2023 Vol.35(5)
Display Mode: |
2023, 35(5): 431-432.
doi: 10.21147/j.issn.1000-9604.2023.05.01
Abstract:
2023, 35(5): 433-437.
doi: 10.21147/j.issn.1000-9604.2023.05.02
Abstract:
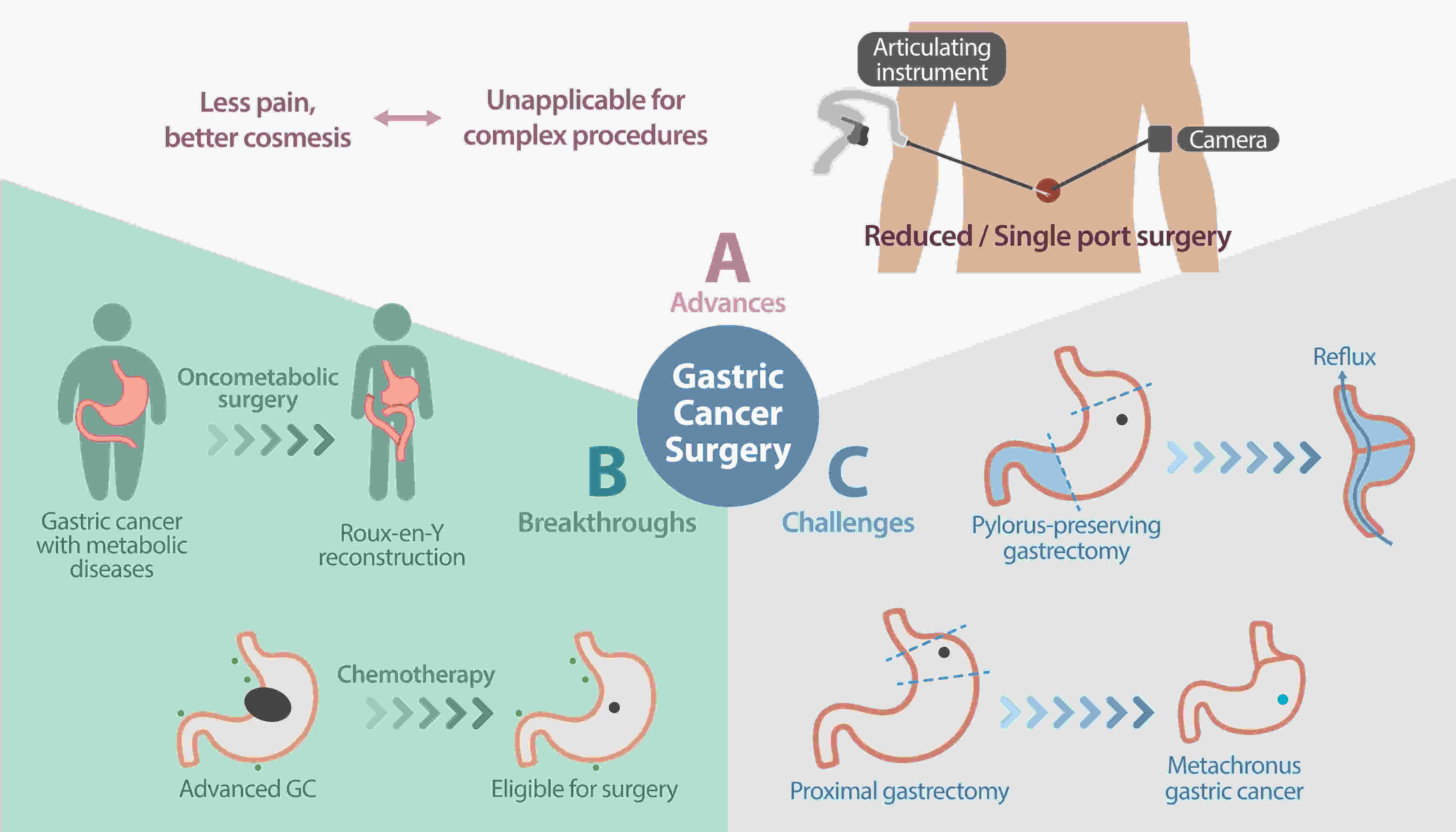
Gastric cancer (GC) remains a substantial health burden worldwide, ranking fifth in incidence and third in mortality among all cancer types. Surgeons have persistently attempted to address this growing burden through surgical management of GC encompassing various aspects of surgery, including advances in surgical techniques and tools for minimally invasive surgery, novel technology for real-time image-guided surgery, and function-preserving and oncometabolic surgeries, aimed at improving patients’ quality of life. The current perspective discusses the five most critical dimensions of the recent technical improvements and conceptual changes in GC surgery. We recommend further exploration of long-term benefits of these advancements, identification of breakthrough solutions to address current challenges, and delivery of the best quality of care.
Gastric cancer (GC) remains a substantial health burden worldwide, ranking fifth in incidence and third in mortality among all cancer types. Surgeons have persistently attempted to address this growing burden through surgical management of GC encompassing various aspects of surgery, including advances in surgical techniques and tools for minimally invasive surgery, novel technology for real-time image-guided surgery, and function-preserving and oncometabolic surgeries, aimed at improving patients’ quality of life. The current perspective discusses the five most critical dimensions of the recent technical improvements and conceptual changes in GC surgery. We recommend further exploration of long-term benefits of these advancements, identification of breakthrough solutions to address current challenges, and delivery of the best quality of care.
2023, 35(5): 438-450.
