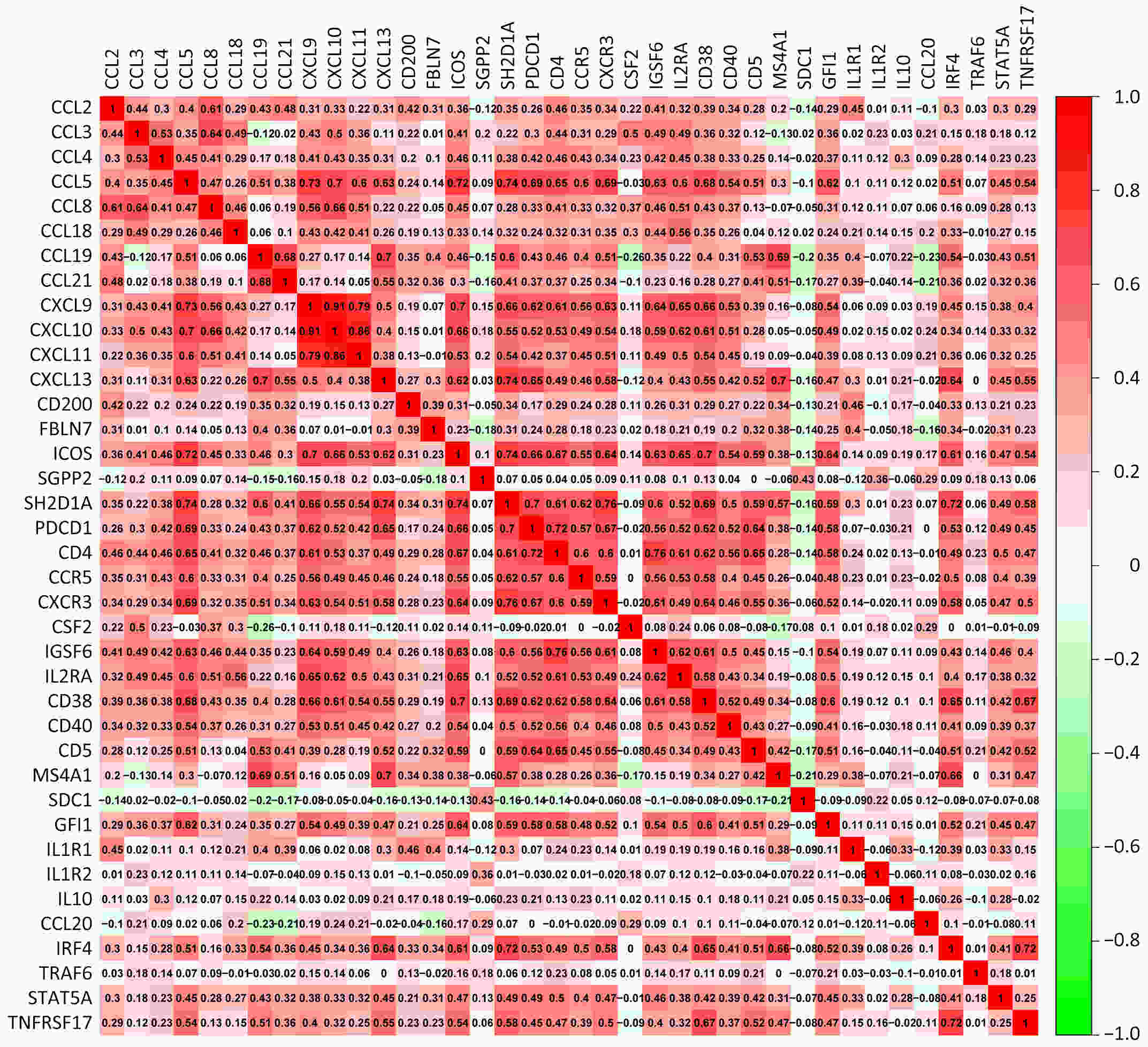
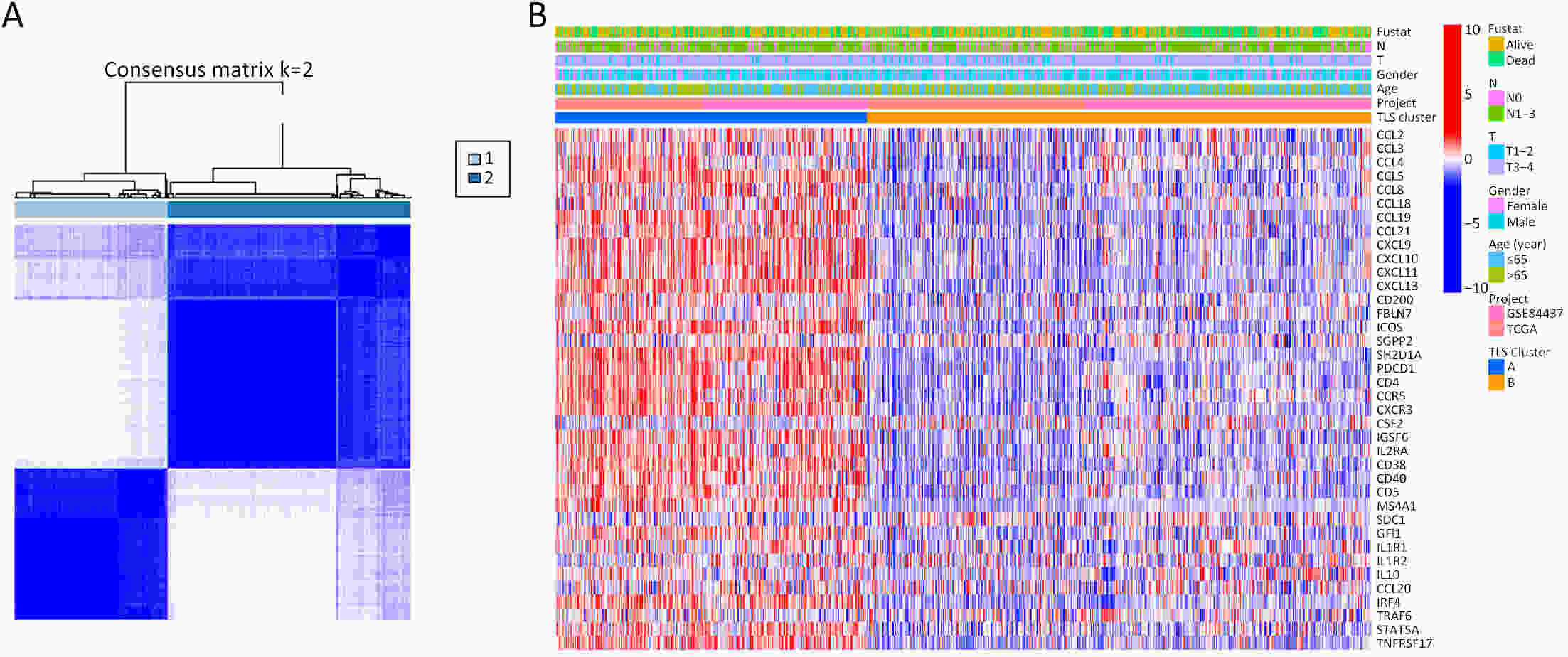
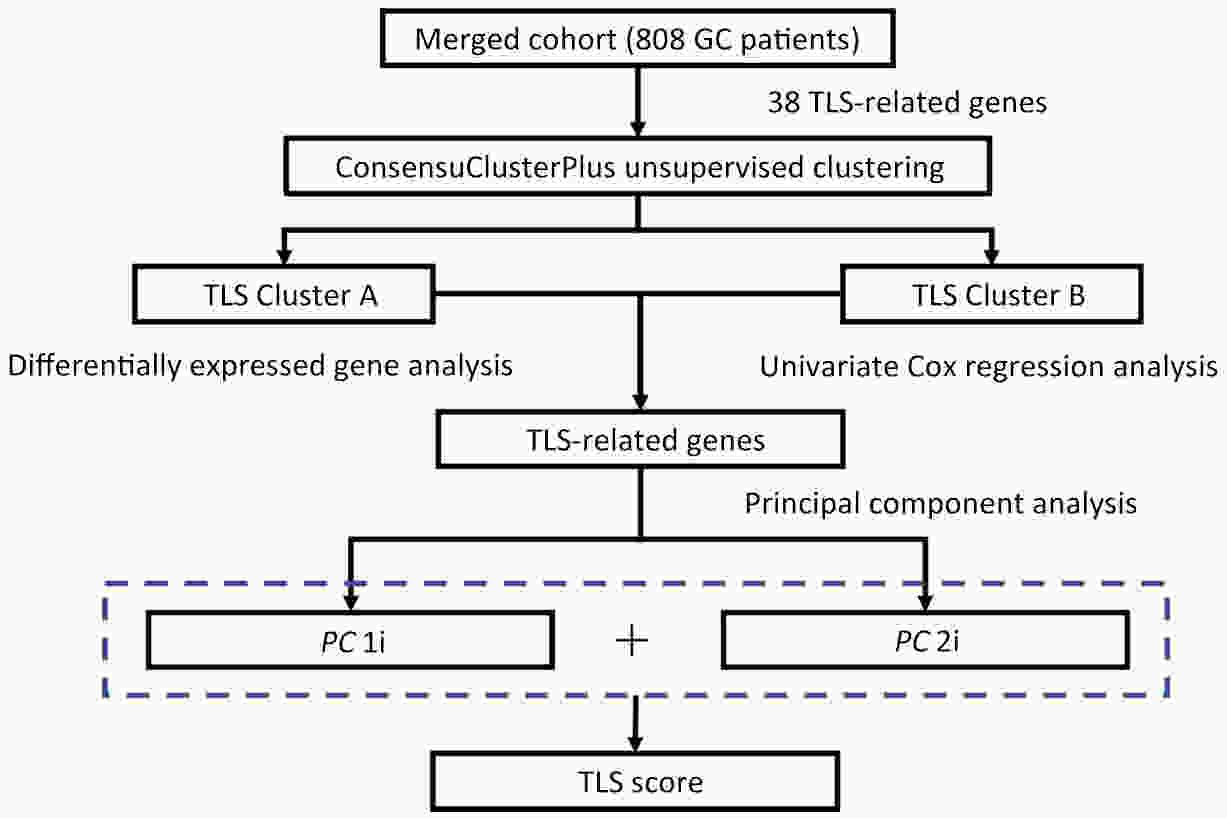
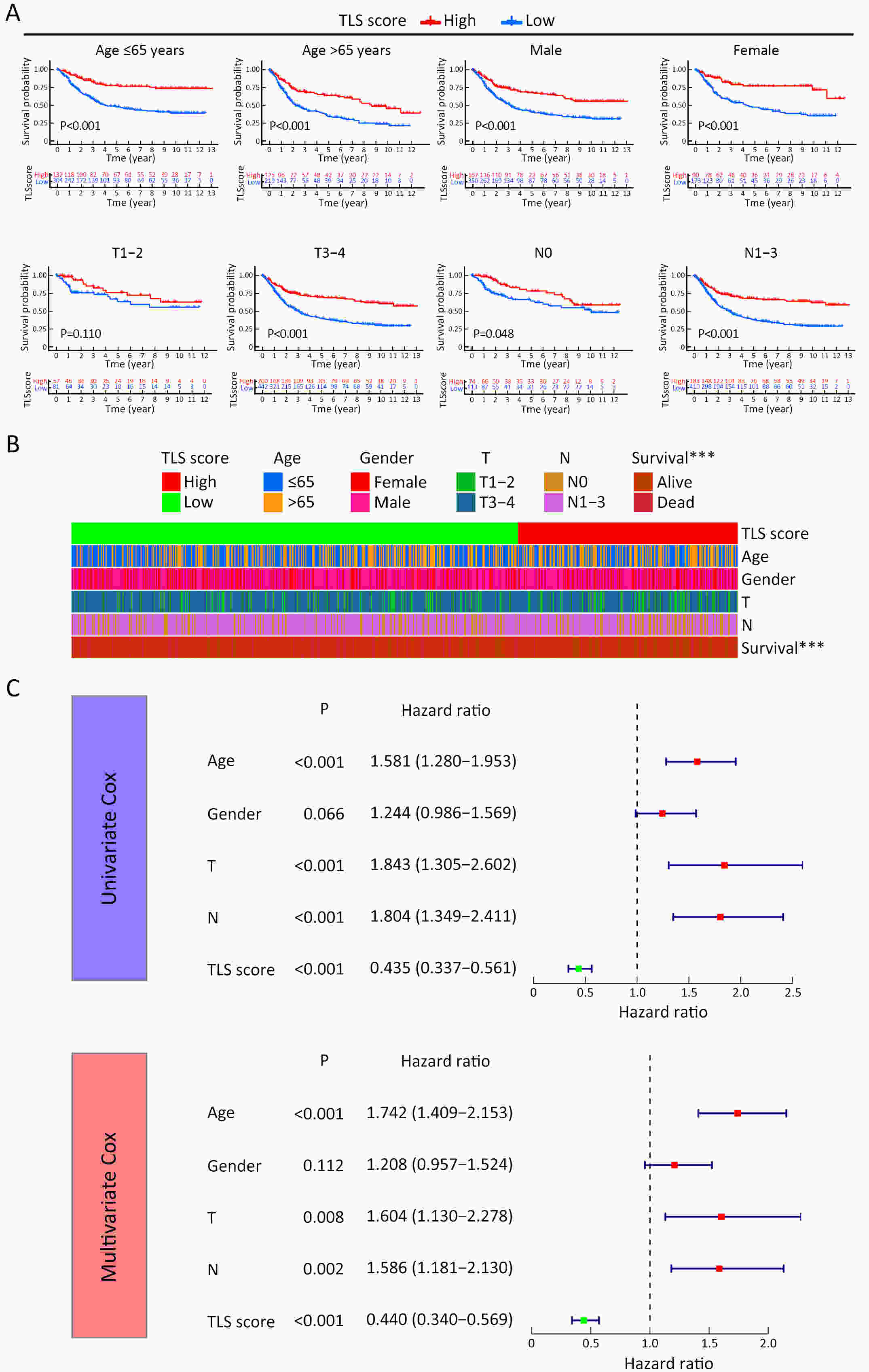
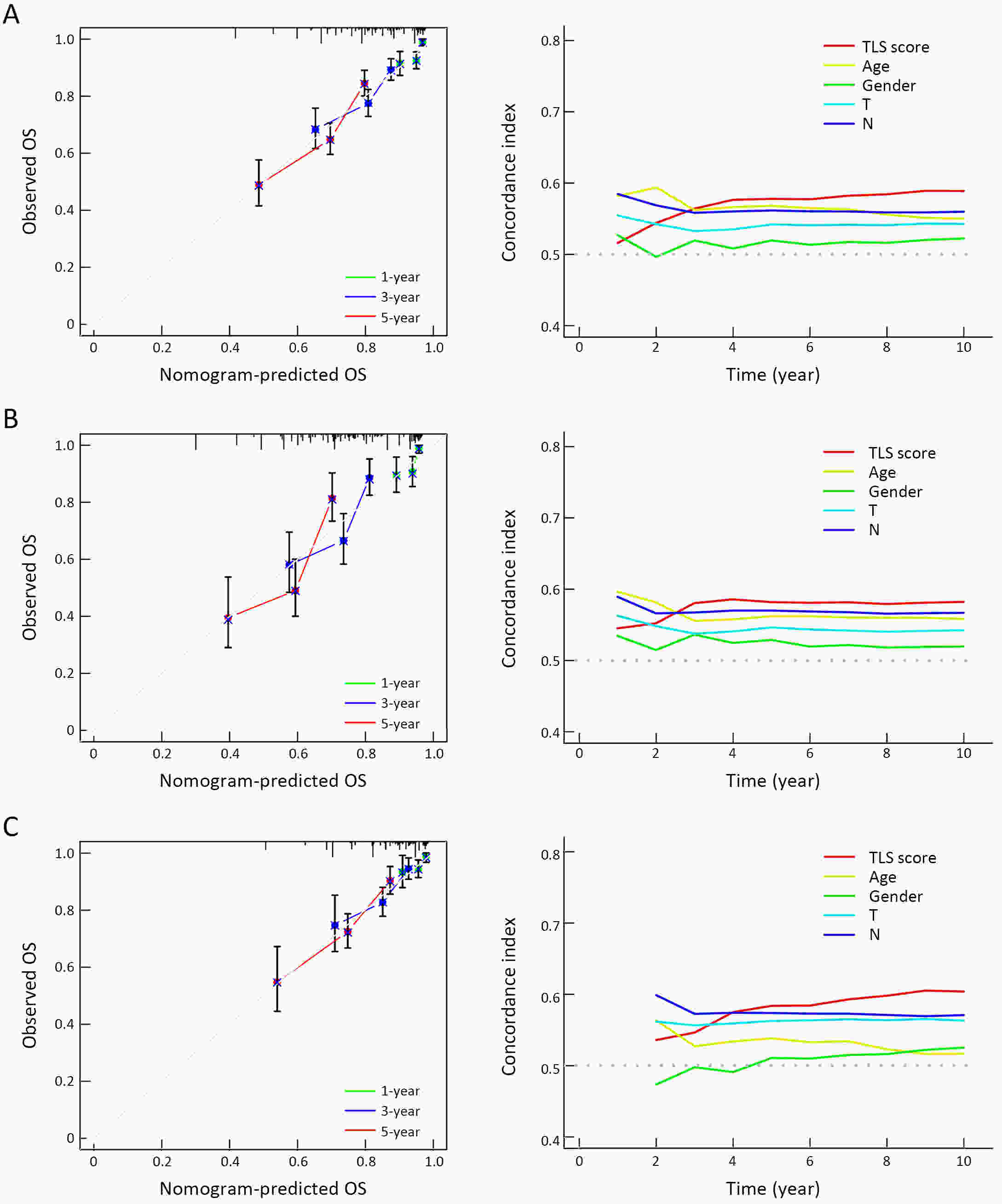
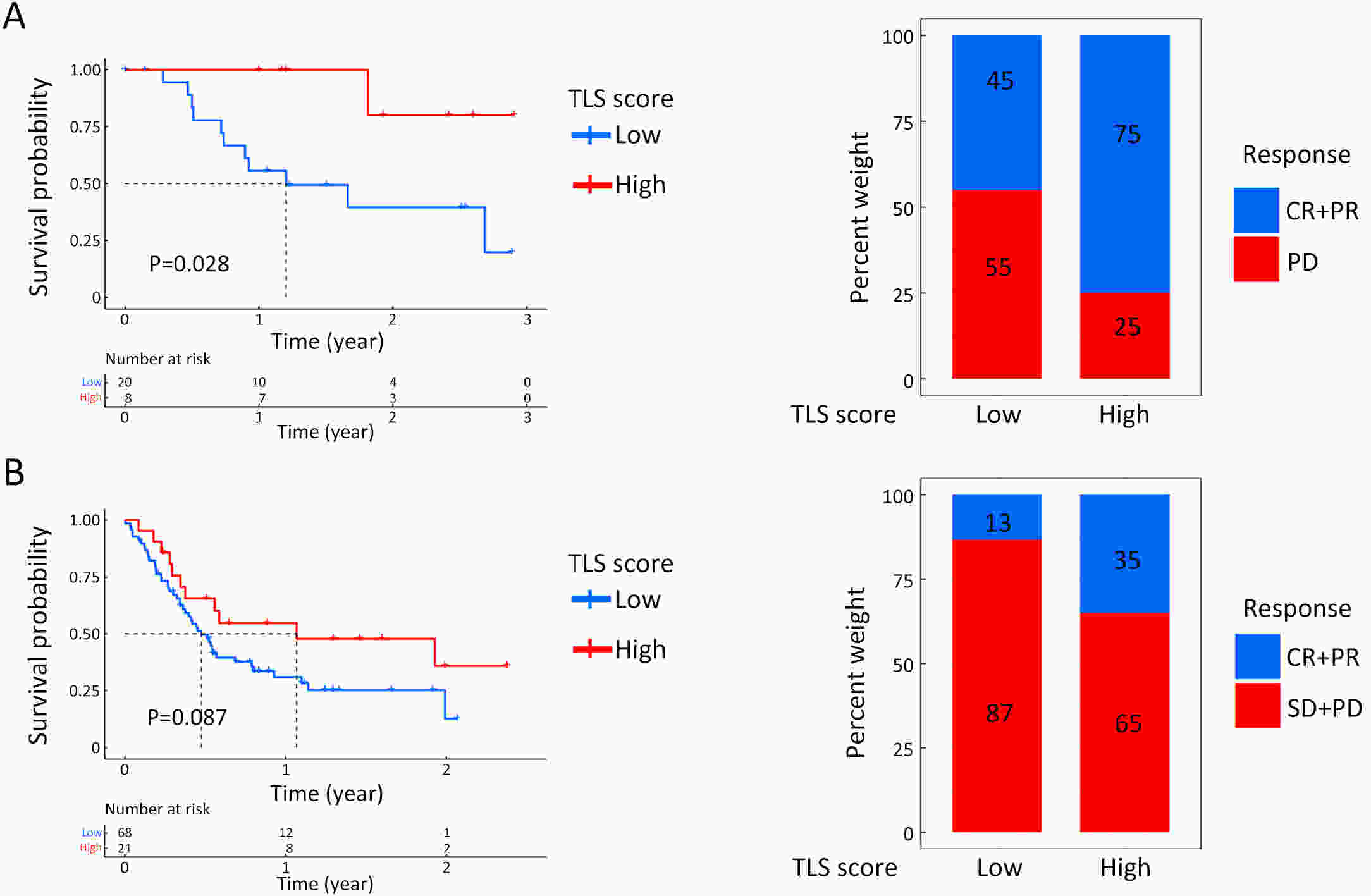
ObjectiveRecent studies have highlighted the distinct value of tertiary lymphoid structure (TLS) for immunotherapeutic response prediction. However, it remains unclear whether TLS could play such roles in gastric cancer (GC). MethodsIn this study, tumor tissue slices from 292 GC patients from Zhongshan Hospital were firstly reviewed to explore the correlation between TLS and clinical characteristics. Subsequently, we curated 38 reported genes that may function as triggers of TLS and performed consensus molecular subtyping in public RNA-seq datasets to determine TLS patterns in GC. Based on the differentially expressed genes acquired from two TLS patterns, we quantified TLS-related genes on the principal component analysis (PCA) algorithm to develop TLS score. A Zhongshan immunotherapy cohort including 13 patients who received programmed cell death 1 (PD1) blockade therapy was established to conduct RNA sequencing analysis and multiplex immunohistochemistry (mIHC) tests using formalin-fixed and paraffin-embedded (FFPE) tissues. The corresponding TLS score and immune cell counts were further compared based on therapeutic response variations. ResultsMature TLS was revealed as an independent prognostic factor in 292 GC patients. Patients with higher TLS score was characterized by prolonged survival time and superior response to immunotherapy. TLS score was correlated with immunotherapy-related characters, such as microsatellite instability (MSI) and tumor mutation burden (TMB). In addition, RNA-seq data analysis in the Zhongshan immunotherapy cohort indicated that a higher TLS score was correlated with a superior response to PD1 blockade therapy. mIHC tests also revealed that PD1+CD8+ T cell counts were significantly increased in the high-TLS score group. ConclusionsThis study highlighted that TLS was significantly associated with immune landscape diversity and complexity. Quantitatively evaluating TLS patterns of individual tumor will strengthen our understanding of TME characteristics and promote more effective immunotherapy strategies.
