2023 Vol.35(1)
Display Mode: |
2023, 35(1): 1-10.
doi: 10.21147/j.issn.1000-9604.2023.01.01
Abstract:
2023, 35(1): 11-14.
doi: 10.21147/j.issn.1000-9604.2023.01.02
Abstract:
2023, 35(1): 15-18.
doi: 10.21147/j.issn.1000-9604.2023.01.03
Abstract:
2023, 35(1): 19-43.
doi: 10.21147/j.issn.1000-9604.2023.01.04
Abstract:
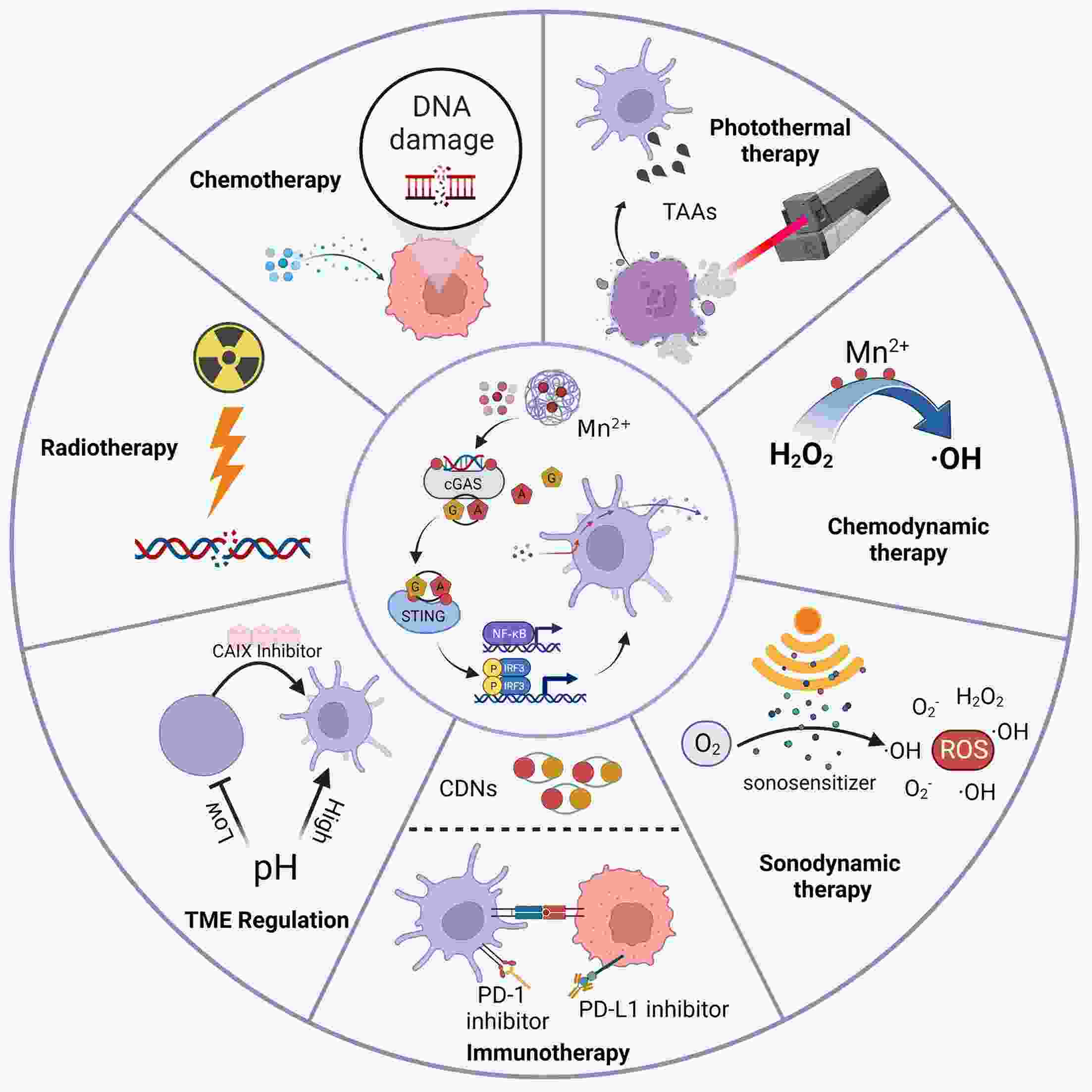
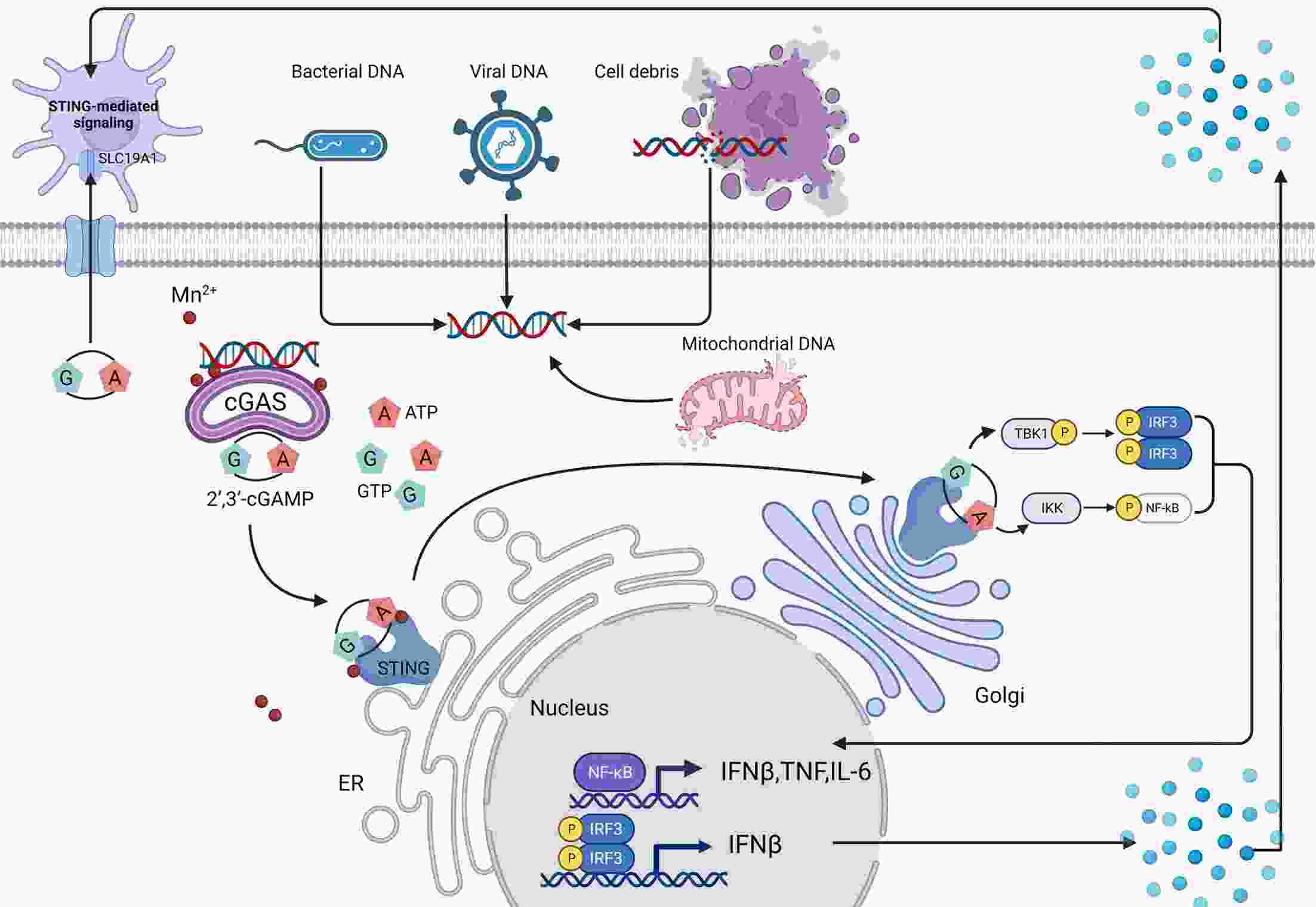
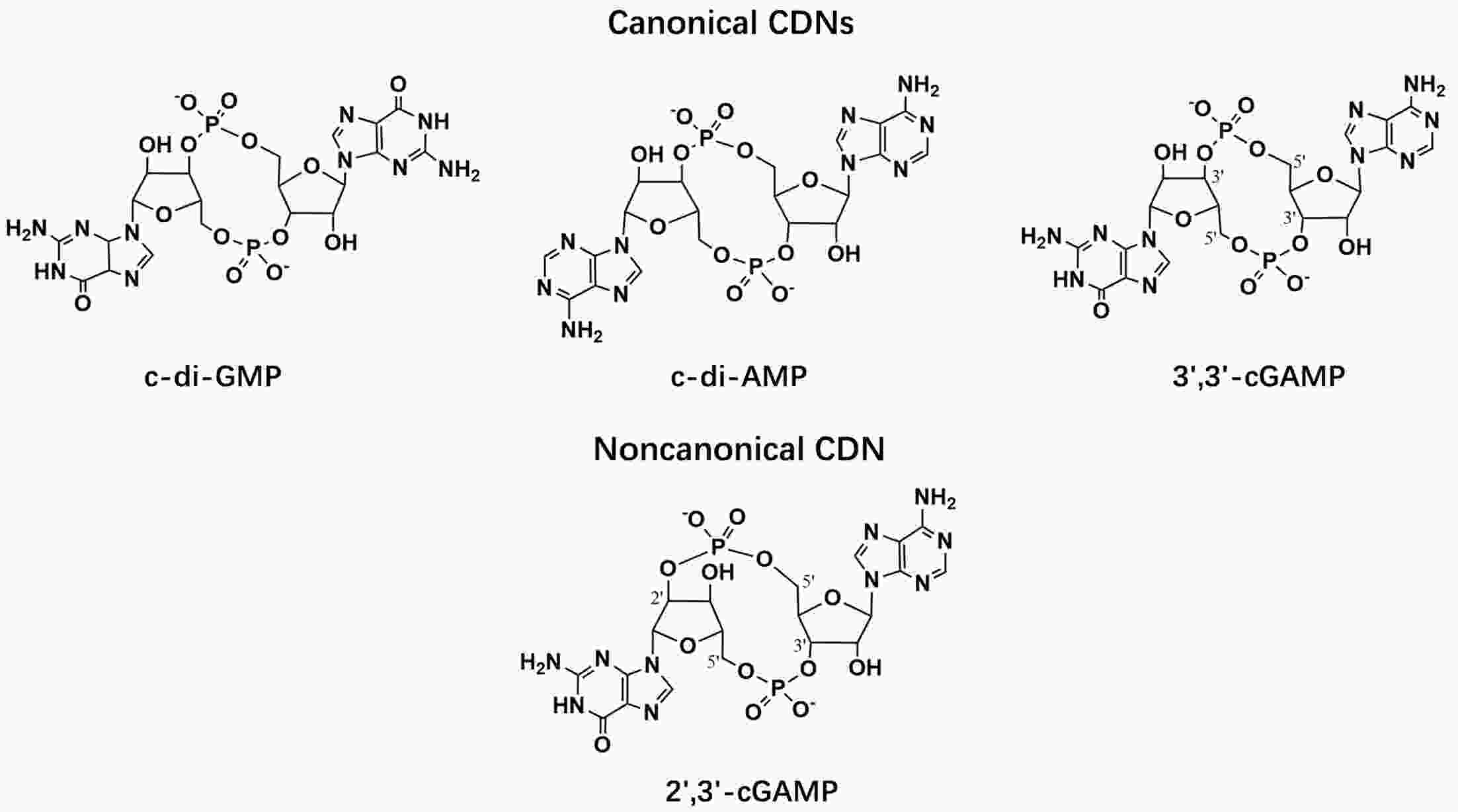
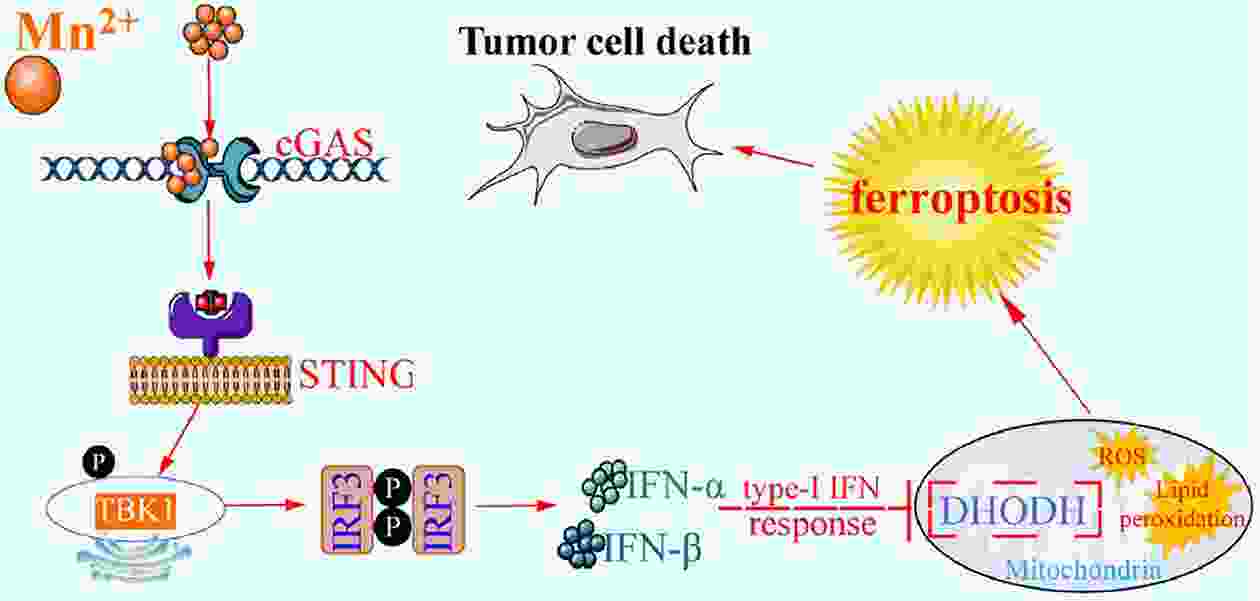
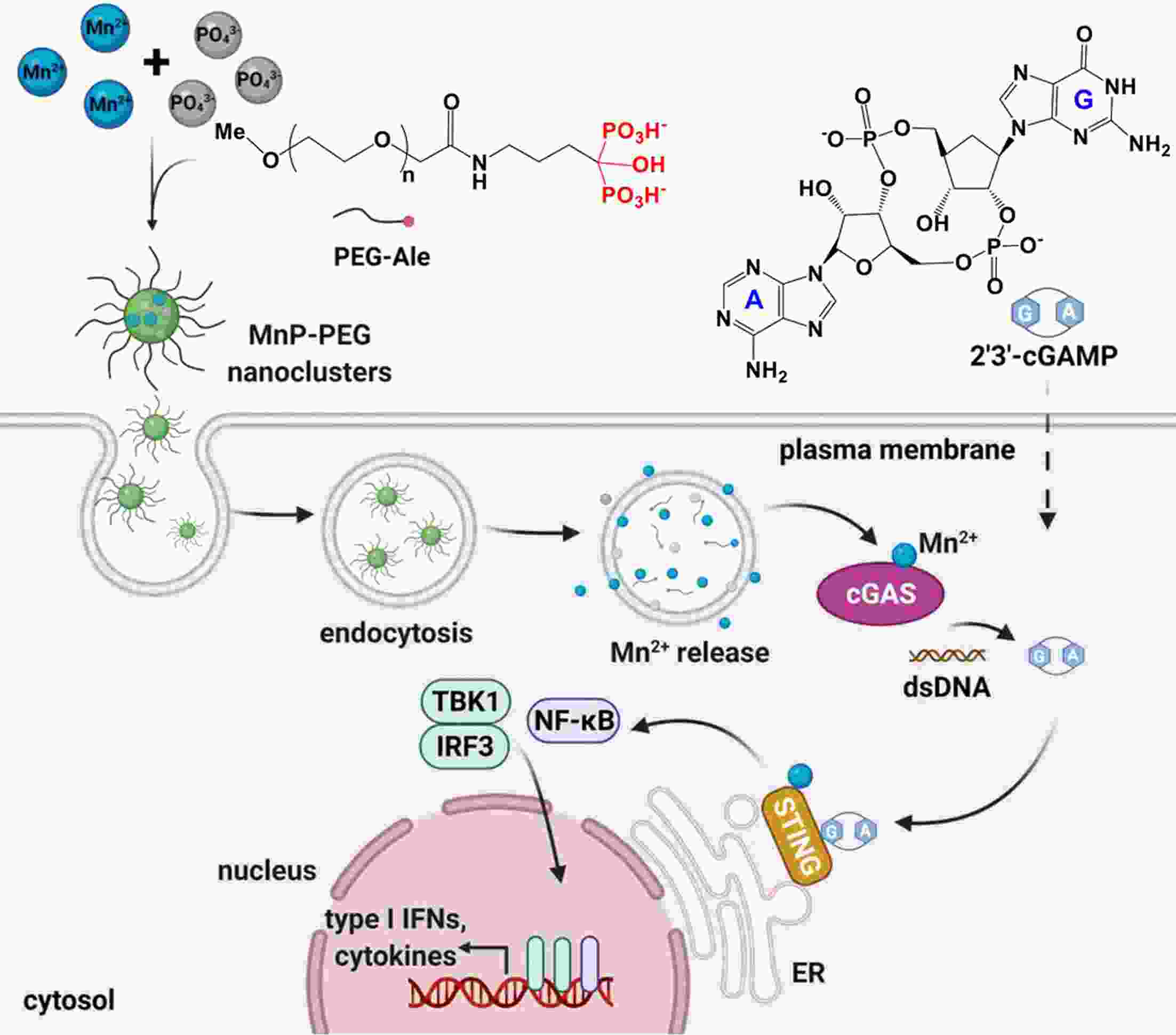
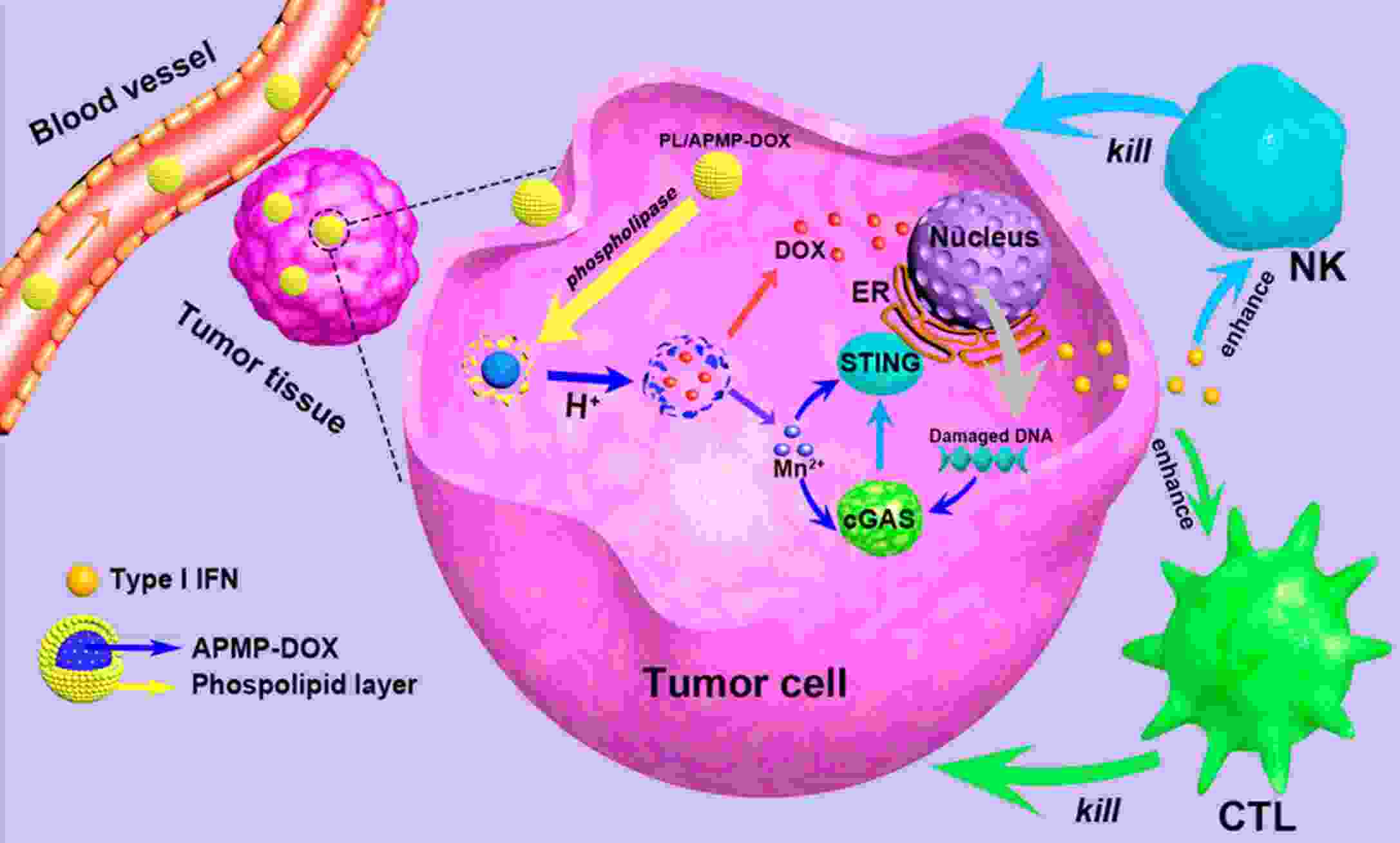
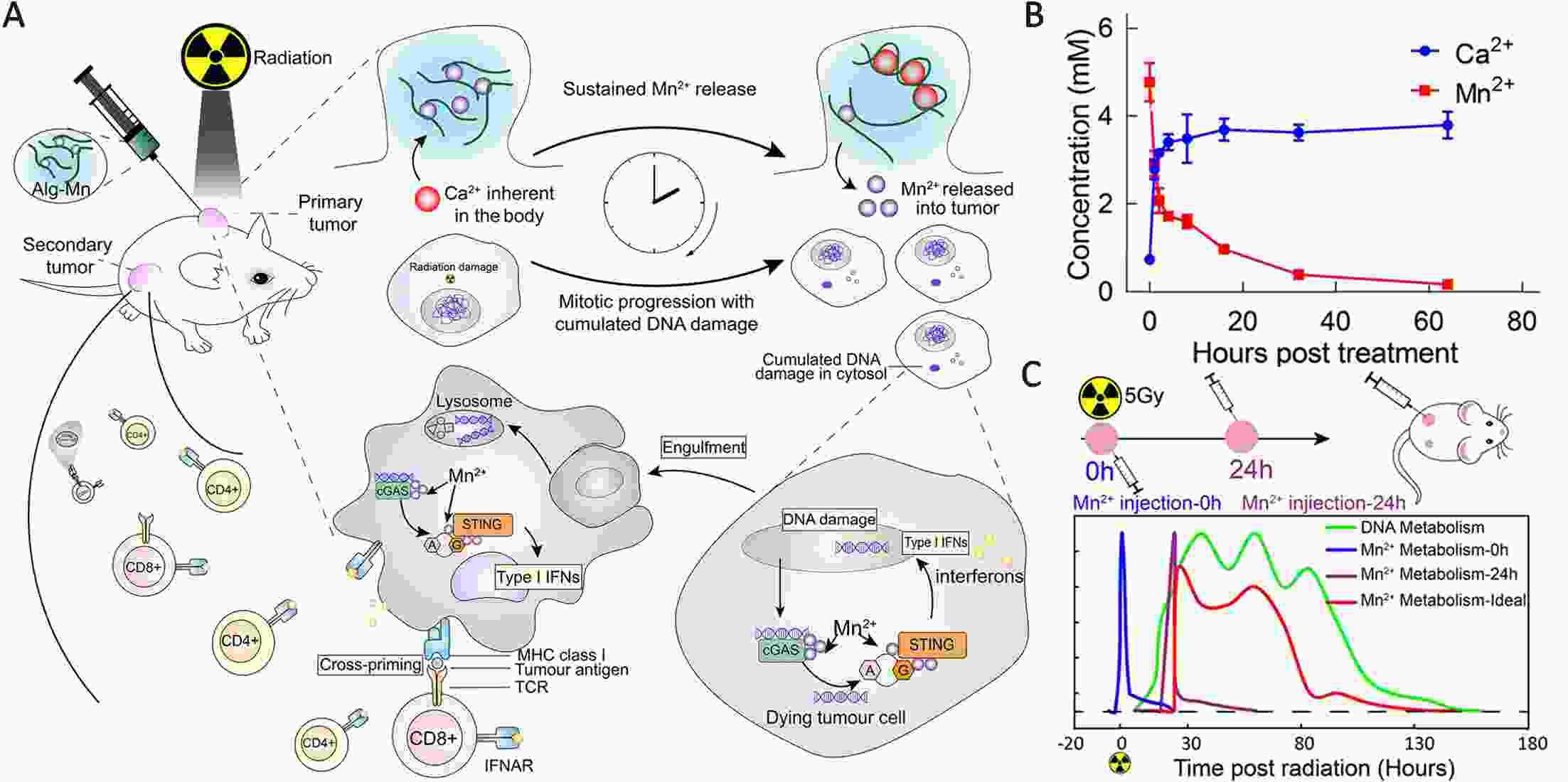
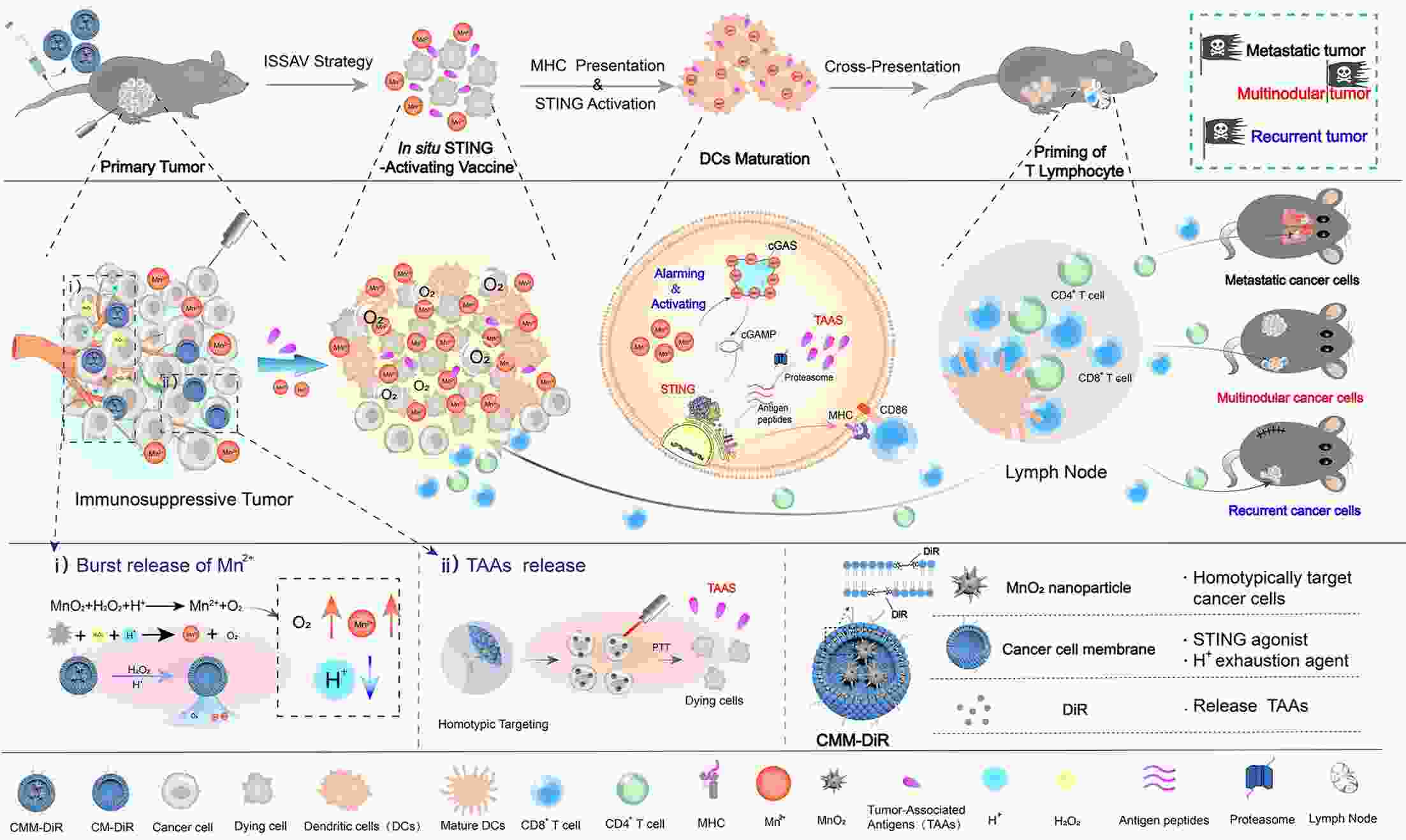
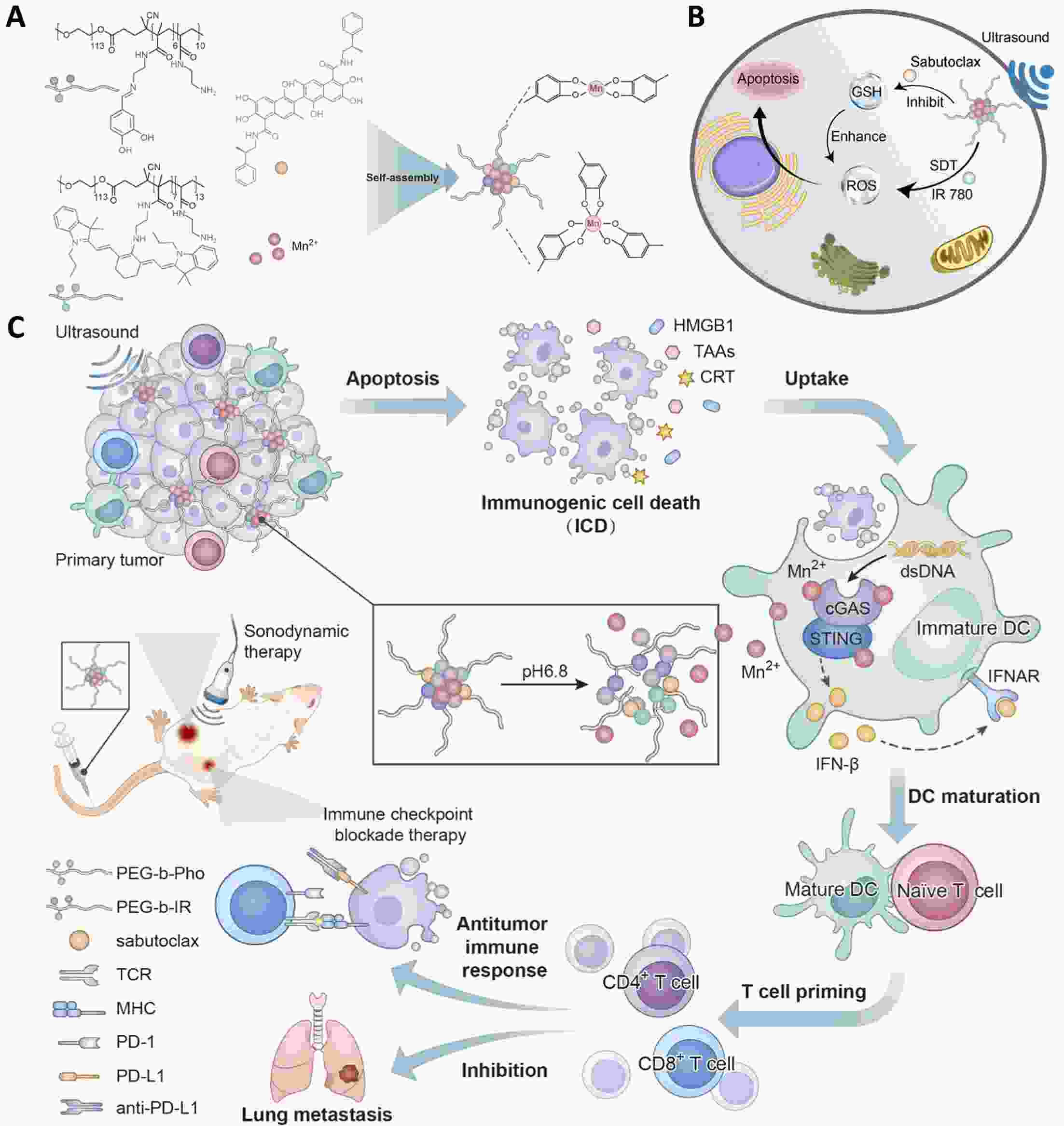
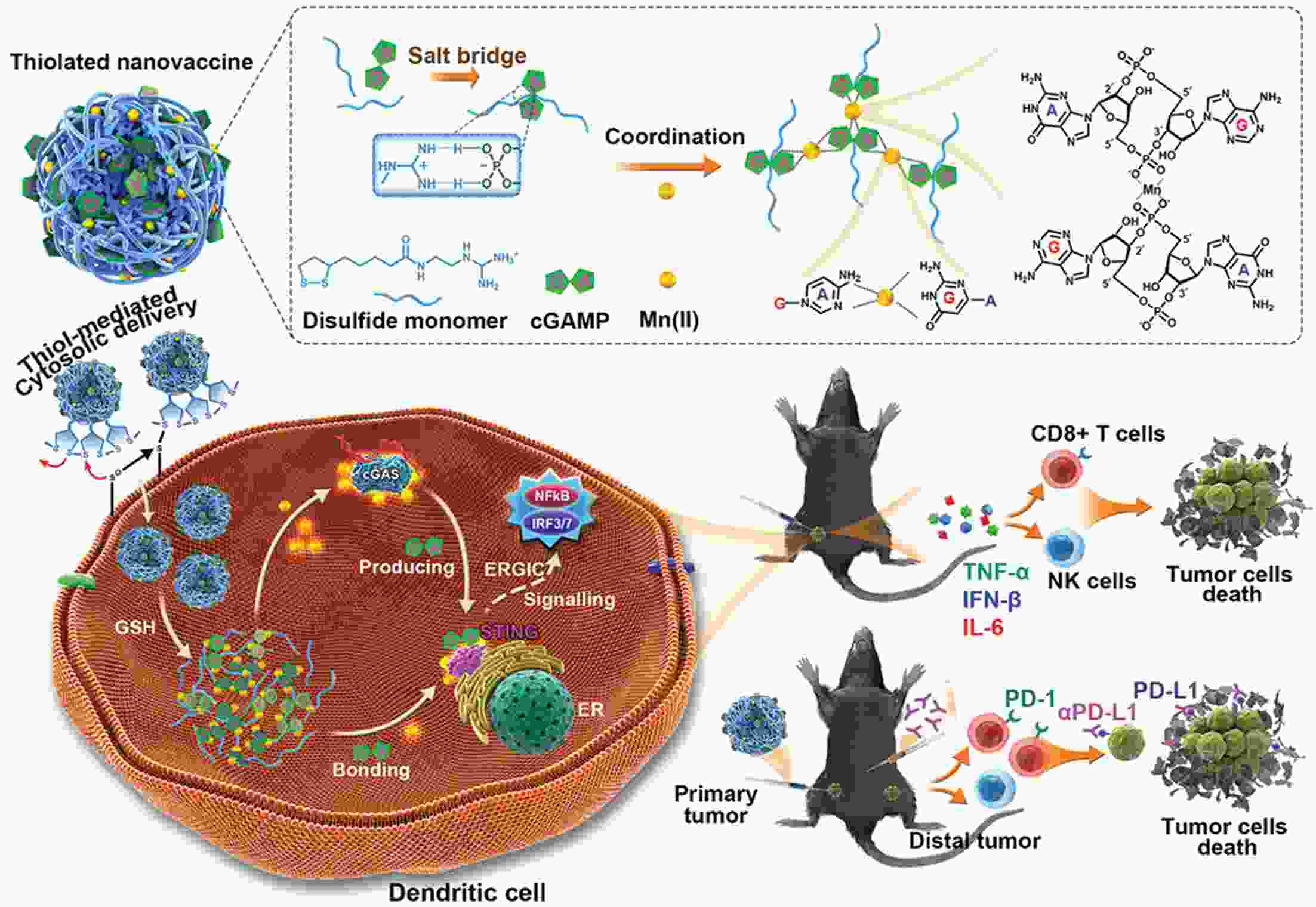
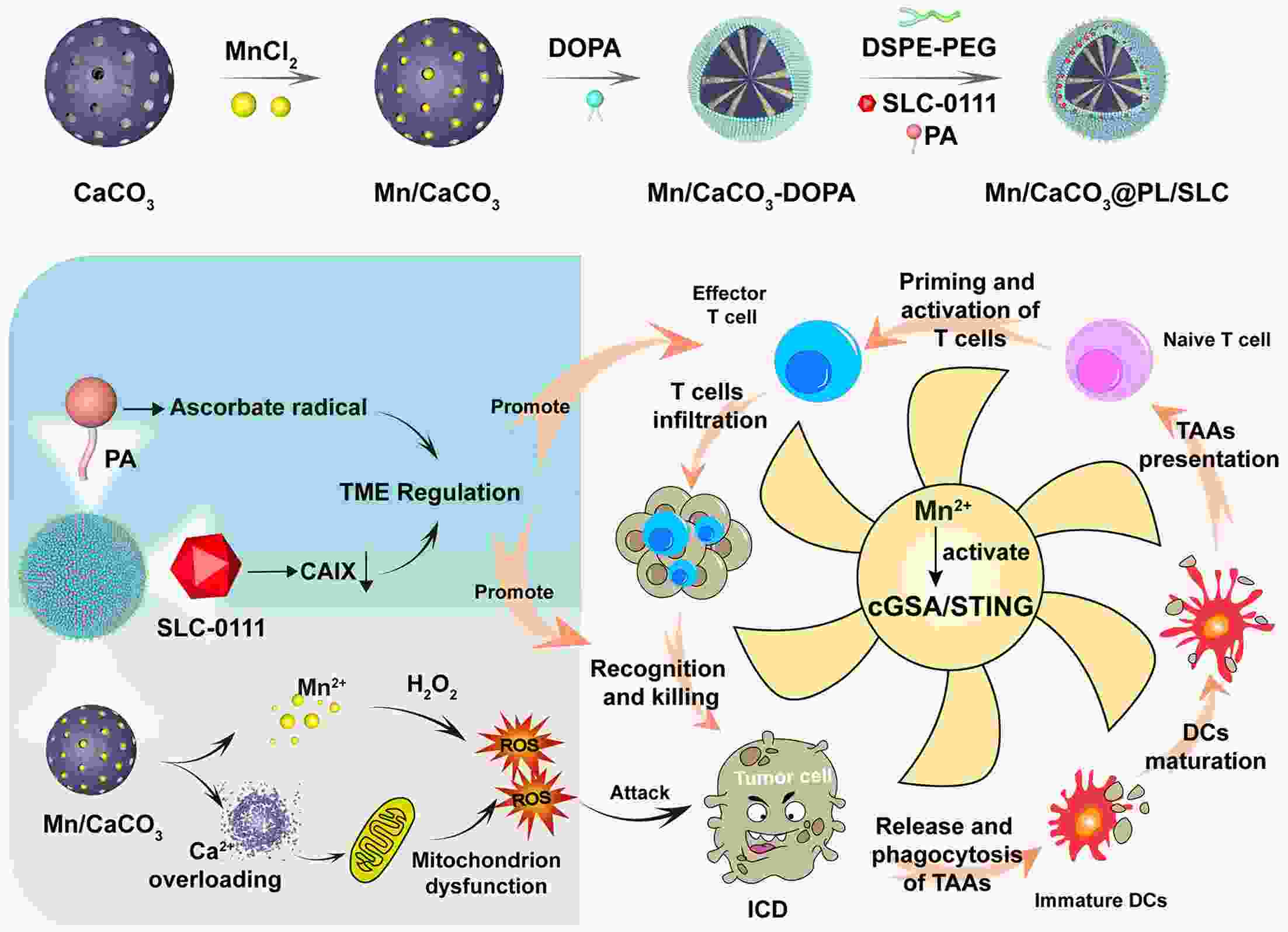
Immunotherapy has efficiently revolutionized the treatment of human neoplastic diseases. However, the overall responsive rate of current immunotherapy is still unsatisfactory, benefiting only a small proportion of patients. Therefore, significant attention has been paid to the modulation of tumor microenvironment (TME) for the enhancement of immunotherapy. Interestingly, recent studies have shown that cyclic GMP-AMP