doi: 10.21147/j.issn.1000-9604.2023.05.03
Abstract:
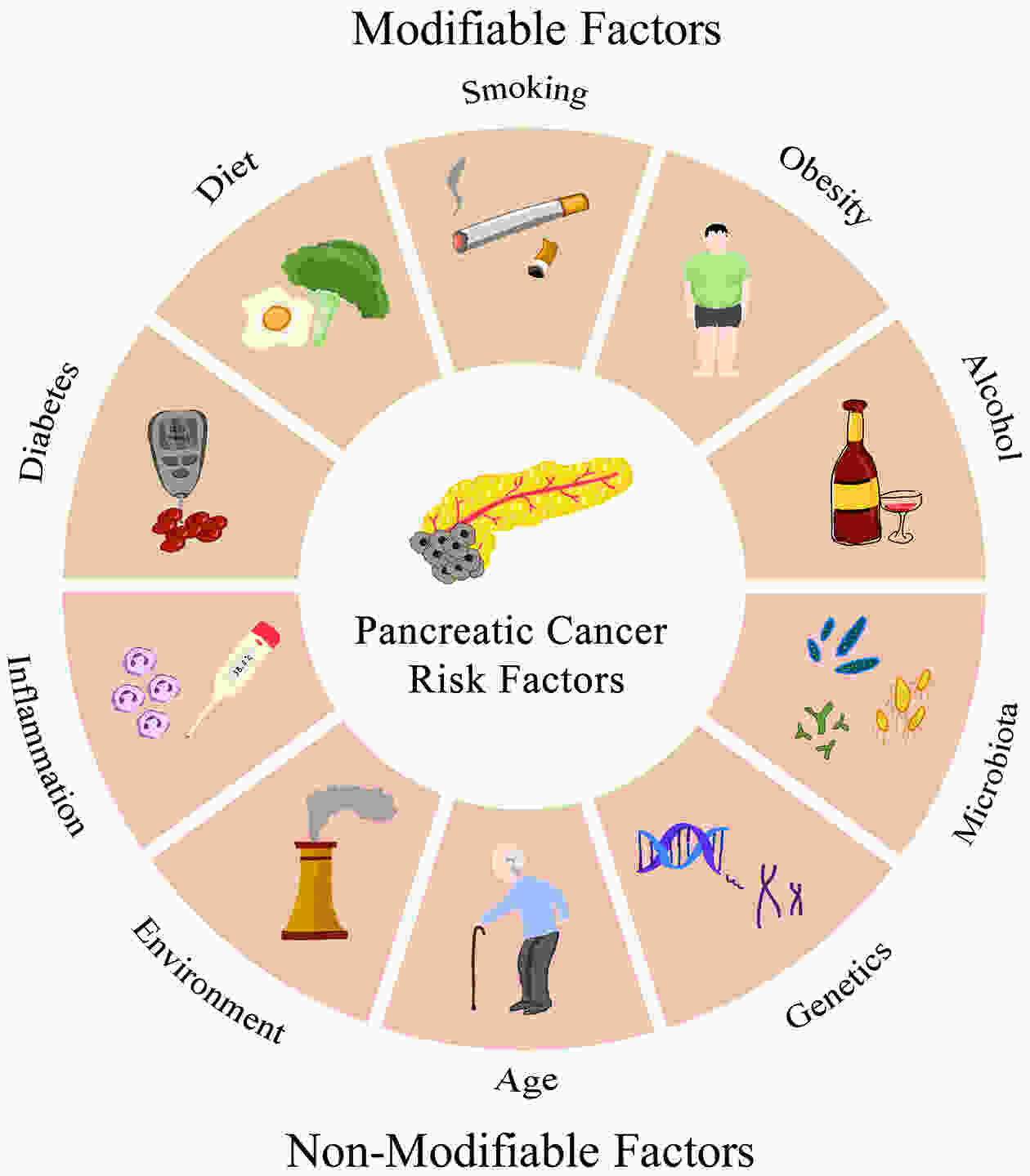
Pancreatic cancer (PC) is a devastating malignancy with an extremely high mortality rate and poses significant challenges to healthcare systems worldwide. The prevalence of PC risk factors spiked over the years, leading to a global increase in PC incidence rates. The contribution of different risk factors, however, varied from region to region due to genetic predisposition, environmental, social, and political factors underlying disease prevalence in addition to public health strategies. This comprehensive review aims to provide a thorough analysis of the epidemiology of PC, discussing its incidence, risk factors, screening strategies and socioeconomic burden. We compiled a wide range of seminal studies as well as epidemiological investigations to serve this review as a comprehensive guide for researchers, healthcare professionals, and policymakers keen for a more profound understanding of PC epidemiology. This review highlights the essentiality of persistent research efforts, interdisciplinary collaboration, and public health initiatives to address the expanding burden of this malignancy.
Pancreatic cancer (PC) is a devastating malignancy with an extremely high mortality rate and poses significant challenges to healthcare systems worldwide. The prevalence of PC risk factors spiked over the years, leading to a global increase in PC incidence rates. The contribution of different risk factors, however, varied from region to region due to genetic predisposition, environmental, social, and political factors underlying disease prevalence in addition to public health strategies. This comprehensive review aims to provide a thorough analysis of the epidemiology of PC, discussing its incidence, risk factors, screening strategies and socioeconomic burden. We compiled a wide range of seminal studies as well as epidemiological investigations to serve this review as a comprehensive guide for researchers, healthcare professionals, and policymakers keen for a more profound understanding of PC epidemiology. This review highlights the essentiality of persistent research efforts, interdisciplinary collaboration, and public health initiatives to address the expanding burden of this malignancy.
2023, 35(5): 451-469.
doi: 10.21147/j.issn.1000-9604.2023.05.04
Abstract:
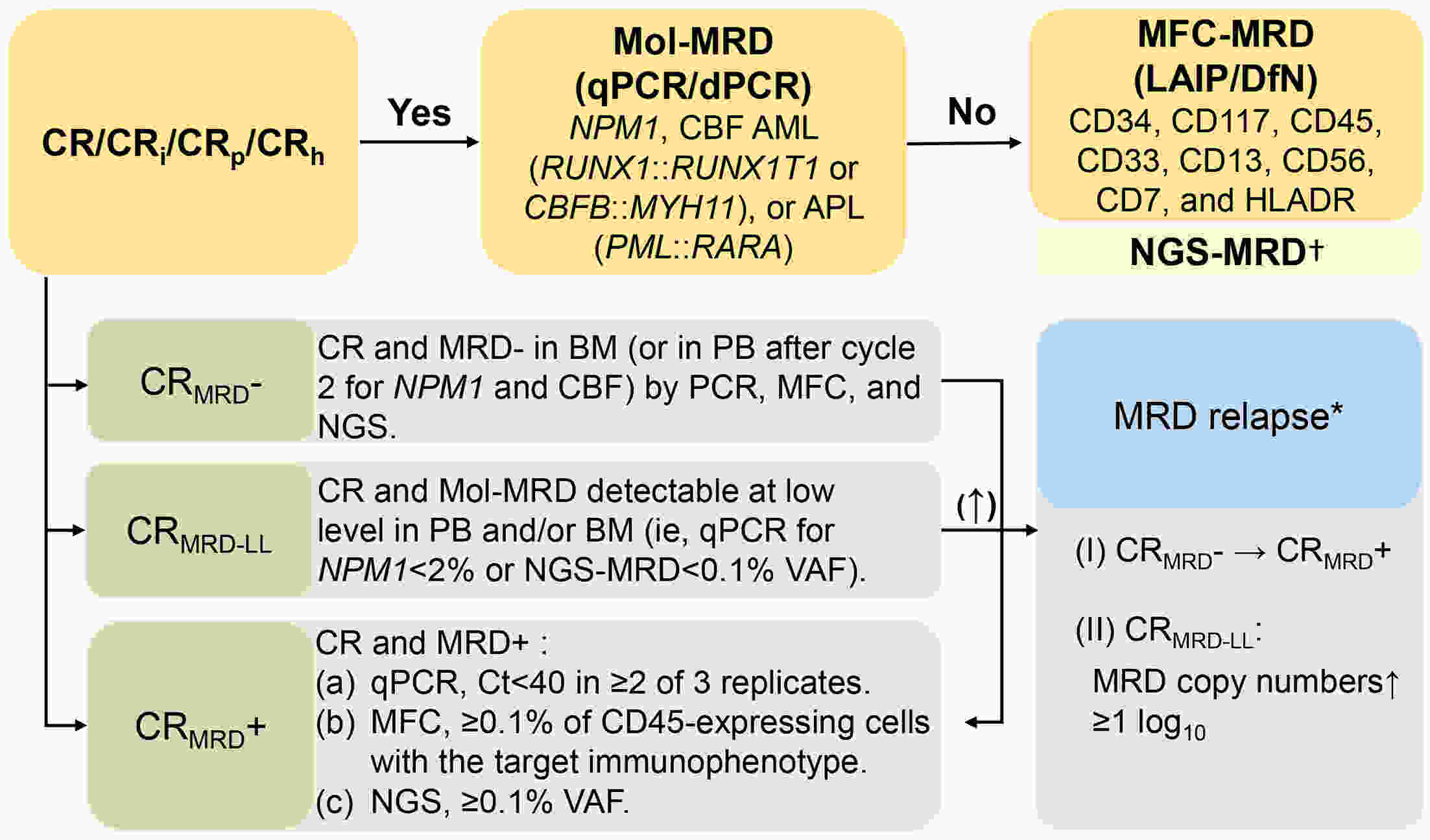
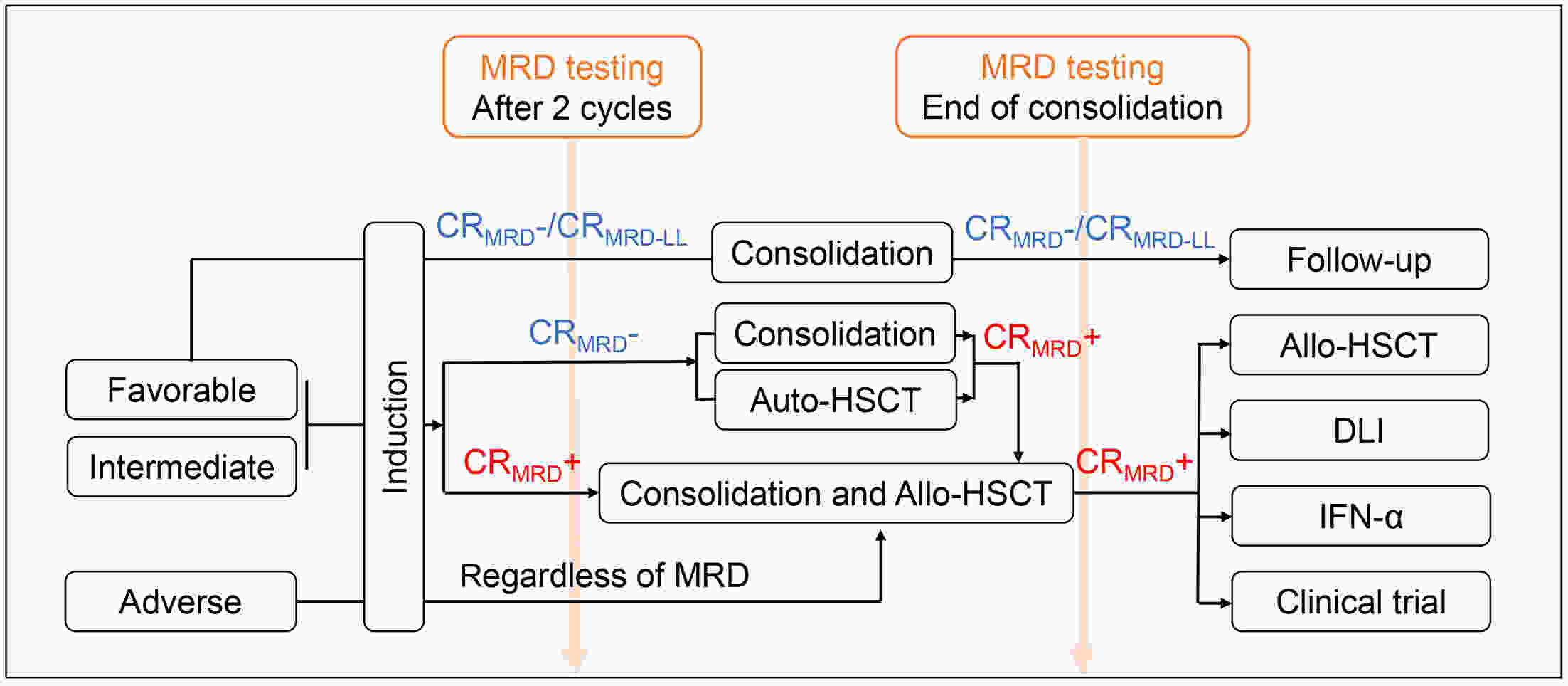
Measurable residual disease (MRD) has been widely recognized as a biomarker for deeply evaluating complete remission (CR), predicting relapse, guiding pre-emptive interventions, and serving as an endpoint surrogate for drug testing. However, despite the emergence of new technologies, there remains a lack of comprehensive understanding regarding the proper techniques, sample materials, and optimal time points for MRD assessment. In this review, we summarized the MRD methods, sample sources, and evaluation frequency according to the risk category of the European Leukemia Net (ELN) 2022. Additionally, we emphasize the importance of properly utilizing and combining these technologies. We have also refined the flowchart outlining each time point for pre-emptive interventions and intervention paths. The evaluation of MRD in acute myeloid leukemia (AML) is sophisticated, clinically applicable, and technology-dependent, and necessitates standardized approaches and further research.