2022, 34(4): 383-394.
doi: 10.21147/j.issn.1000-9604.2022.04.06
Abstract:
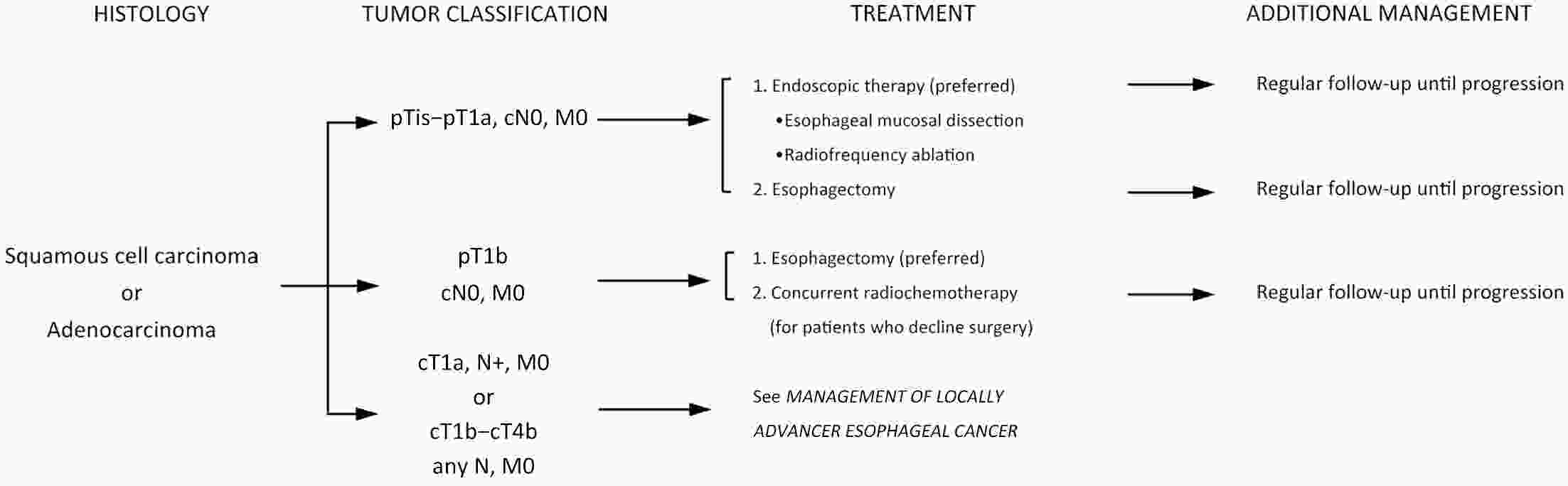
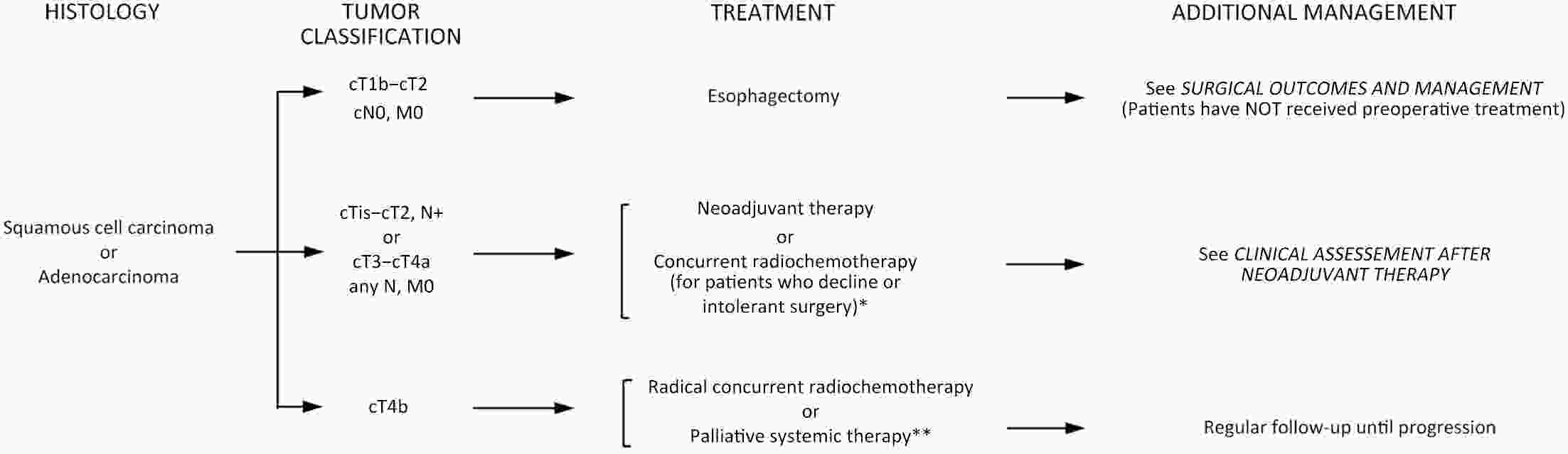
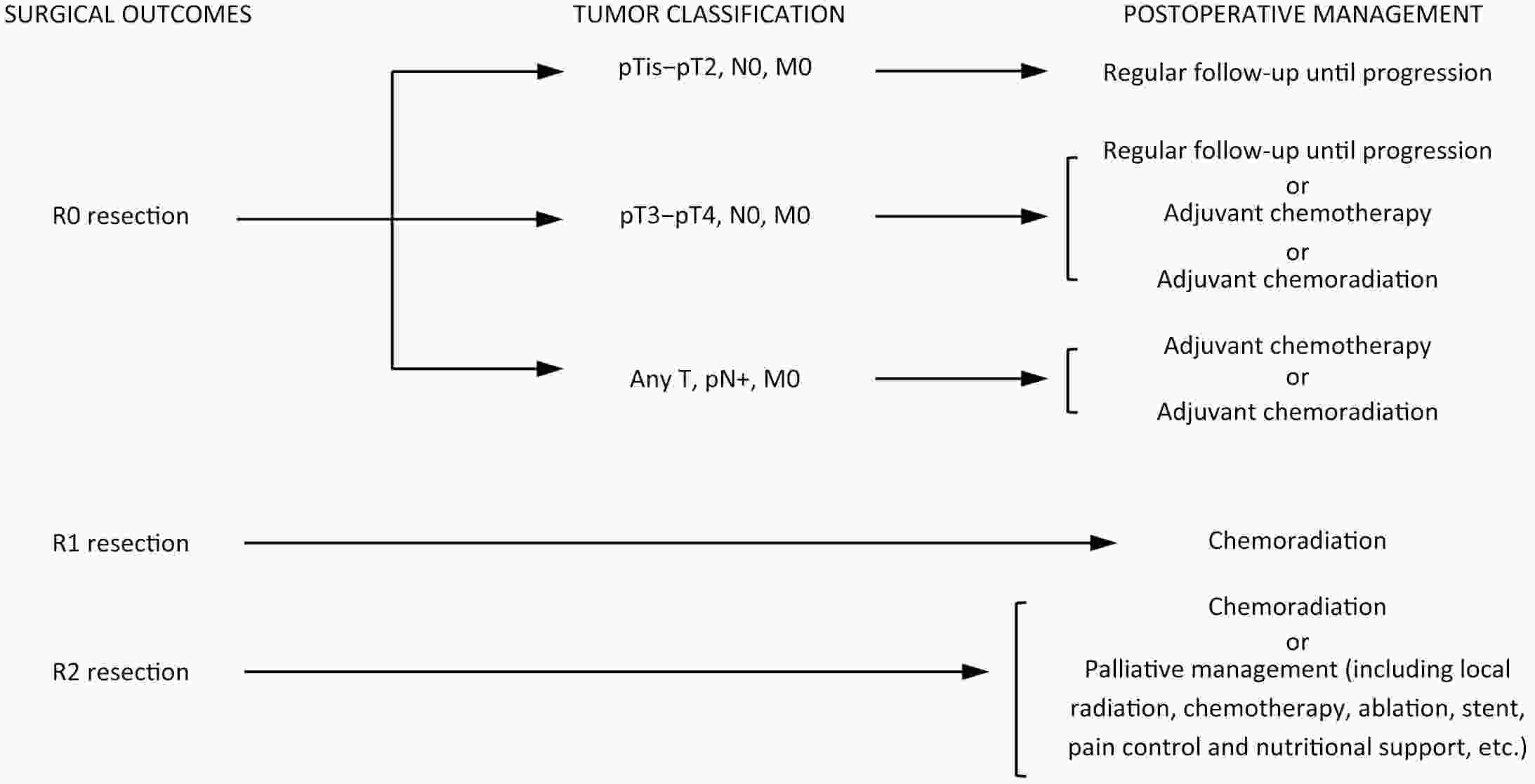
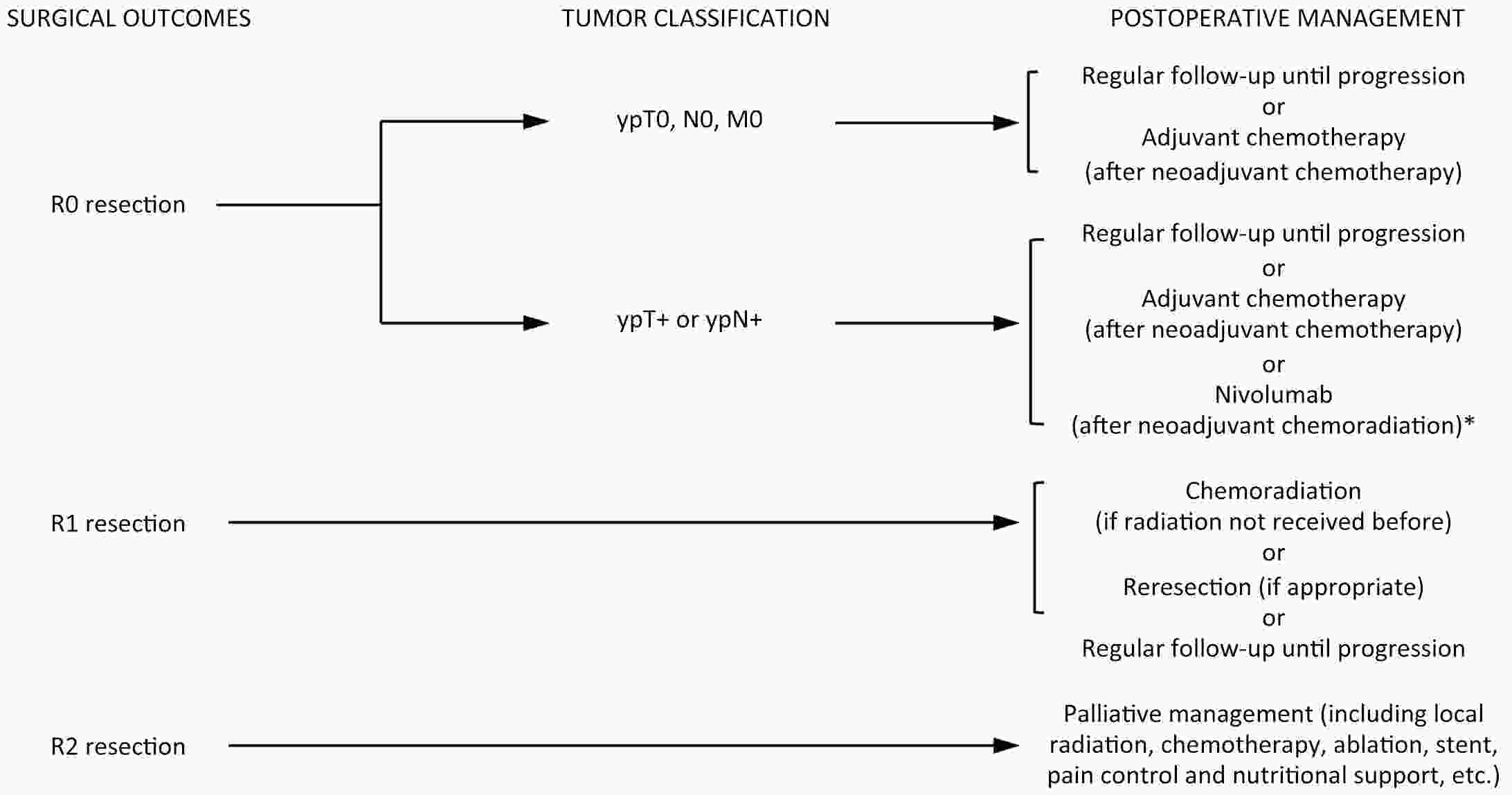
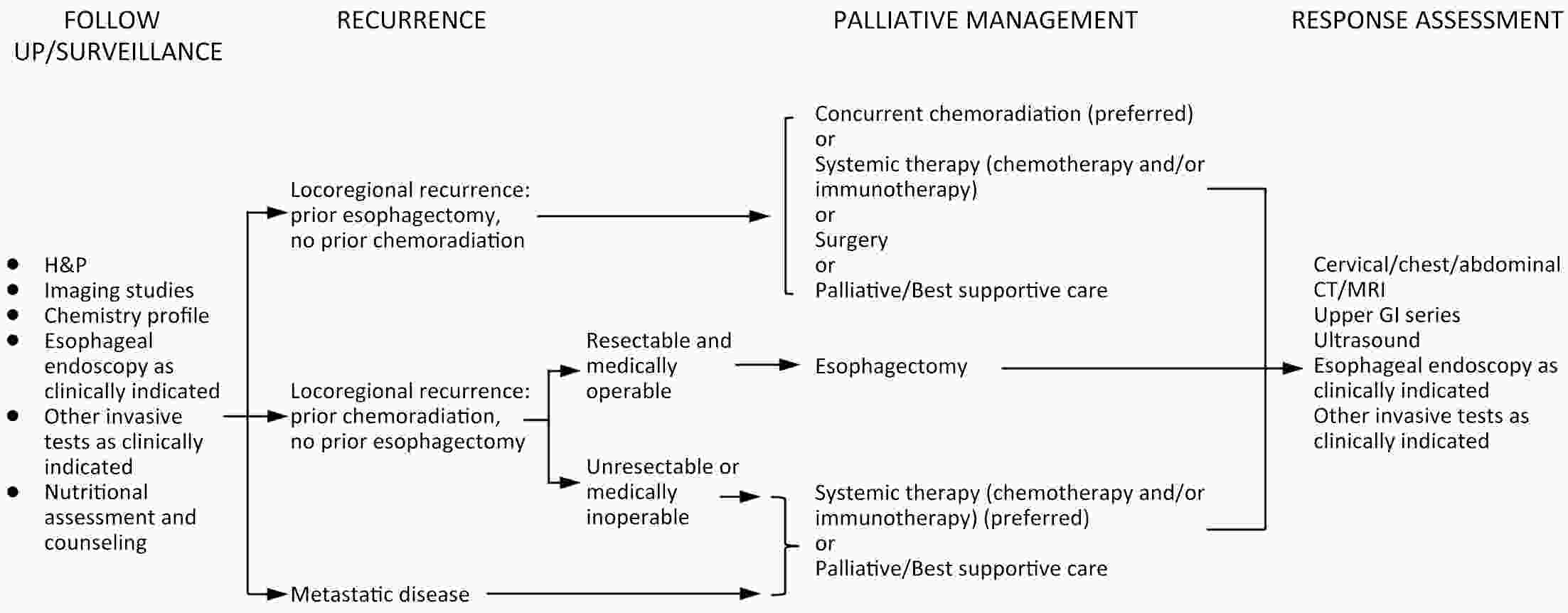
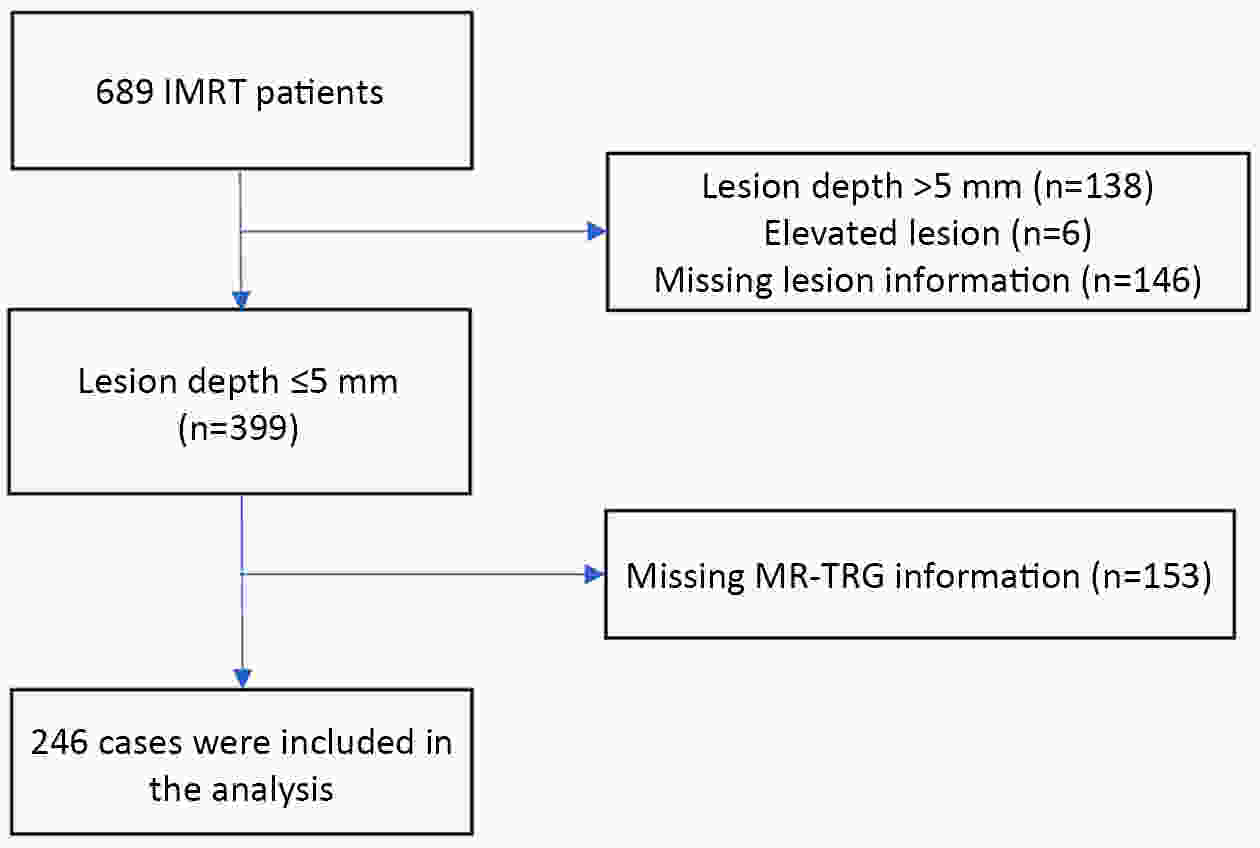
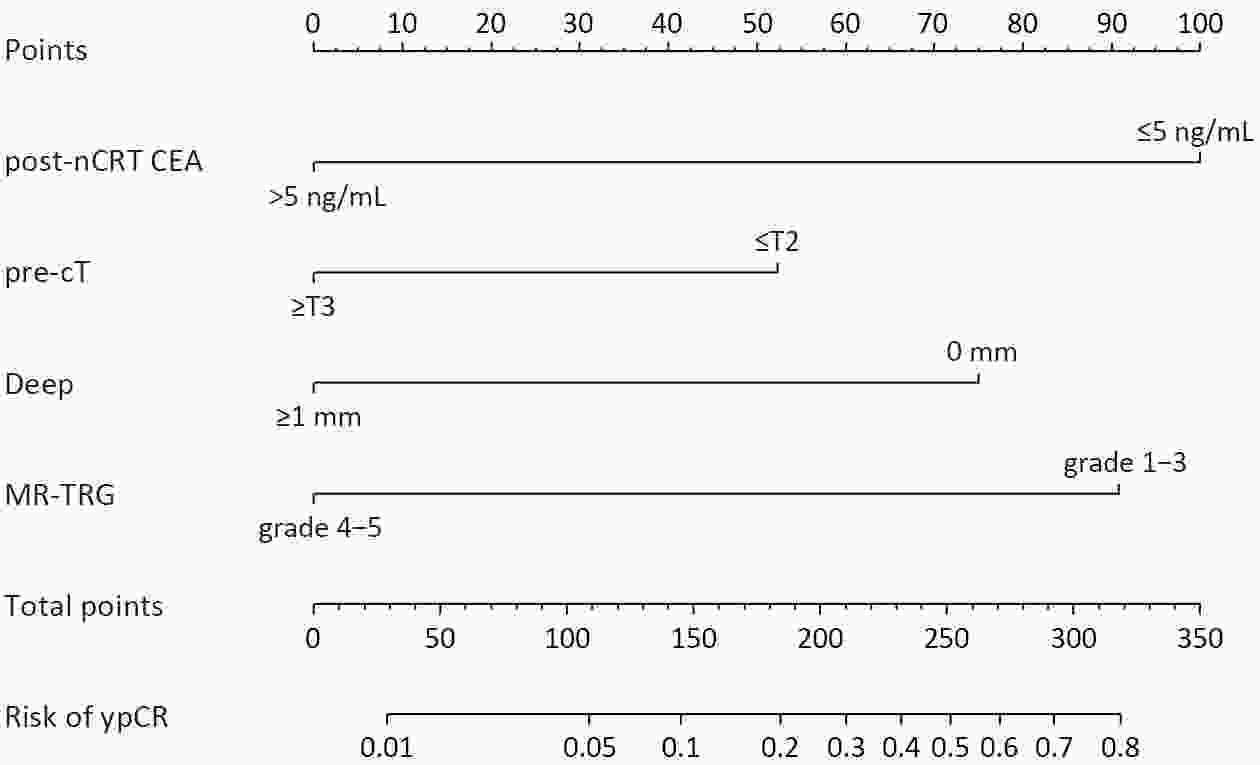
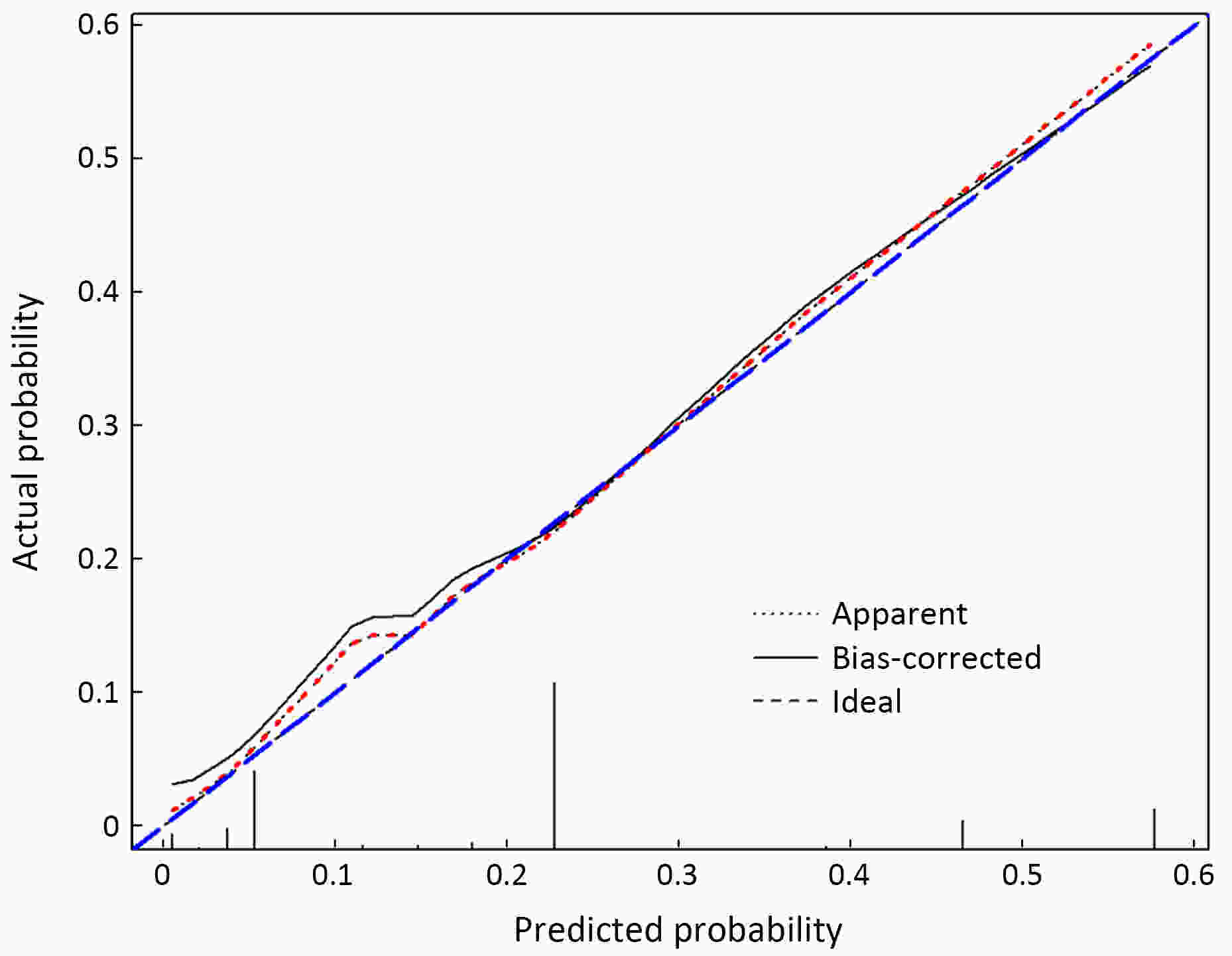
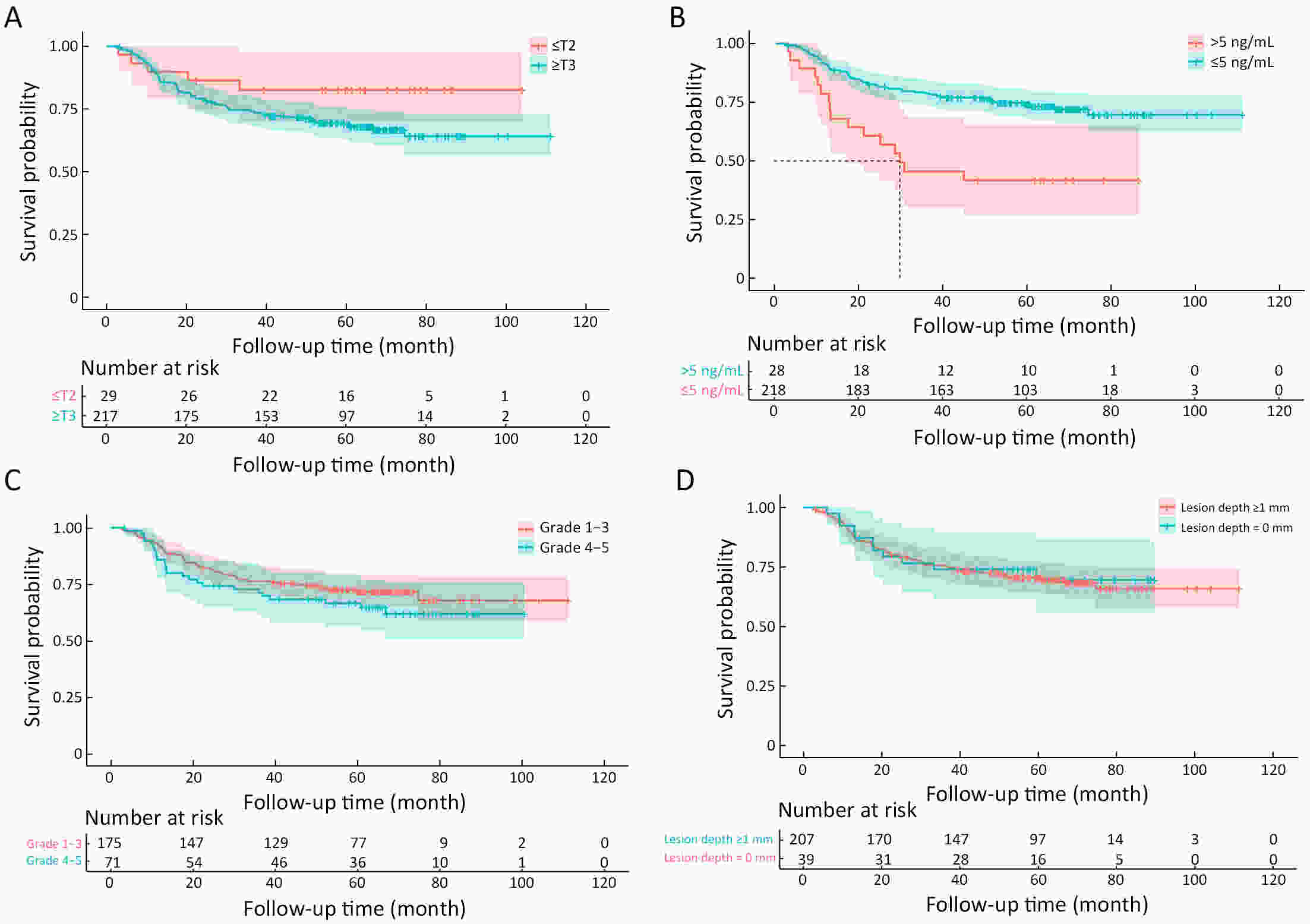
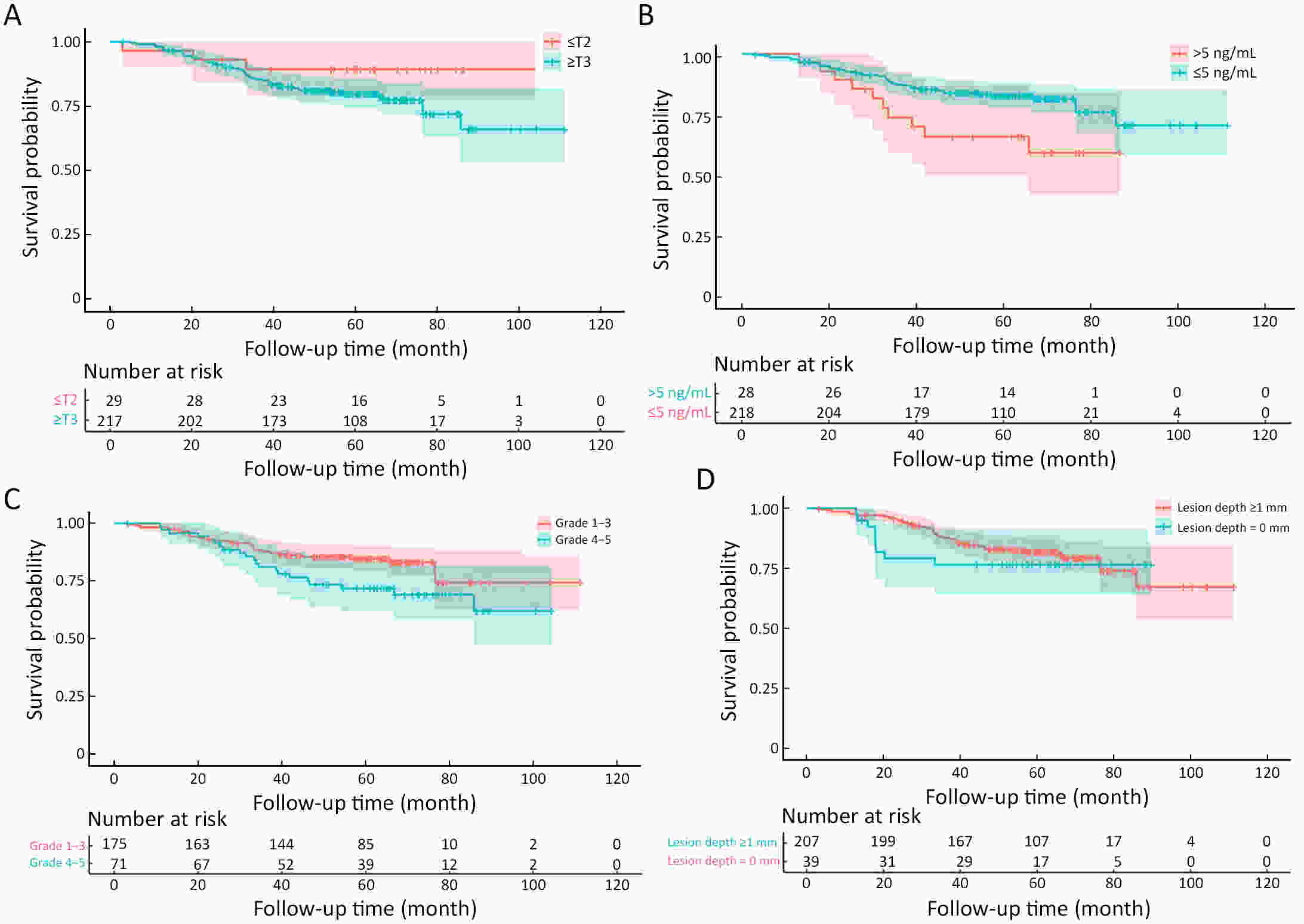
ObjectiveThe accurate prediction of tumor response to neoadjuvant chemoradiotherapy (nCRT) remains challenging. Few studies have investigated pathologic complete response (ypCR) prediction in patients with residual flat mucosal lesions after treatment. This study aimed to identify variables for predicting ypCR in patients with residual