synthase-stimulator of interferon gene (cGAS-STING) was initially found as an innate immune sensor to recognize cytoplasmic DNA (such as bacterial, viral, micronuclei, and mitochondrial). It is a promising signaling pathway to activate antitumor immune responses via type I interferon production. Notably, Mn2+ was found to be a critical molecule to sensitize the activation of the cGAS-STING pathway for better immunotherapy. This activation led to the development of Mn2+-based strategies for tumor immunotherapy via the activation of the cGAS-STING pathway. In this critical review, we aimed to summarize the recent progress of this field, focusing on the following three aspects. First, we briefly introduced the signaling pathway of cGAS-STING activation, and its regulation effect on the antitumor immunity cycle has been discussed. Along with this, several agonists of the cGAS-STING pathway were introduced with their potential as immunotherapeutic drugs. Then, the basic biological functions of Mn2+ have been illustrated, focusing on its critical roles in the cGAS-STING pathway activation. Next, we systematically reviewed the Mn2+-based strategies for tumor immunotherapy, which can be classified by the methods based on Mn2+ alone or Mn2+ combined with other therapeutic modalities. We finally speculated the future perspectives of the field and provided rational suggestions to develop better Mn2+-based therapeutics.
Immunotherapy has efficiently revolutionized the treatment of human neoplastic diseases. However, the overall responsive rate of current immunotherapy is still unsatisfactory, benefiting only a small proportion of patients. Therefore, significant attention has been paid to the modulation of tumor microenvironment (TME) for the enhancement of immunotherapy. Interestingly, recent studies have shown that cyclic GMP-AMP synthase-stimulator of interferon gene (cGAS-STING) was initially found as an innate immune sensor to recognize cytoplasmic DNA (such as bacterial, viral, micronuclei, and mitochondrial). It is a promising signaling pathway to activate antitumor immune responses via type I interferon production. Notably, Mn2+ was found to be a critical molecule to sensitize the activation of the cGAS-STING pathway for better immunotherapy. This activation led to the development of Mn2+-based strategies for tumor immunotherapy via the activation