Measurable residual disease (MRD) has been widely recognized as a biomarker for deeply evaluating complete remission (CR), predicting relapse, guiding pre-emptive interventions, and serving as an endpoint surrogate for drug testing. However, despite the emergence of new technologies, there remains a lack of comprehensive understanding regarding the proper techniques, sample materials, and optimal time points for MRD assessment. In this review, we summarized the MRD methods, sample sources, and evaluation frequency according to the risk category of the European Leukemia Net (ELN) 2022. Additionally, we emphasize the importance of properly utilizing and combining these technologies. We have also refined the flowchart outlining each time point for pre-emptive interventions and intervention paths. The evaluation of MRD in acute myeloid leukemia (AML) is sophisticated, clinically applicable, and technology-dependent, and necessitates standardized approaches and further research.
2023, 35(5): 470-482.
doi: 10.21147/j.issn.1000-9604.2023.05.05
Abstract:
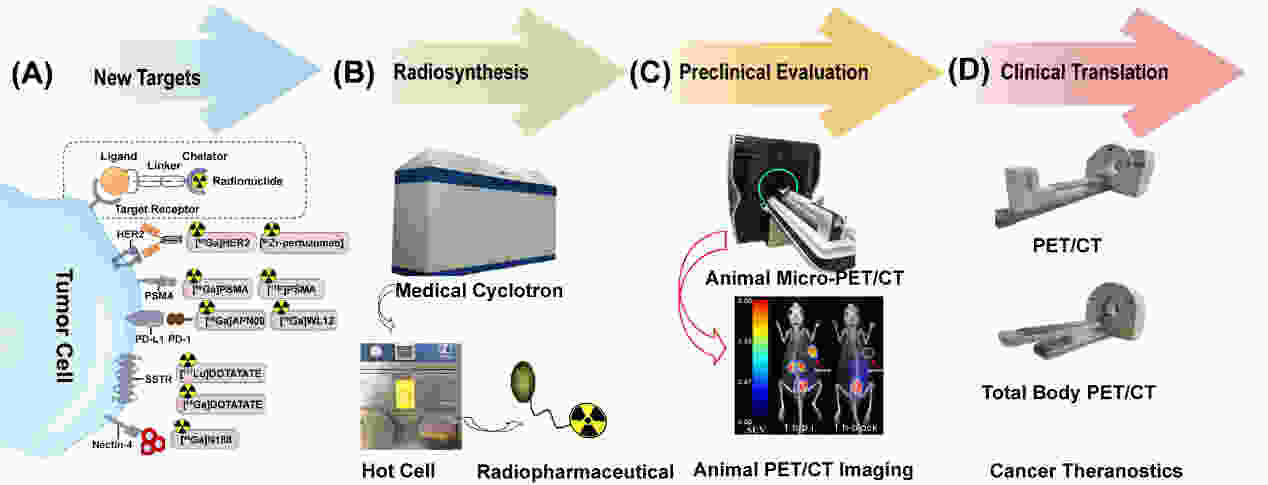
Nuclear medicine plays an irreplaceable role in the diagnosis and treatment of tumors. Radiopharmaceuticals are important components of nuclear medicine. Among the radiopharmaceuticals approved by the Food and Drug Administration (FDA), radio-tracers targeting prostate-specific membrane antigen (PSMA) and somatostatin receptor (SSTR) have held essential positions in the diagnosis and treatment of prostate cancers and neuroendocrine neoplasms, respectively. In recent years, FDA-approved serials of immune-therapy and targeted therapy drugs targeting programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1), human epidermal growth factor receptor 2 (HER2), and nectin cell adhesion molecule 4 (Nectin 4). How to screen patients suitable for these treatments and monitor the therapy? Nuclear medicine with specific radiopharmaceuticals can visualize the expression level of those targets in systemic lesions and evaluate the efficacy of treatment. In addition to radiopharmaceuticals, imaging equipment is also a key step for nuclear medicine. Advanced equipment including total-body positron emission tomography/computed tomography (PET/CT) and positron emission tomography/magnetic resonance imaging (PET/MRI) has been developed, which contribute to the diagnosis and treatment of tumors, as well as the development of new radiopharmaceuticals. Here, we conclude most recently advances of radiopharmaceuticals in nuclear medicine, and they substantially increase the “arsenal” of clinicians for tumor therapy.