flat mucosal lesions after nCRT for locally advanced rectal cancer (LARC). MethodsData of patients with residual flat mucosal lesions after nCRT who underwent radical resection between 2009 and 2015 were retrospectively collected from the LARC database at Peking University Cancer Hospital. Univariate and multivariate analyses of the association between clinicopathological factors and ypCR were performed, and a nomogram was constructed by incorporating the significant predictors. ResultsOf the 246 patients with residual flat mucosal lesions included in the final analysis, 56 (22.8%) had ypCR. Univariate and multivariate analyses showed that pretreatment cT stage (pre-cT) ≤T2 (P=0.016), magnetic resonance tumor regression grade (MR-TRG) 1−3 (P=0.001) and residual mucosal lesion depth =0 mm (P<0.001) were associated with a higher rate of ypCR. A nomogram was developed with a concordance index (C-index) of 0.759 and the calibration curve showed that the nomogram model had good predictive consistency. The follow-up time ranged from 3.0 to 113.3 months, with a median follow-up time of 63.77 months. The multivariate Cox regression model showed that the four variables in the nomogram model were not risk factors for disease-free survival (DFS) or overall survival (OS). ConclusionsCompletely flat mucosa, early cT stage and good MR-TRG were predictive factors for ypCR instead of DFS or OS in patients with LARC with residual flat mucosal lesions after nCRT. Endoscopic mucosal re-evaluation before surgery is important, as it may contribute to decision-making and facilitate nonoperative management or organ preservation.