of the cGAS-STING pathway. In this critical review, we aimed to summarize the recent progress of this field, focusing on the following three aspects. First, we briefly introduced the signaling pathway of cGAS-STING activation, and its regulation effect on the antitumor immunity cycle has been discussed. Along with this, several agonists of the cGAS-STING pathway were introduced with their potential as immunotherapeutic drugs. Then, the basic biological functions of Mn2+ have been illustrated, focusing on its critical roles in the cGAS-STING pathway activation. Next, we systematically reviewed the Mn2+-based strategies for tumor immunotherapy, which can be classified by the methods based on Mn2+ alone or Mn2+ combined with other therapeutic modalities. We finally speculated the future perspectives of the field and provided rational suggestions to develop better Mn2+-based therapeutics.
2023, 35(1): 44-57.
doi: 10.21147/j.issn.1000-9604.2023.01.05
Abstract:
Cancers derived from the gastrointestinal (GI) tract are often treated with radical surgery to achieve a cure. However, recent advances in the management of GI cancers involve the use of a combination of neoadjuvant radiation and chemotherapy followed by surgical intervention to achieve improved local control and cure. Interestingly, a small proportion of patients with highly sensitive tumors achieved a pathological complete response (pCR) (no residual tumor cells in the resected specimen) to neoadjuvant chemoradiation therapy (nCRT). The desire for organ preservation and avoidance of surgical morbidity brings the idea of a nonoperative management (NOM) strategy. Because of the different nature of tumor biology, GI cancers present diverse responses to nCRT, ranging from high sensitivity (anal cancer) to low sensitivity (gastric/esophageal cancer). There is an increasing attention to NOM of localized GI cancers; however, without the use of biomarkers/imaging parameters to select such patients, NOM will remain a challenge. Therefore, this review intends to summarize some of the recent updates from the aspect of current nCRT regimens, criteria for patient selection and active surveillance schedules. We also hope to review significant sequelae of radical surgery and the complications of nCRT to clarify the directions for optimization of nCRT and NOM for oncologic outcomes and quality of life.