Nuclear medicine plays an irreplaceable role in the diagnosis and treatment of tumors. Radiopharmaceuticals are important components of nuclear medicine. Among the radiopharmaceuticals approved by the Food and Drug Administration (FDA), radio-tracers targeting prostate-specific membrane antigen (PSMA) and somatostatin receptor (SSTR) have held essential positions in the diagnosis and treatment of prostate cancers and neuroendocrine neoplasms, respectively. In recent years, FDA-approved serials of immune-therapy and targeted therapy drugs targeting programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1), human epidermal growth factor receptor 2 (HER2), and nectin cell adhesion molecule 4 (Nectin 4). How to screen patients suitable for these treatments and monitor the therapy? Nuclear medicine with specific radiopharmaceuticals can visualize the expression level of those targets in systemic lesions and evaluate the efficacy of treatment. In addition to radiopharmaceuticals, imaging equipment is also a key step for nuclear medicine. Advanced equipment including total-body positron emission tomography/computed tomography (PET/CT) and positron emission tomography/magnetic resonance imaging (PET/MRI) has been developed, which contribute to the diagnosis and treatment of tumors, as well as the development of new radiopharmaceuticals. Here, we conclude most recently advances of radiopharmaceuticals in nuclear medicine, and they substantially increase the “arsenal” of clinicians for tumor therapy.
2023, 35(5): 483-500.
doi: 10.21147/j.issn.1000-9604.2023.05.06
Abstract:
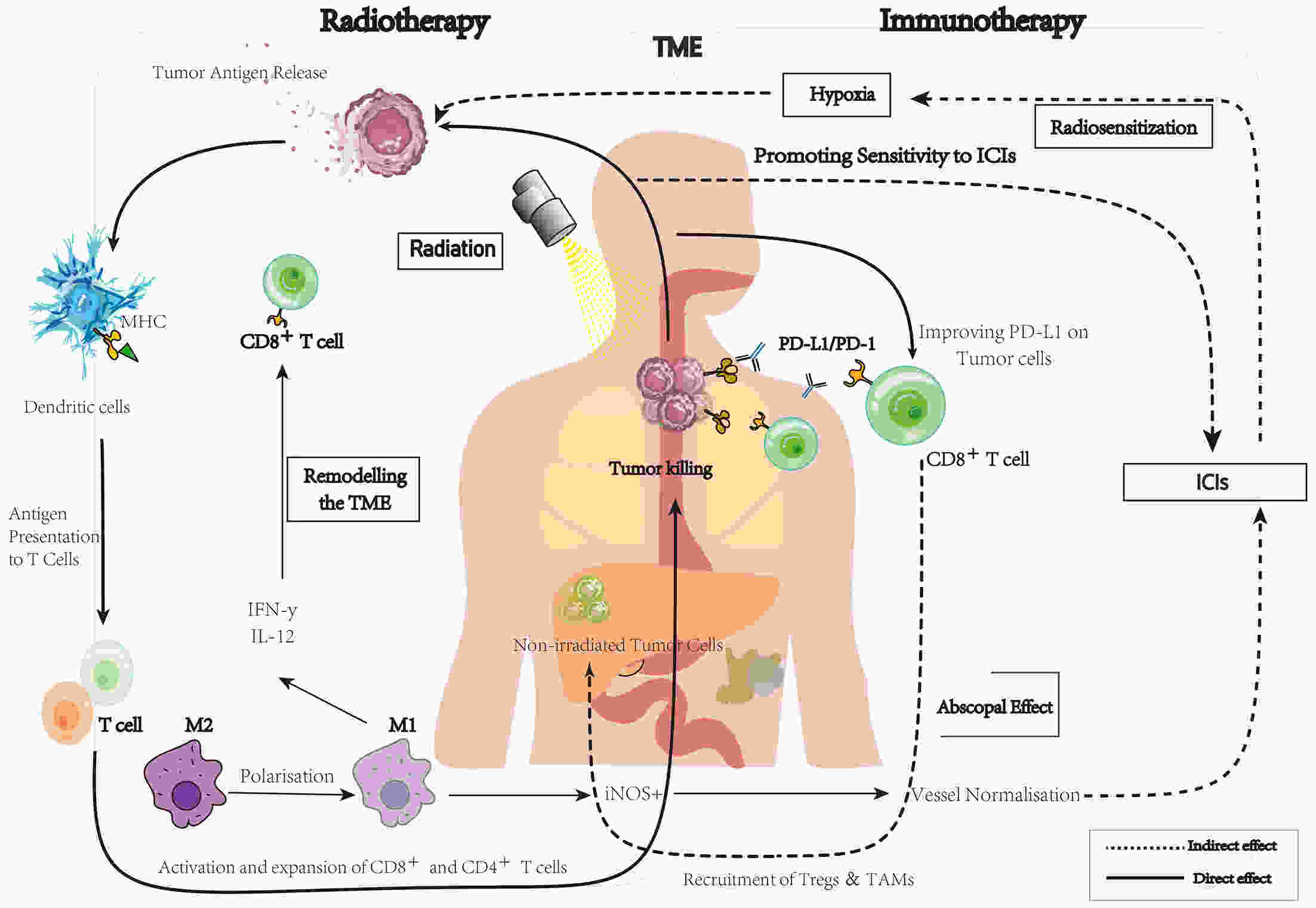
Esophageal cancer usually has a poor prognosis. Given the significant breakthrough with tumor immunotherapy, an increasing number of clinical studies have demonstrated that the combination of radiotherapy and immune checkpoint inhibitors (ICIs) may have a synergistic effect and good outcome in esophageal cancer. Clinical studies of immunoradiotherapy (iRT) for esophageal cancer have proliferated enormously from 2021 to the present. However, a summary of the efficacy and toxicity of combined therapy to guide esophageal cancer treatment in clinical practice is lacking. For this review, we integrate the latest data to analyze and assess the efficacy and safety of iRT for esophageal cancer. In addition, we discuss better predictive biomarkers, therapeutic options for specific populations, and other challenges to identify directions for future research design.