2022, 34(4): 395-405.
doi: 10.21147/j.issn.1000-9604.2022.04.07
Abstract:
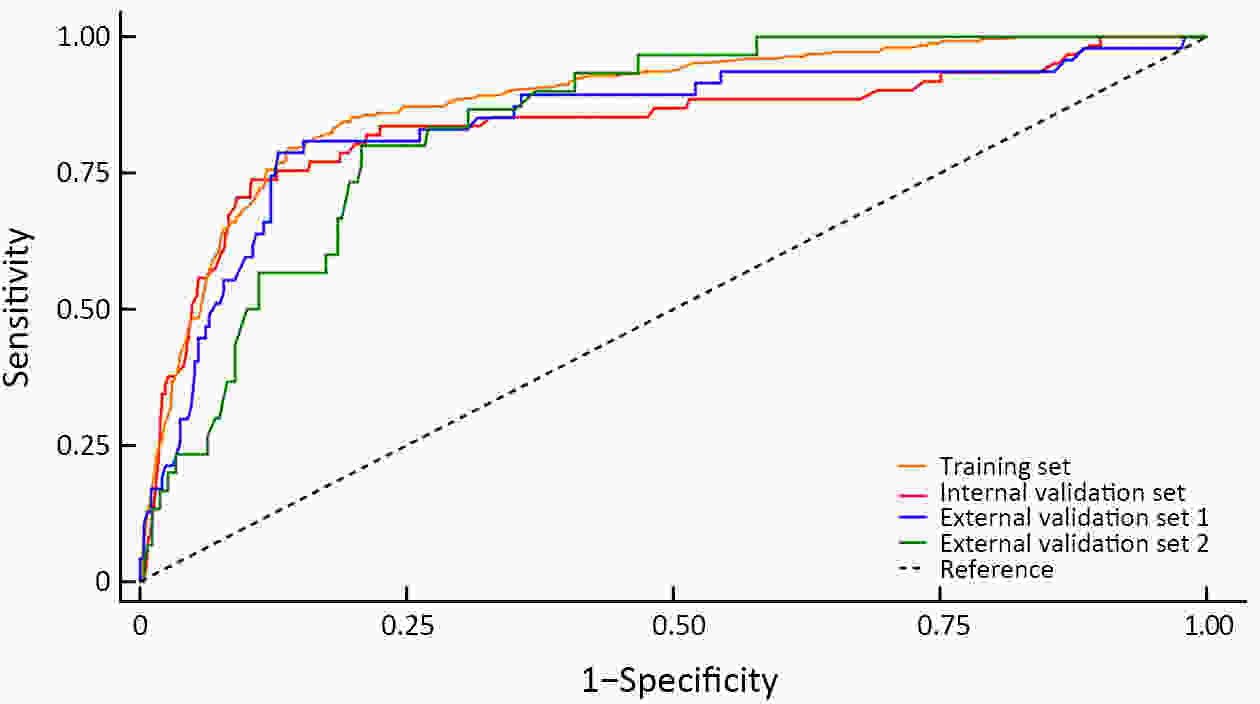
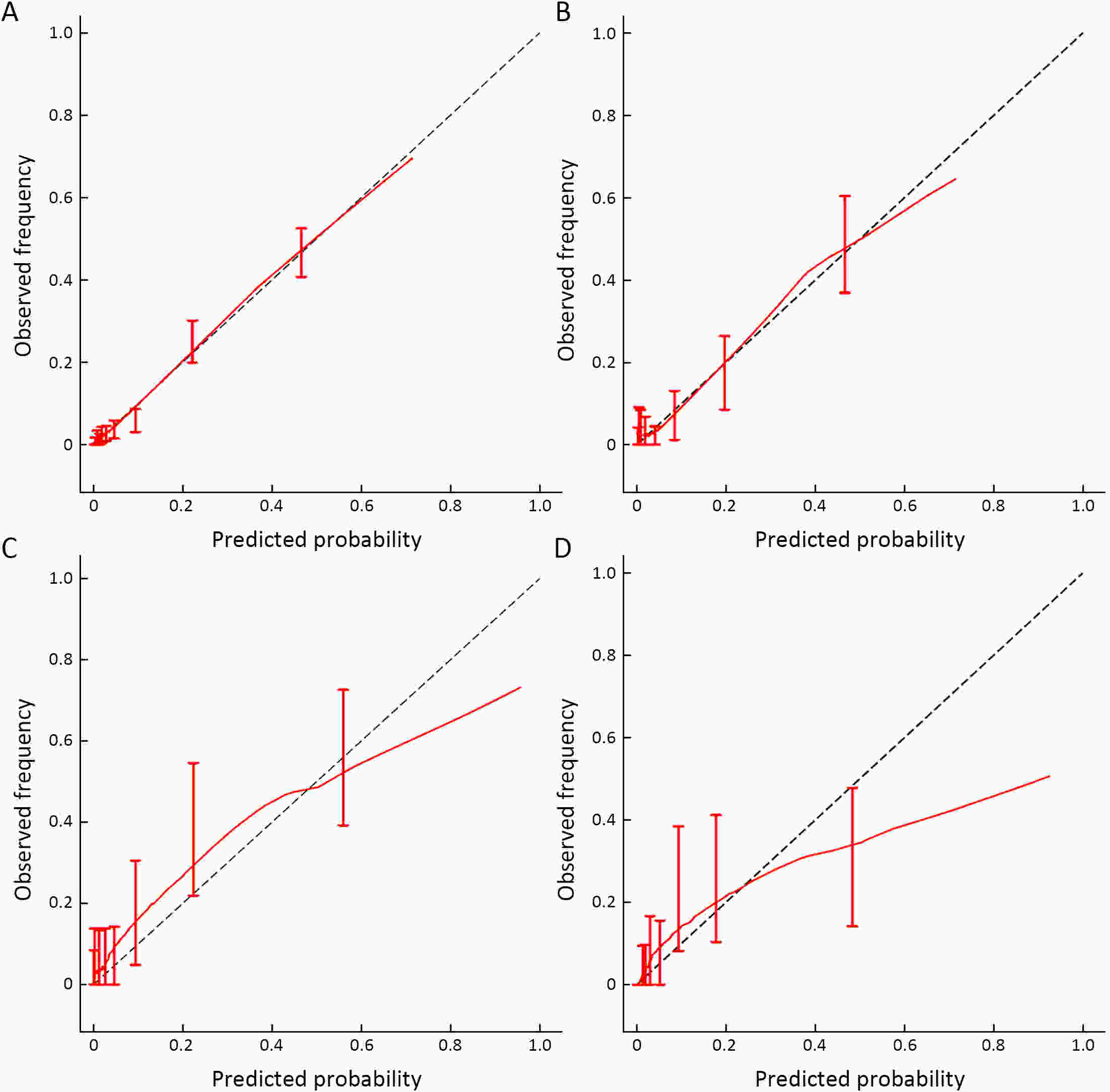
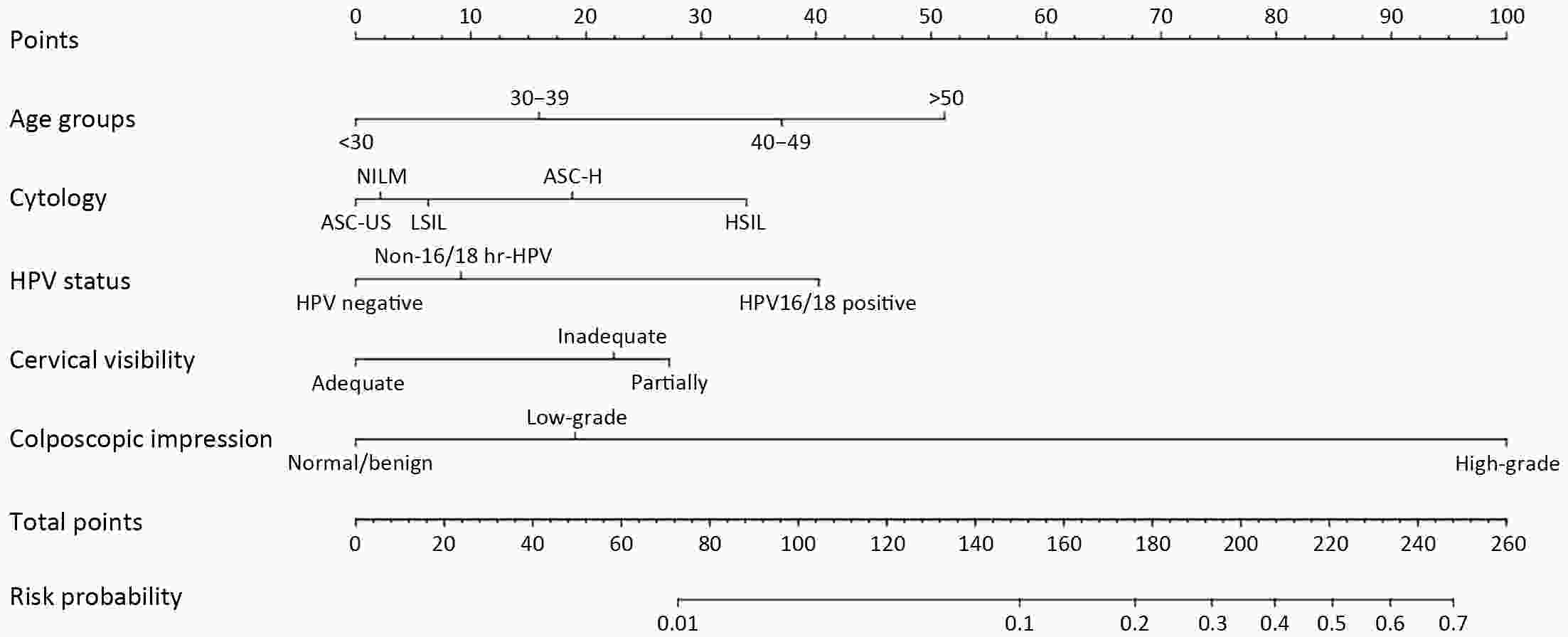
ObjectiveThis study aimed to develop a nomogram that can predict occult high-grade squamous intraepithelial lesions or worse (HSIL+) and determine the need for endocervical curettage (ECC) in patients referred for colposcopy. MethodsThis retrospective multicenter study included 4,149 patients who were referred to any one of six tertiary hospitals in China for colposcopy between January 2020 and November 2021 because of abnormal screening results. ECC data were extracted from the medical records. Univariate and multivariate logistic regression analyses were performed to identify factors that could predict HSIL+ on ECC. Patients were randomly assigned to a training set or to an internal validation set for performance and comparability testing. The model was externally validated and tested in patients from two additional hospitals. The nomogram was assessed in terms of discrimination and calibration and subjected to decision curve analysis. ResultsHSIL+ was found on ECC in 38.8% (n=388) of cases. Our predictive nomogram included age group, cytology, human papillomavirus (HPV) status, visibility of the cervix and colposcopic impression. The nomogram had good overall discrimination, which was internally validated [area under the receiver-operator characteristic (AUC), 0.839; 95% confidence interval (95% CI), 0.773−0.904]. In terms of external validation, the AUC was 0.843 (95% CI, 0.773−0.912) for the consecutive sample and 0.843 (95% CI, 0.783−0.902) for the comparative sample. Calibration analysis suggested good consistency between predicted and observed probabilities. Decision curve analysis suggested this nomogram would be clinically useful with almost the entire range of threshold probabilities. ConclusionsThis internally and externally validated nomogram can be easily applied and incorporates multiple clinically relevant variables that can be used to identify patients with occult HSIL+ who need ECC.