Cancers derived from the gastrointestinal (GI) tract are often treated with radical surgery to achieve a cure. However, recent advances in the management of GI cancers involve the use of a combination of neoadjuvant radiation and chemotherapy followed by surgical intervention to achieve improved local control and cure. Interestingly, a small proportion of patients with highly sensitive tumors achieved a pathological complete response (pCR) (no residual tumor cells in the resected specimen) to neoadjuvant chemoradiation therapy (nCRT). The desire for organ preservation and avoidance of surgical morbidity brings the idea of a nonoperative management (NOM) strategy. Because of the different nature of tumor biology, GI cancers present diverse responses to nCRT, ranging from high sensitivity (anal cancer) to low sensitivity (gastric/esophageal cancer). There is an increasing attention to NOM of localized GI cancers; however, without the use of biomarkers/imaging parameters to select such patients, NOM will remain a challenge. Therefore, this review intends to summarize some of the recent updates from the aspect of current nCRT regimens, criteria for patient selection and active surveillance schedules. We also hope to review significant sequelae of radical surgery and the complications of nCRT to clarify the directions for optimization of nCRT and NOM for oncologic outcomes and quality of life.
2023, 35(1): 58-65.
doi: 10.21147/j.issn.1000-9604.2023.01.06
Abstract:
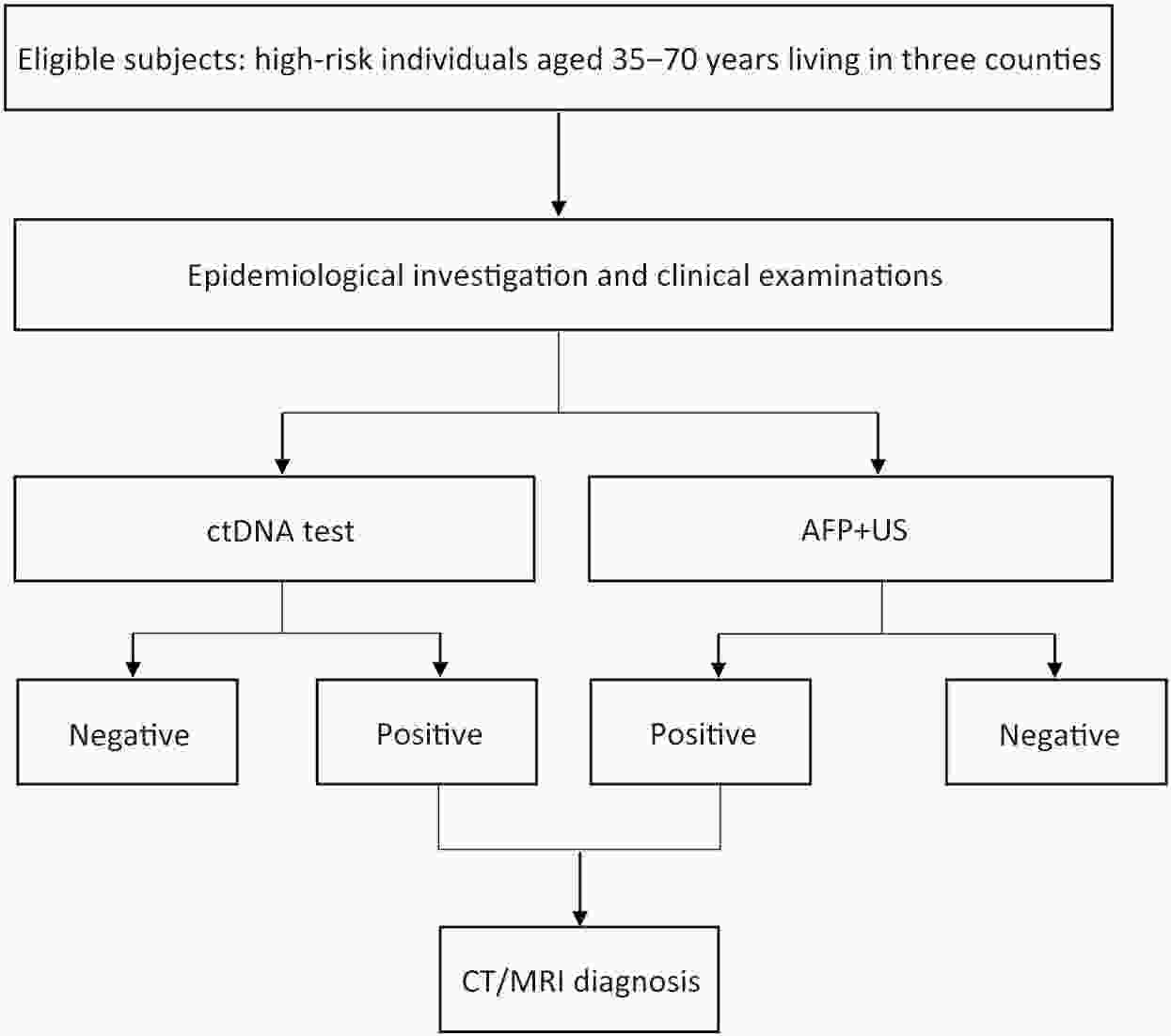
ObjectiveCirculating tumor DNA (ctDNA) and alpha-fetoprotein (AFP) plus ultrasound (US) have been considered to have high diagnostic accuracy for cancer detection, however, the efficacy of ctDNA methylation combined with the traditional detection modality of liver cancer has not been tested in a Chinese independent cohort. MethodsThe high-risk