Esophageal cancer usually has a poor prognosis. Given the significant breakthrough with tumor immunotherapy, an increasing number of clinical studies have demonstrated that the combination of radiotherapy and immune checkpoint inhibitors (ICIs) may have a synergistic effect and good outcome in esophageal cancer. Clinical studies of immunoradiotherapy (iRT) for esophageal cancer have proliferated enormously from 2021 to the present. However, a summary of the efficacy and toxicity of combined therapy to guide esophageal cancer treatment in clinical practice is lacking. For this review, we integrate the latest data to analyze and assess the efficacy and safety of iRT for esophageal cancer. In addition, we discuss better predictive biomarkers, therapeutic options for specific populations, and other challenges to identify directions for future research design.
2023, 35(5): 501-510.
doi: 10.21147/j.issn.1000-9604.2023.05.07
Abstract:
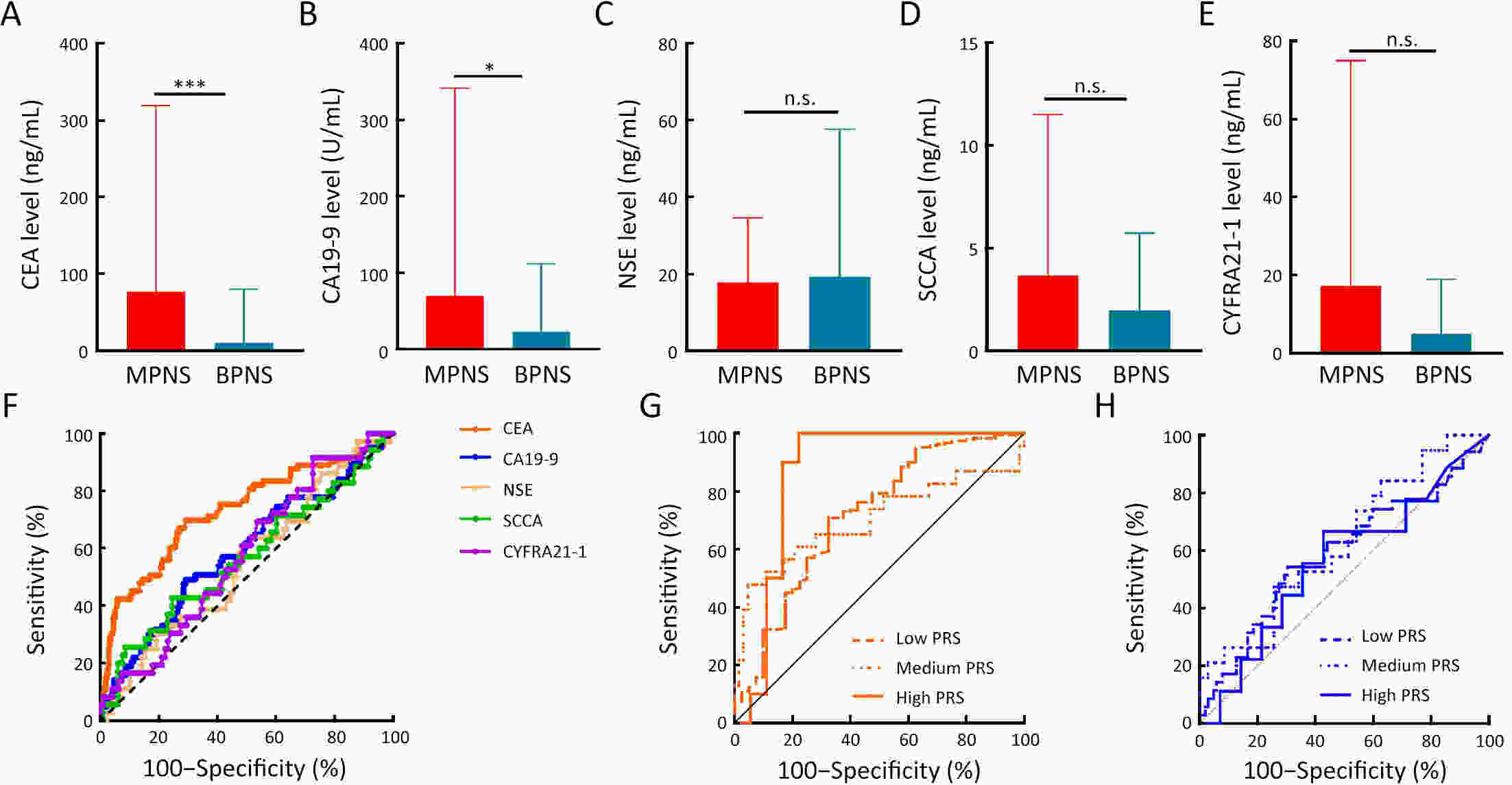
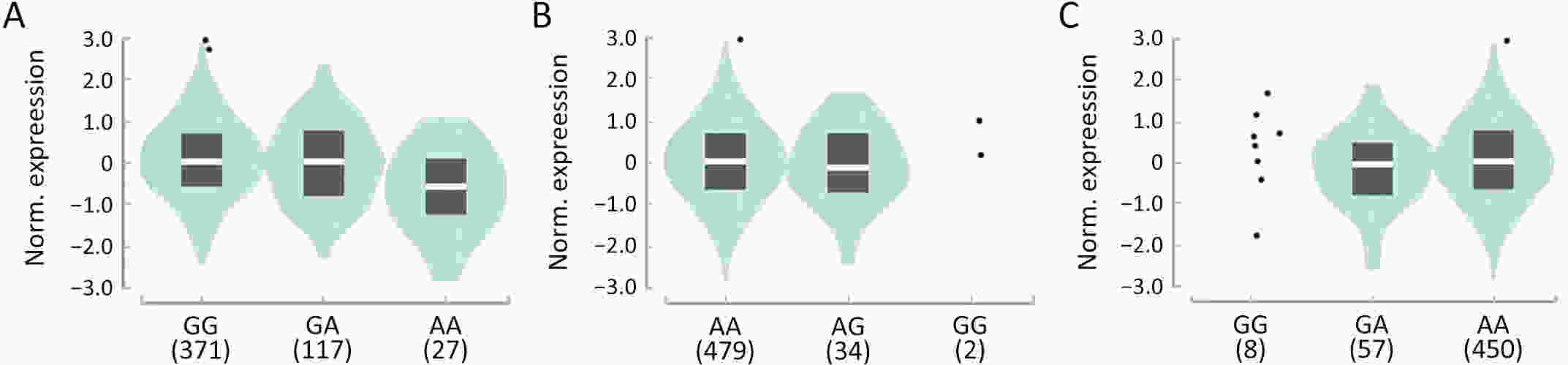
ObjectiveThe heightened prevalence of pulmonary nodules (PN) has escalated its significance as a public health concern. While the precise identification of high-risk PN carriers for malignancy remains an ongoing challenge, genetic variants hold potentials as determinants of disease susceptibility that can aid in diagnosis. Yet, current understanding of the genetic loci associated with malignant PN (MPN) risk is limited. MethodsA frequency-matched case-control study was performed, comprising 247 MPN cases and 412 benign NP (BNP) controls. We genotyped 11 established susceptibility loci for lung cancer in a Chinese cohort. Loci associated with MPN risk were utilized to compute a polygenic risk score (PRS). This PRS was subsequently incorporated into the diagnostic evaluation of MPNs, with emphasis on serum tumor biomarkers. ResultsLoci rs10429489G>A, rs17038564A>G, and rs12265047A>G were identified as being associated with an increased risk of MPNs. The PRS, formulated from the cumulative risk effects of these loci, correlated with the malignant risk of PNs in a dose-dependent fashion. A high PRS was found to amplify the MPN risk by 156% in comparison to a low PRS [odds ratio (OR)=2.56, 95% confidence interval (95% CI), 1.40−4.67]. Notably, the PRS was observed to enhance the diagnostic accuracy of serum carcinoembryonic antigen (CEA) in distinguishing MPNs from BPNs, with diagnostic values rising from 0.716 to 0.861 across low- to high-PRS categories. Further bioinformatics investigations pinpointed rs10429489G>A as an expression quantitative trait locus. ConclusionsLoci rs10429489G>A, rs17038564A>G, and rs12265047A>G contribute to MPN risk and augment the diagnostic precision for MPNs based on serum CEA concentrations.