2022, 34(4): 406-414.
doi: 10.21147/j.issn.1000-9604.2022.04.08
Abstract:
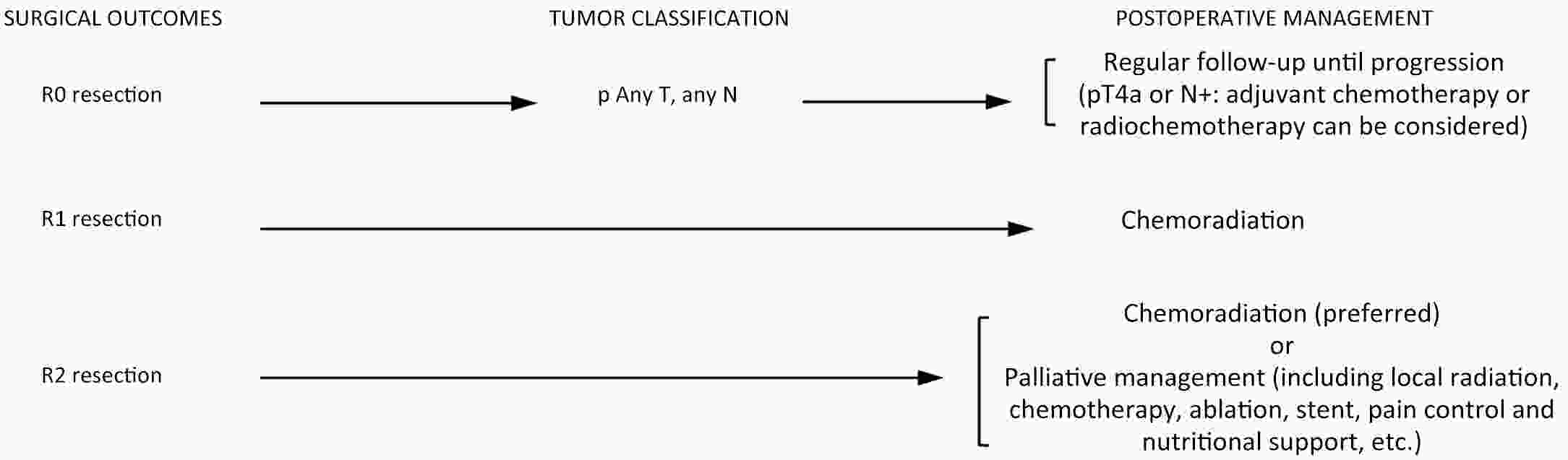
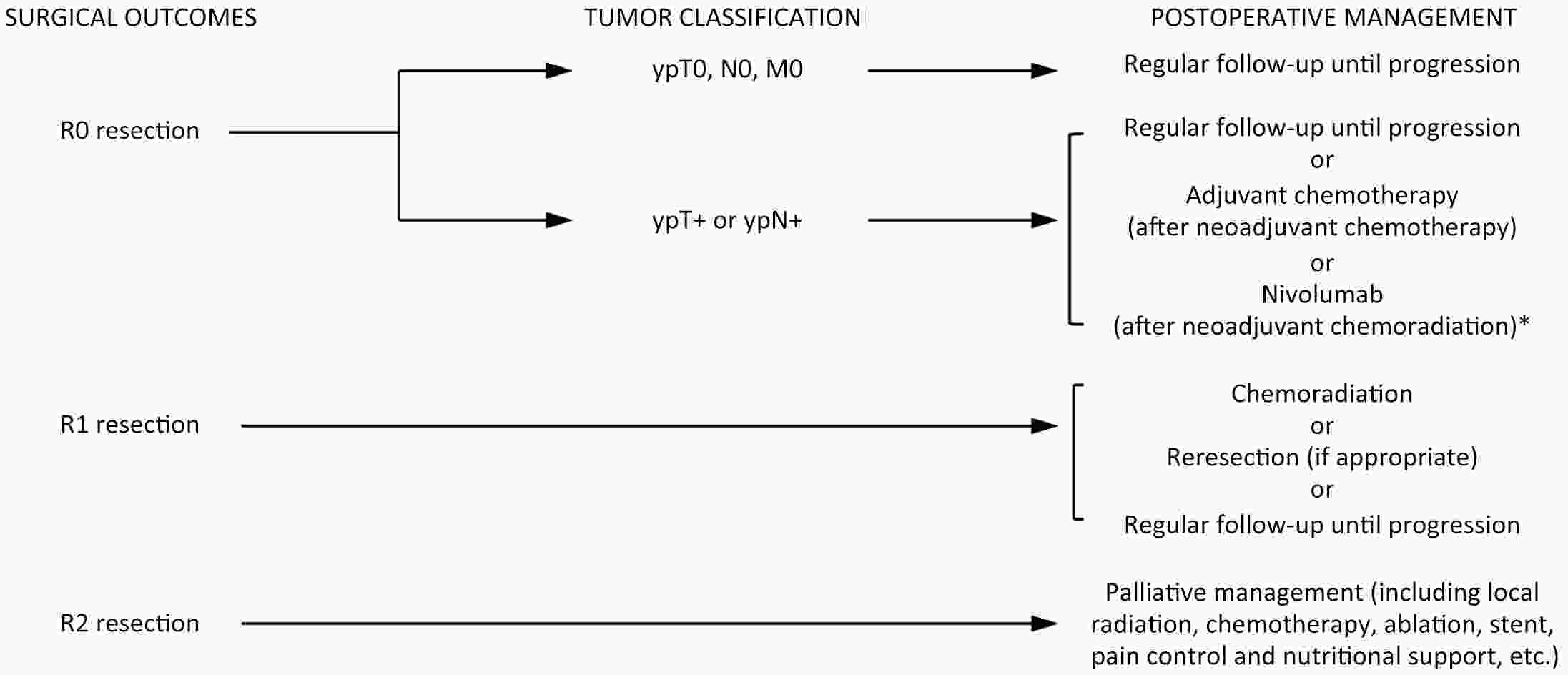
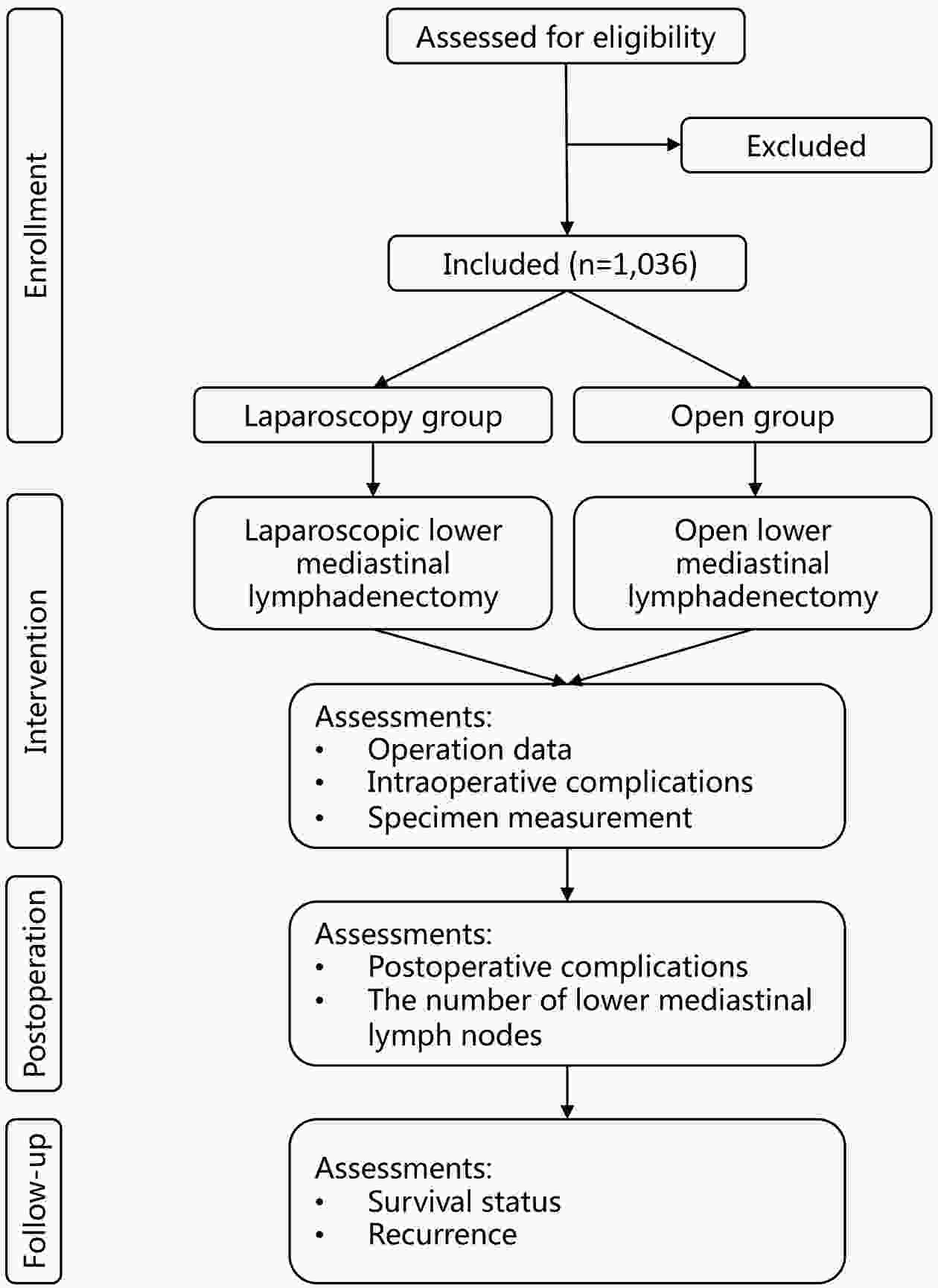
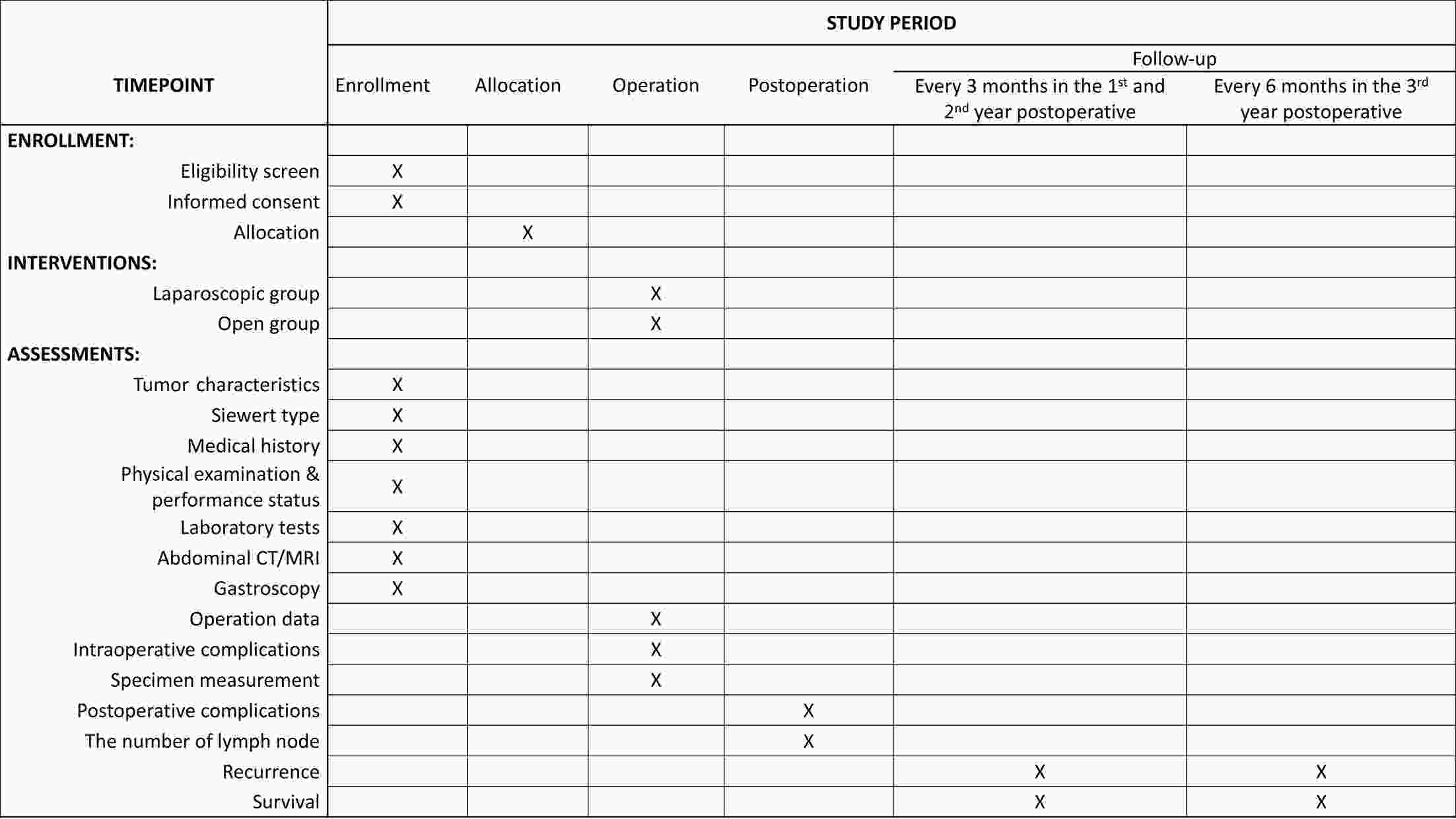
ObjectiveThis study aims to verify the feasibility and efficacy of laparoscopic lower mediastinal lymphadenectomy for Siewert type II/III adenocarcinoma of esophagogastric junction (AEG). Setting An exploratory, observational, prospective, cohort study will be carried out under the Idea, Development, Exploration, Assessment and Long-term Follow-up (IDEAL) framework (stage 2b). Participants The study will recruit 1,036 patients with cases of locally advanced AEG (Siewert type II/III, clinical stage cT2−4aN0−3M0), and 518 will be assigned to either the laparoscopy group or the open group. Interventions Patients will receive lower mediastinal lymphadenectomy along with either total or proximal gastrectomy. Primary and secondary outcome measures The primary endpoint is the number of lower mediastinal lymph nodes retrieved, and the secondary endpoints are the surgical safety and prognosis, including intraoperative and postoperative lower-mediastinal-lymphadenectomy-related morbidity and mortality, rate of rehospitalization, R0 resection rate, 3-year local recurrence rate, and 3-year overall survival. ConclusionsThe study will provide data for the guidance and development of surgical treatment strategies for AEG. Trial registration number The study has been registered in ClinicalTrials.gov (No. NCT04443478).
2022, 34(4): 415-421.
doi: 10.21147/j.issn.1000-9604.2022.04.09
Abstract:
Several phosphoinositide 3-kinase (PI3K) inhibitors are currently approved to treat hematolymphatic malignant diseases worldwide, and many drugs that have the same target are in the clinical research stage. In March 2022, duvelisib became the first PI3K inhibitor approved in China indicated for the treatment of hematolymphatic malignant diseases. Meanwhile, linperlisib and copanlisib have almost completed the technical review of the clinical specialty. The Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) found that class I PI3K inhibitors can cause various degrees of immune-related adverse events, which are associated with action mechanisms, affecting the benefit-risk assessment of the drugs. On April 21, 2021, the United States Food and Drug Administration (FDA) convened the Oncologic Drugs Advisory Committee (ODAC) meeting to discuss the safety of PI3K inhibitors indicated for hematolymphatic malignancies and their related risk of death. The hematological tumor group of CDE of the China NMPA summarized and combined the data on PI3K inhibitors listed or under technical review for marketing authorization applications and found that such products may have unique efficacy and safety characteristics in Chinese patients with malignant lymphoma.