individuals aged between 35 and 70 years who were diagnosed with liver cirrhosis or had moderate and severe fatty liver were eligible for inclusion. All participants were invited to receive a traditional examination [referring to AFP plus US], and ctDNA methylation, respectively. The sensitivity and specificity of different diagnostic tools were calculated. The logistic regression model was applied to estimate the area under the curve (AUC), which was further validated by 10-fold internal cross-validation. ResultsA total of 1,205 individuals were recruited in our study, and 39 participants were diagnosed with liver cancer. The sensitivity of AFP, US, US plus AFP, and the combination of US, AFP, and ctDNA methylation was 33.33%, 56.41%, 66.67%, and 87.18%, respectively. The corresponding specificity of AFP, US, US plus AFP, and the combination of all modalities was 98.20%, 99.31%, 97.68%, and 97.68%, respectively. The AUCs of AFP, US, US plus AFP, and the combination of AFP, US, and ctDNA methylation were 65.77%, 77.86%, 82.18%, and 92.43%, respectively. The internally validated AUCs of AFP, US, US plus AFP, and the combination of AFP, US, and ctDNA methylation were 67.57%, 83.26%, 86.54%, and 93.35%, respectively. ConclusionsThe ctDNA methylation is a good complementary to AFP and US for the detection of liver cancer.
2023, 35(1): 66-80.
doi: 10.21147/j.issn.1000-9604.2023.01.07
Abstract:
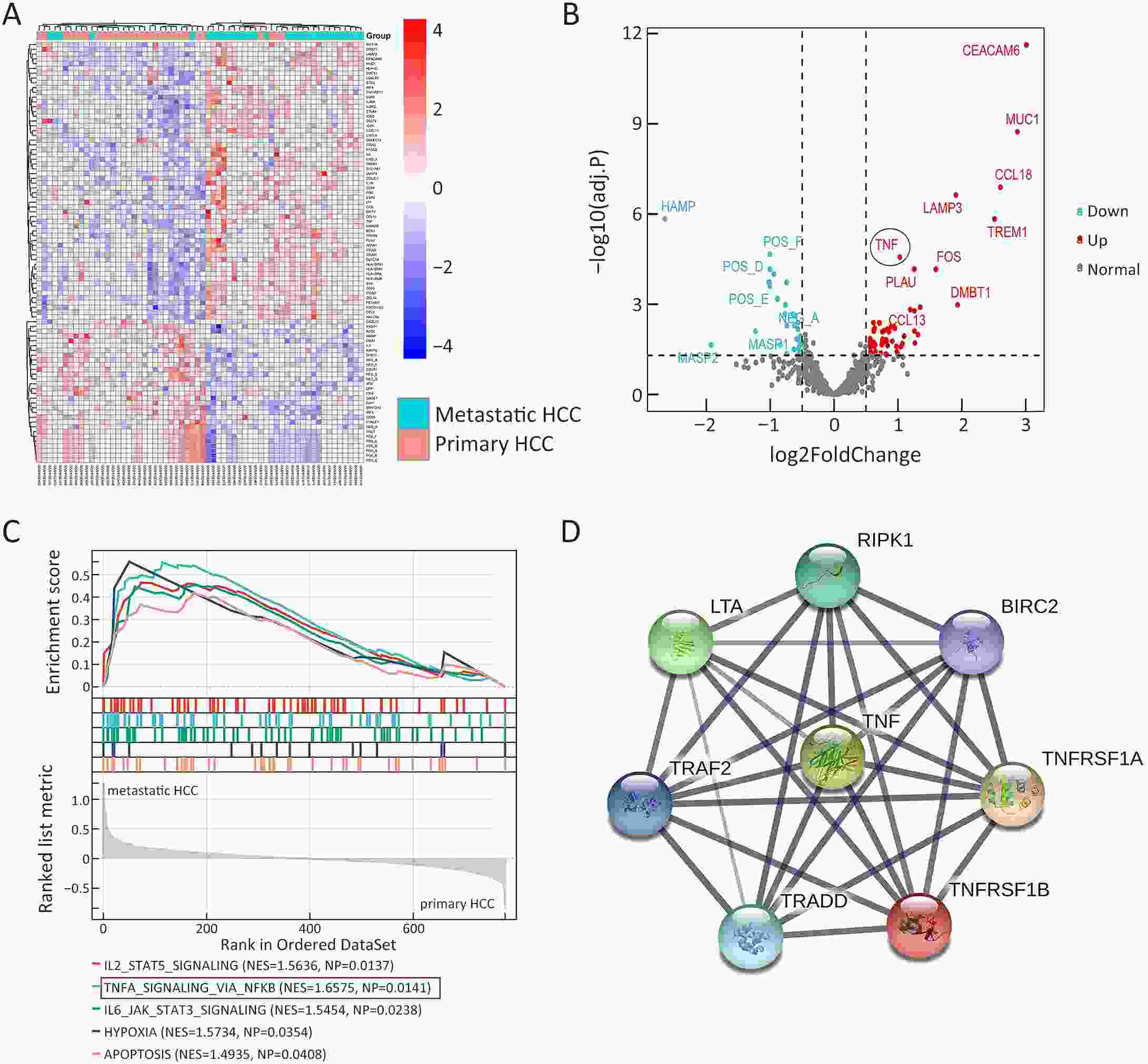
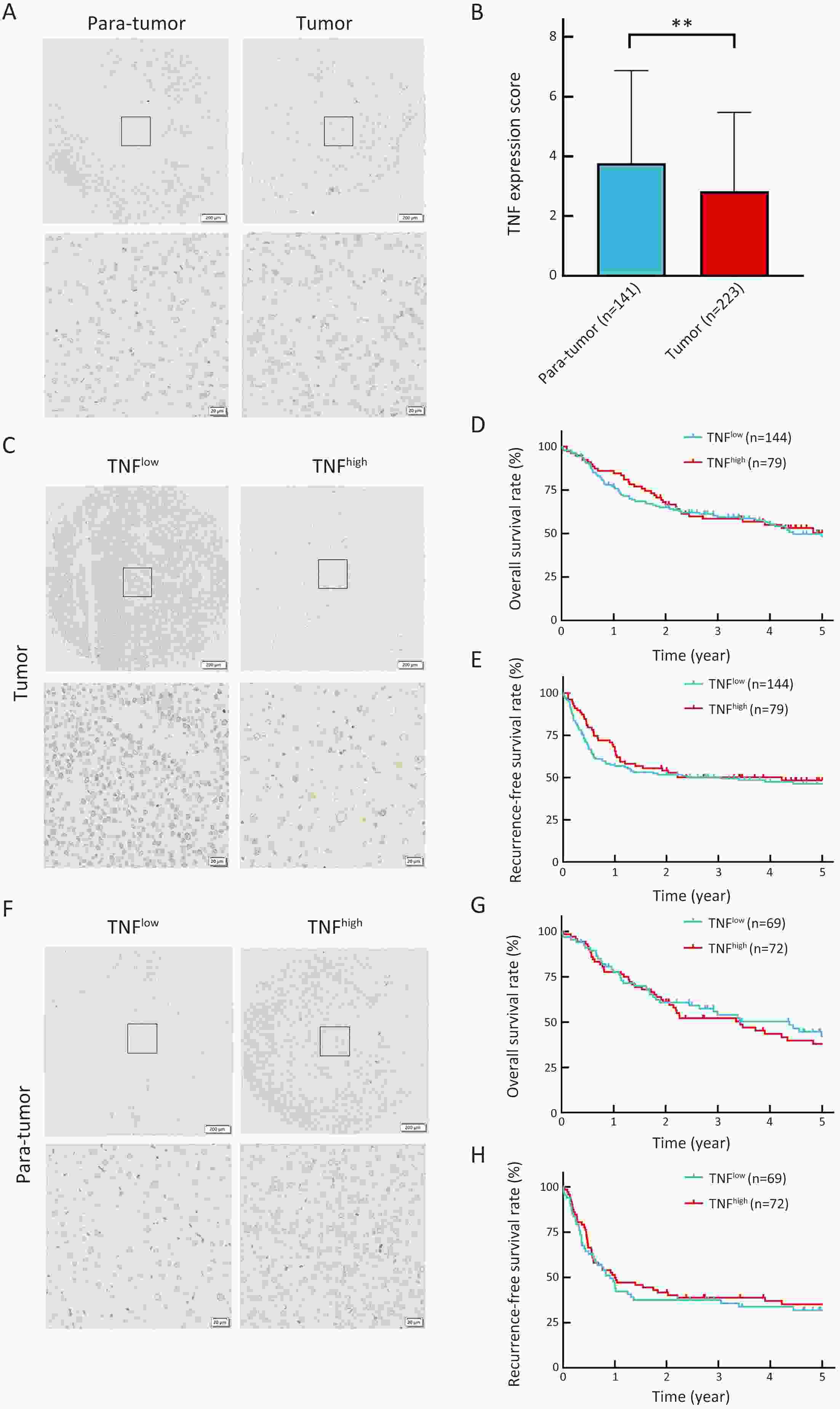
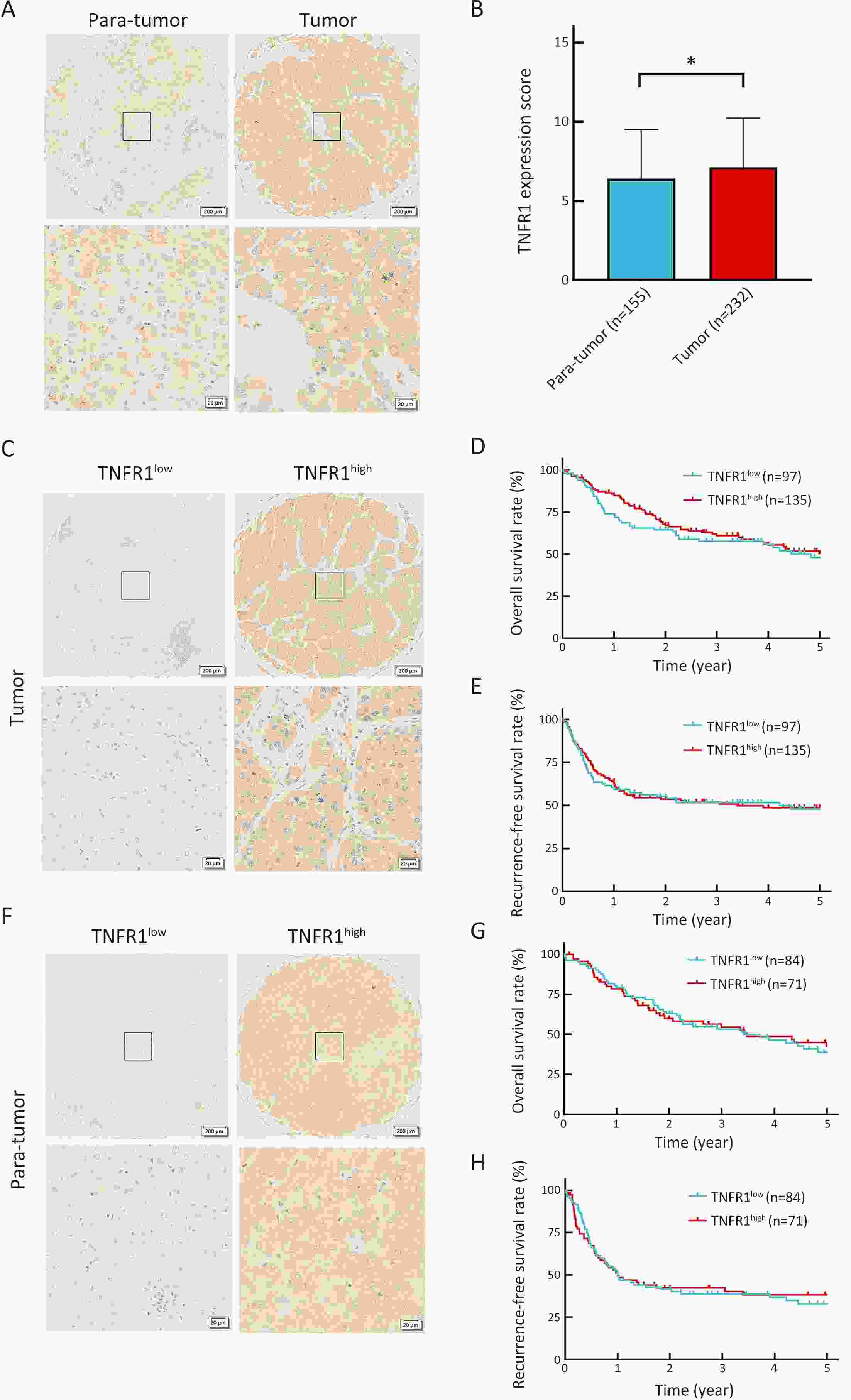
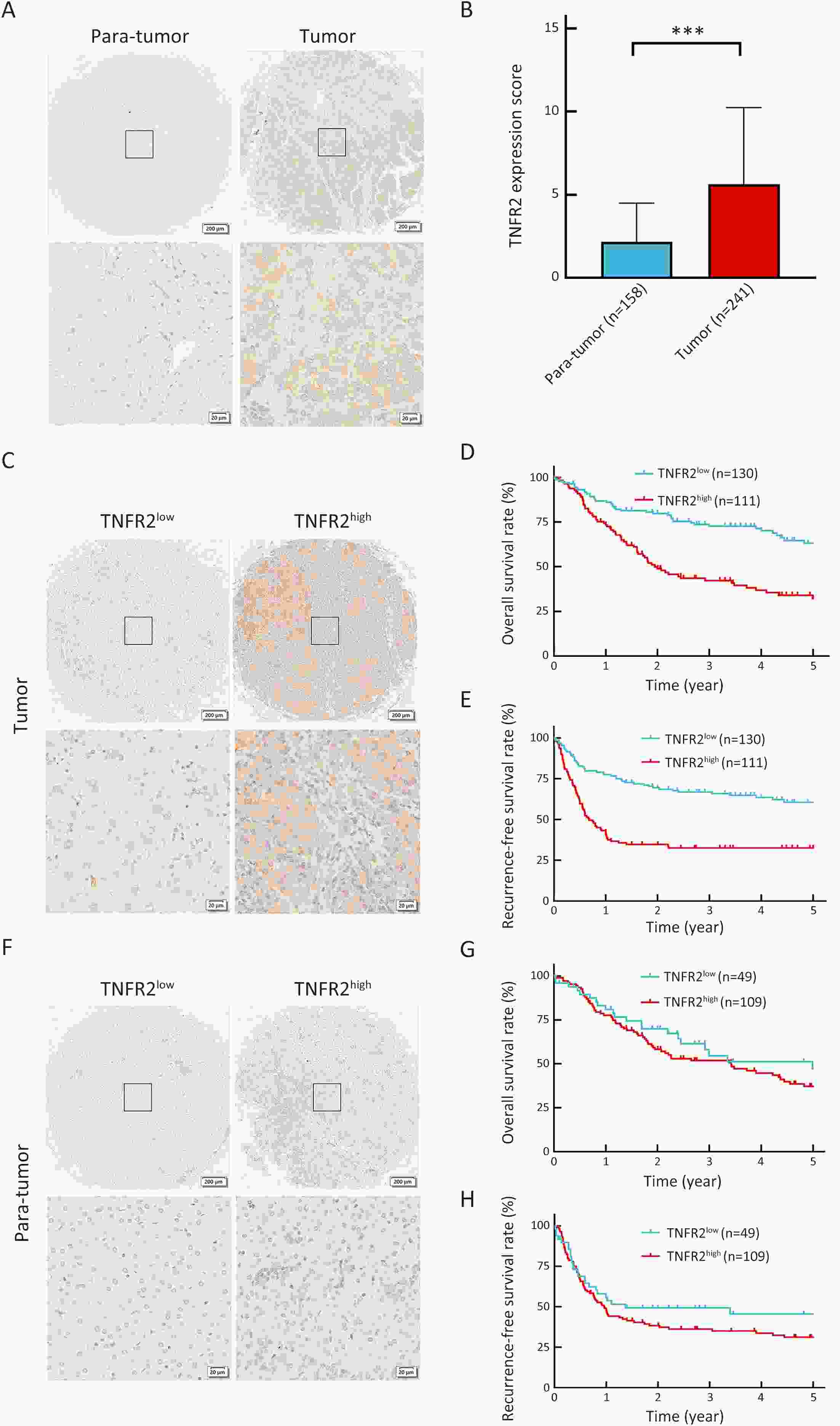
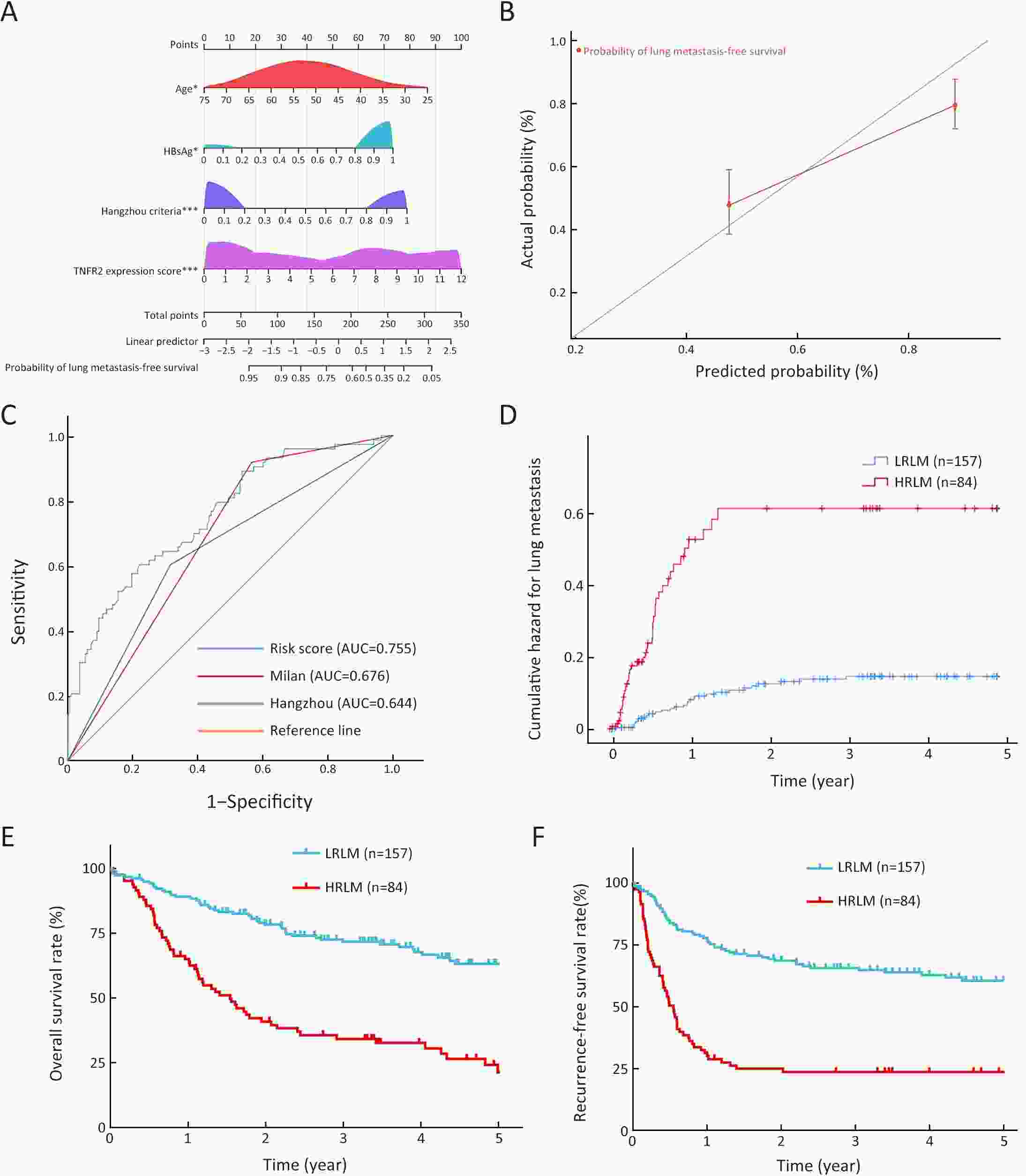