2023, 35(5): 511-525.
doi: 10.21147/j.issn.1000-9604.2023.05.08
Abstract:
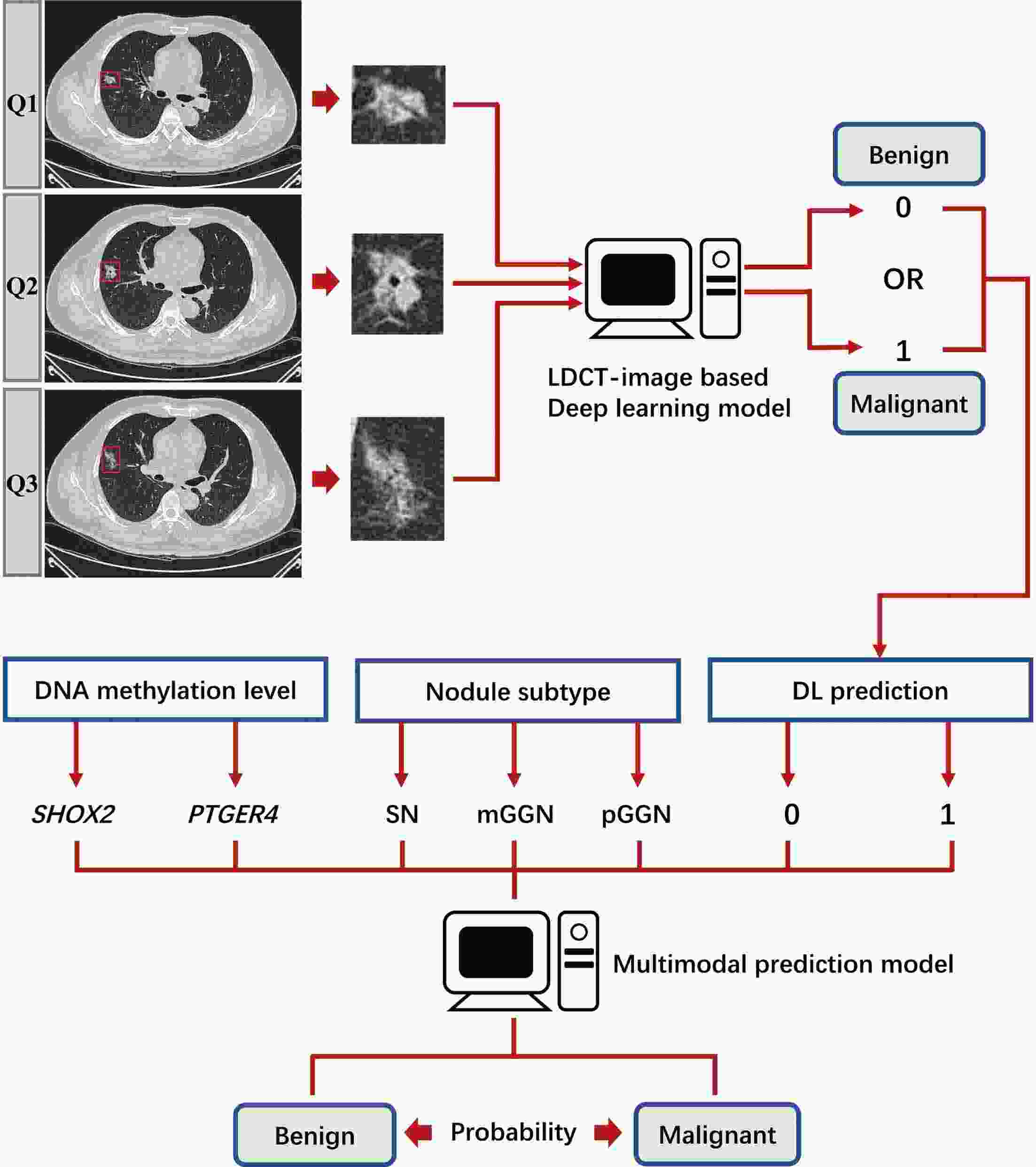
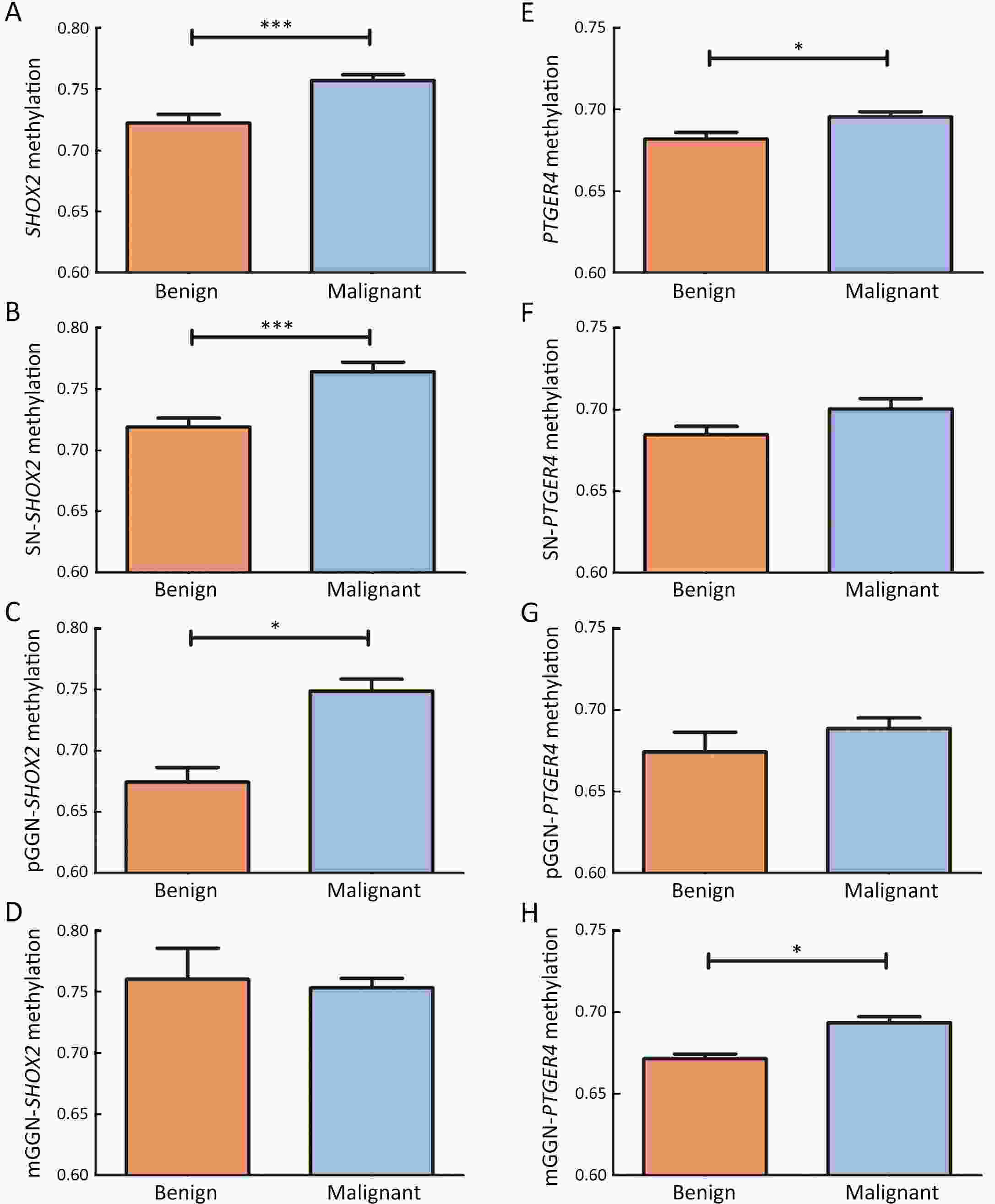
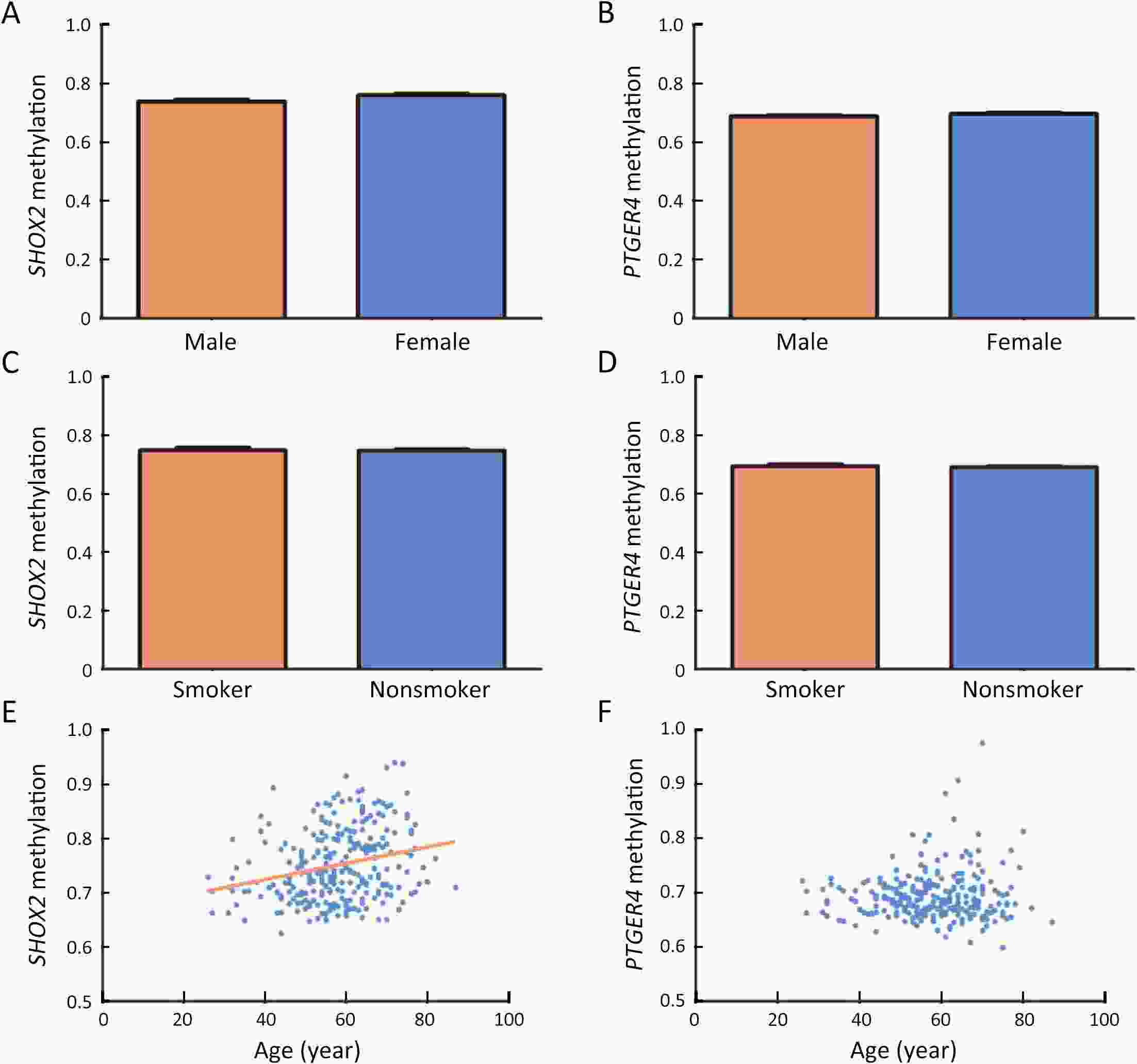
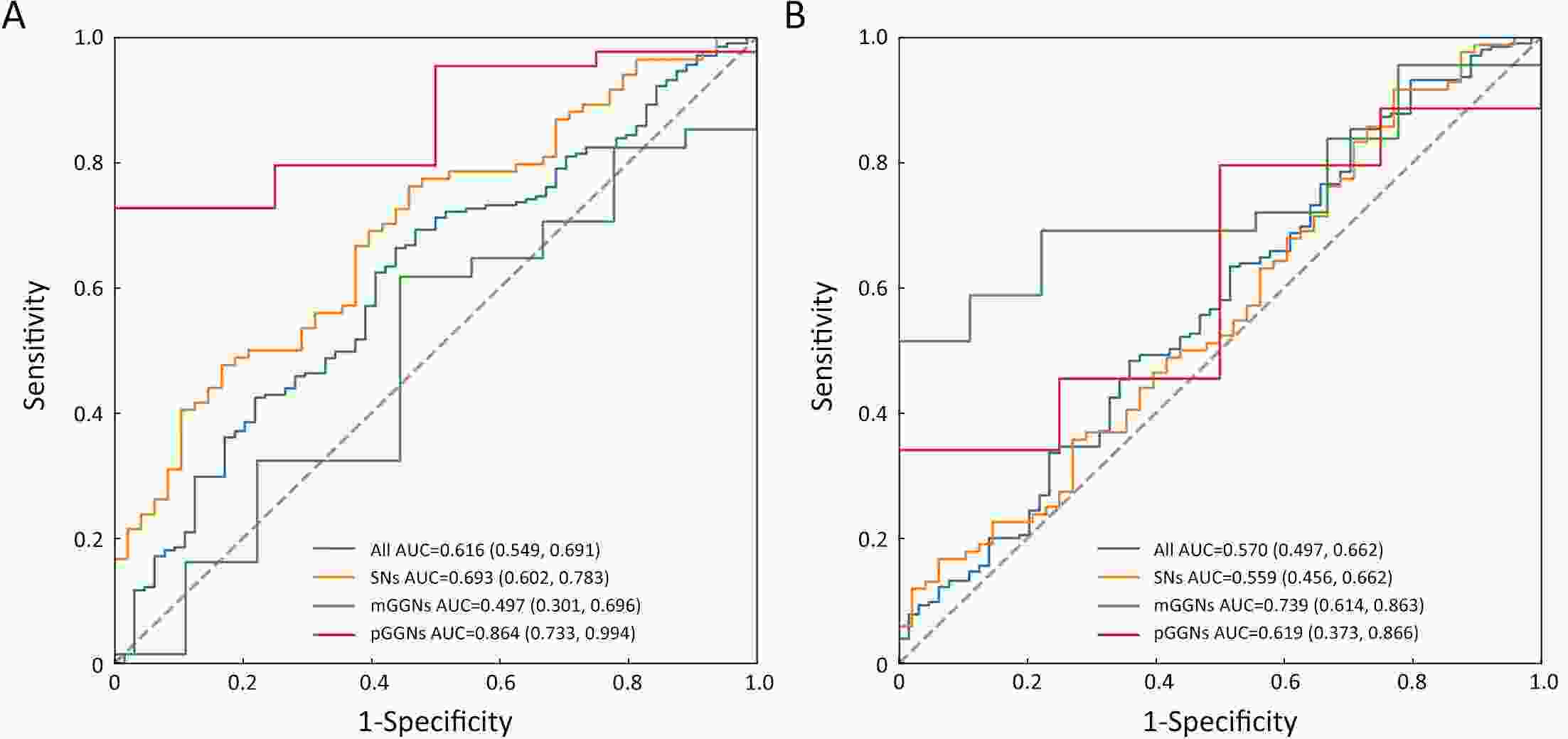
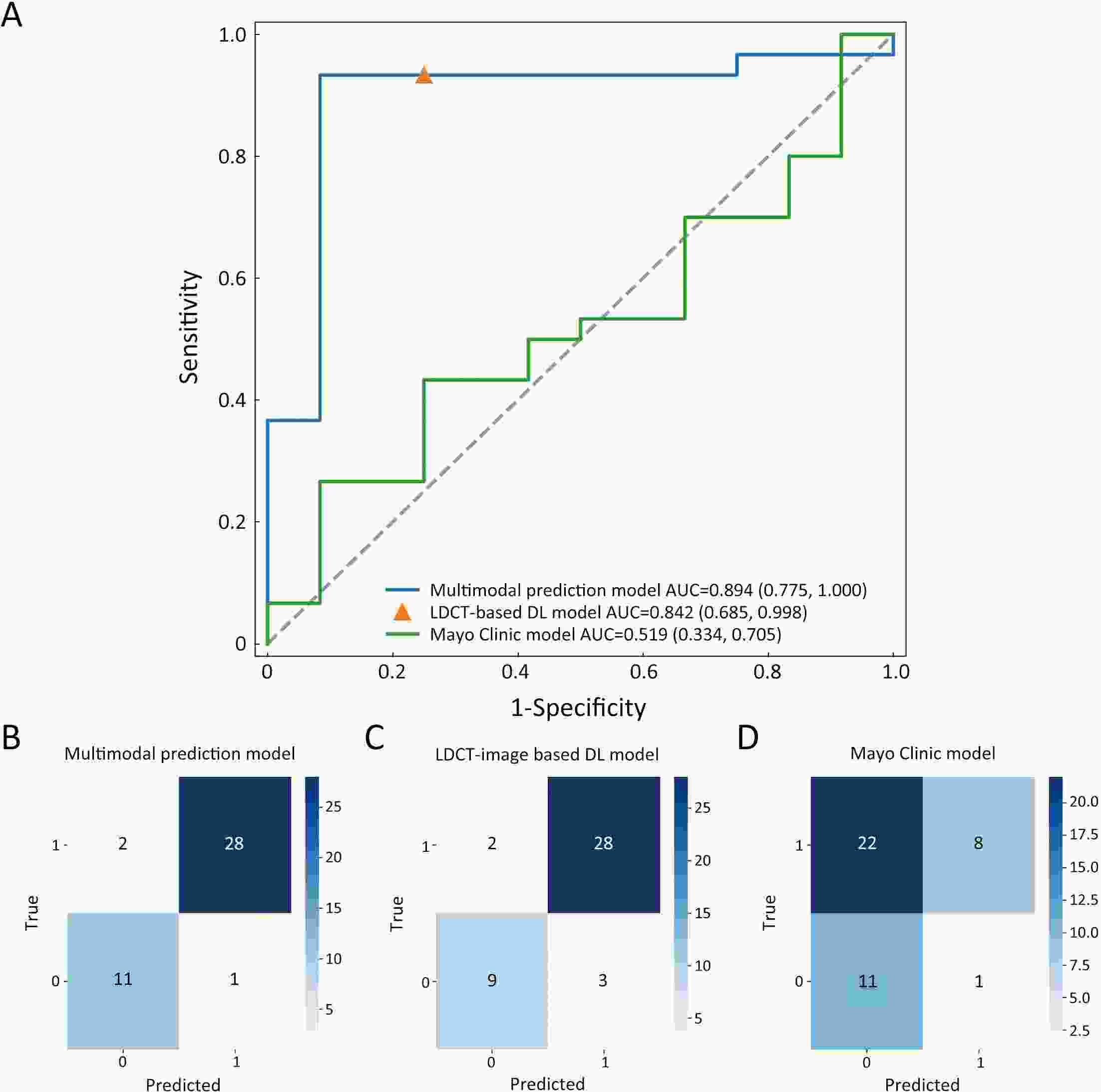
ObjectiveDNA methylation alterations are early events in carcinogenesis and immune signalling in lung cancer. This study aimed to develop a model based on short stature homeobox 2 gene (SHOX2)/prostaglandin E receptor 4 gene (PTGER4) DNA methylation in plasma, appearance subtype of pulmonary nodules (PNs) and low-dose computed tomography (LDCT) images to distinguish early-stage lung cancers. MethodsWe developed a multimodal