Several phosphoinositide 3-kinase (PI3K) inhibitors are currently approved to treat hematolymphatic malignant diseases worldwide, and many drugs that have the same target are in the clinical research stage. In March 2022, duvelisib became the first PI3K inhibitor approved in China indicated for the treatment of hematolymphatic malignant diseases. Meanwhile, linperlisib and copanlisib have almost completed the technical review of the clinical specialty. The Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) found that class I PI3K inhibitors can cause various degrees of immune-related adverse events, which are associated with action mechanisms, affecting the benefit-risk assessment of the drugs. On April 21, 2021, the United States Food and Drug Administration (FDA) convened the Oncologic Drugs Advisory Committee (ODAC) meeting to discuss the safety of PI3K inhibitors indicated for hematolymphatic malignancies and their related risk of death. The hematological tumor group of CDE of the China NMPA summarized and combined the data on PI3K inhibitors listed or under technical review for marketing authorization applications and found that such products may have unique efficacy and safety characteristics in Chinese patients with malignant lymphoma.
2022, 34(4): 422-424.
doi: 10.21147/j.issn.1000-9604.2022.04.10
Abstract:
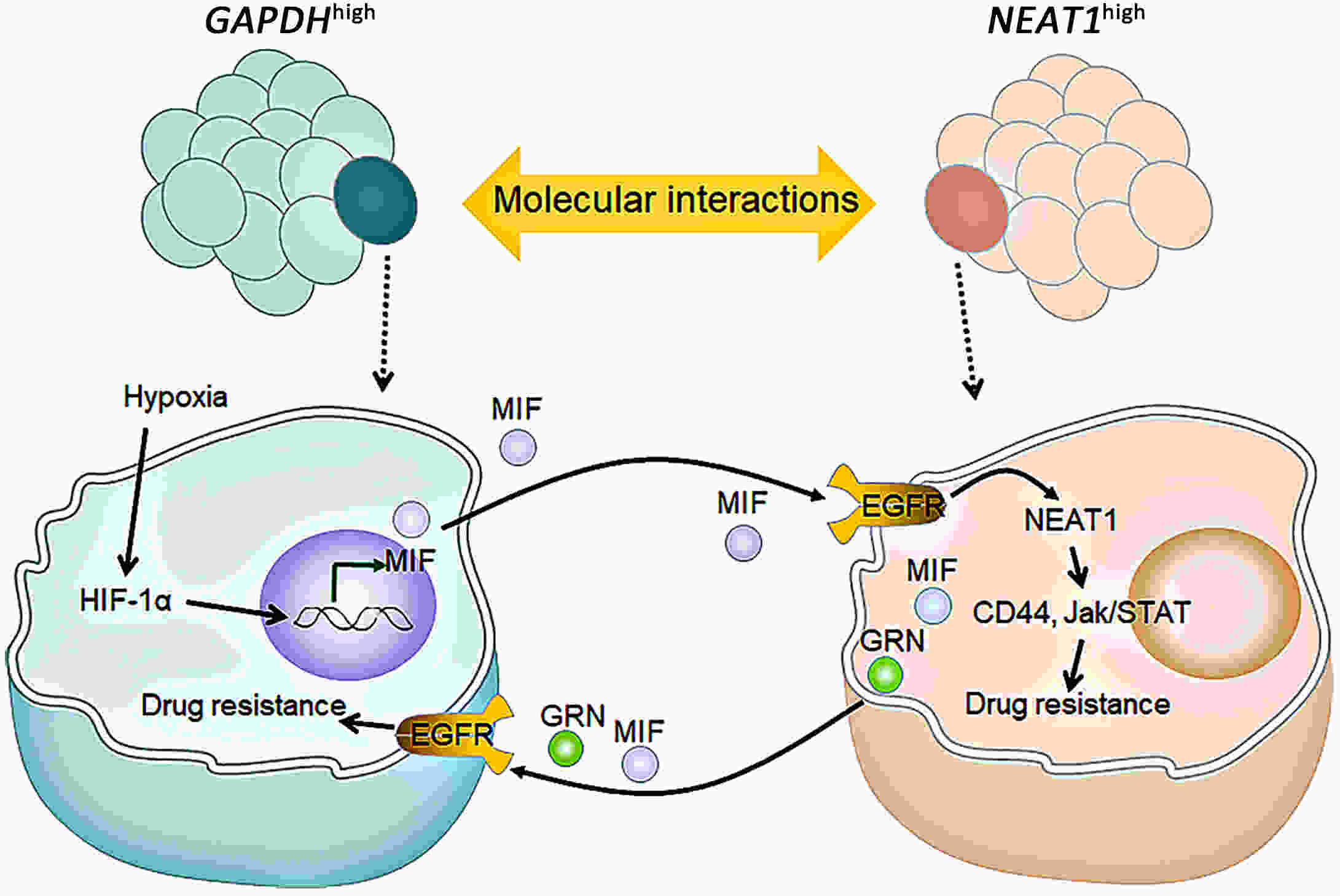
Hepatobiliary tumors are of high grade of heterogeneity, which is recognized as a key contributor to drug resistance and poor disease prognosis. However, the intrinsic mechanism between heterogeneity and drug response in hepatobiliary tumor is still largely unknown. Using tumor organoid models, Wang and her colleagues have found that cooperation among distinct subpopulations might be a key mechanism for drug resistance in hepatobiliary tumor.
Hepatobiliary tumors are of high grade of heterogeneity, which is recognized as a key contributor to drug resistance and poor disease prognosis. However, the intrinsic mechanism between heterogeneity and drug response in hepatobiliary tumor is still largely unknown. Using tumor organoid models, Wang and her colleagues have found that cooperation among distinct subpopulations might be a key mechanism for drug resistance in hepatobiliary tumor.

 Abstract
Abstract FullText HTML
FullText HTML PDF 2765KB
PDF 2765KB