ObjectiveLung metastasis is a common and fatal complication of liver transplantation for hepatocellular carcinoma (HCC). The precise prediction of post-transplant lung metastasis in the early phase is of great value. MethodsThe mRNA profiles of primary and paired lung metastatic lesions were analyzed to determine key signaling pathways. We enrolled 241 HCC patients who underwent liver transplantation from three centers. Tissue microarrays were used to evaluate the prognostic capacity of tumor necrosis factor (TNF), tumor necrosis factor receptor 1 (TNFR1), and TNFR2, particularly for post-transplant lung metastasis. ResultsComparison of primary and lung metastatic lesions revealed that the TNF-dependent signaling pathway was related to lung metastasis of HCC. The expression of TNF was degraded in comparison to that in para-tumor tissues (P<0.001). The expression of key receptors in the TNF-dependent signaling pathway, TNFR1 and TNFR2, was higher in HCC tissues than in para-tumor tissues (P<0.001). TNF and TNFR1 showed no relationship with patients’ outcomes, whereas elevated TNFR2 in tumor tissue was significantly associated with worse overall survival (OS) and increased recurrence risk (5-year OS rate: 31.9% vs. 62.5%, P<0.001). Notably, elevated TNFR2 levels were also associated with an increased risk of post-transplant lung metastasis (hazard ratio: 1.146; P<0.001). Cox regression analysis revealed that TNFR2, Hangzhou criteria, age, and hepatitis B surface antigen were independent risk factors for post-transplant lung metastasis, and a novel nomogram was established accordingly. The nomogram achieved excellent prognostic efficiency (area under time-dependent receiver operating characteristic =0.755, concordance-index =0.779) and was superior to conventional models, such as the Milan criteria. ConclusionsTNFR2 is a potent prognostic biomarker for predicting post-transplant lung metastasis in patients with HCC. A nomogram incorporating TNFR2 deserves to be a helpful prognostic tool in liver transplantation for HCC.

 Abstract
Abstract FullText HTML
FullText HTML PDF 2203KB
PDF 2203KB