prediction model with a training set of 257 individuals. The performance of the multimodal prediction model was further validated in an independent validation set of 42 subjects. In addition, we explored the association between SHOX2/PTGER4 DNA methylation and driver gene mutations in lung cancer based on data from The Cancer Genome Atlas (TCGA) portal. ResultsThere were significant differences between the early-stage lung cancers and benign groups in the methylation levels. The area under a receiver operator characteristic curve (AUC) of SHOX2 in patients with solid nodules, mixed ground-glass opacity nodules and pure ground-glass opacity nodules were 0.693, 0.497 and 0.864, respectively, while the AUCs of PTGER4 were 0.559, 0.739 and 0.619, respectively. With the highest AUC of 0.894, the novel multimodal prediction model outperformed the Mayo Clinic model (0.519) and LDCT-based deep learning model (0.842) in the independent validation set. Database analysis demonstrated that patients with SHOX2/PTGER4 DNA hypermethylation were enriched in TP53 mutations. ConclusionsThe present multimodal prediction model could more efficiently distinguish early-stage lung cancer from benign PNs. A prognostic index based on DNA methylation and lung cancer driver gene alterations may separate the patients into groups with good or poor prognosis.
2023, 35(5): 526-535.
doi: 10.21147/j.issn.1000-9604.2023.05.09
Abstract:
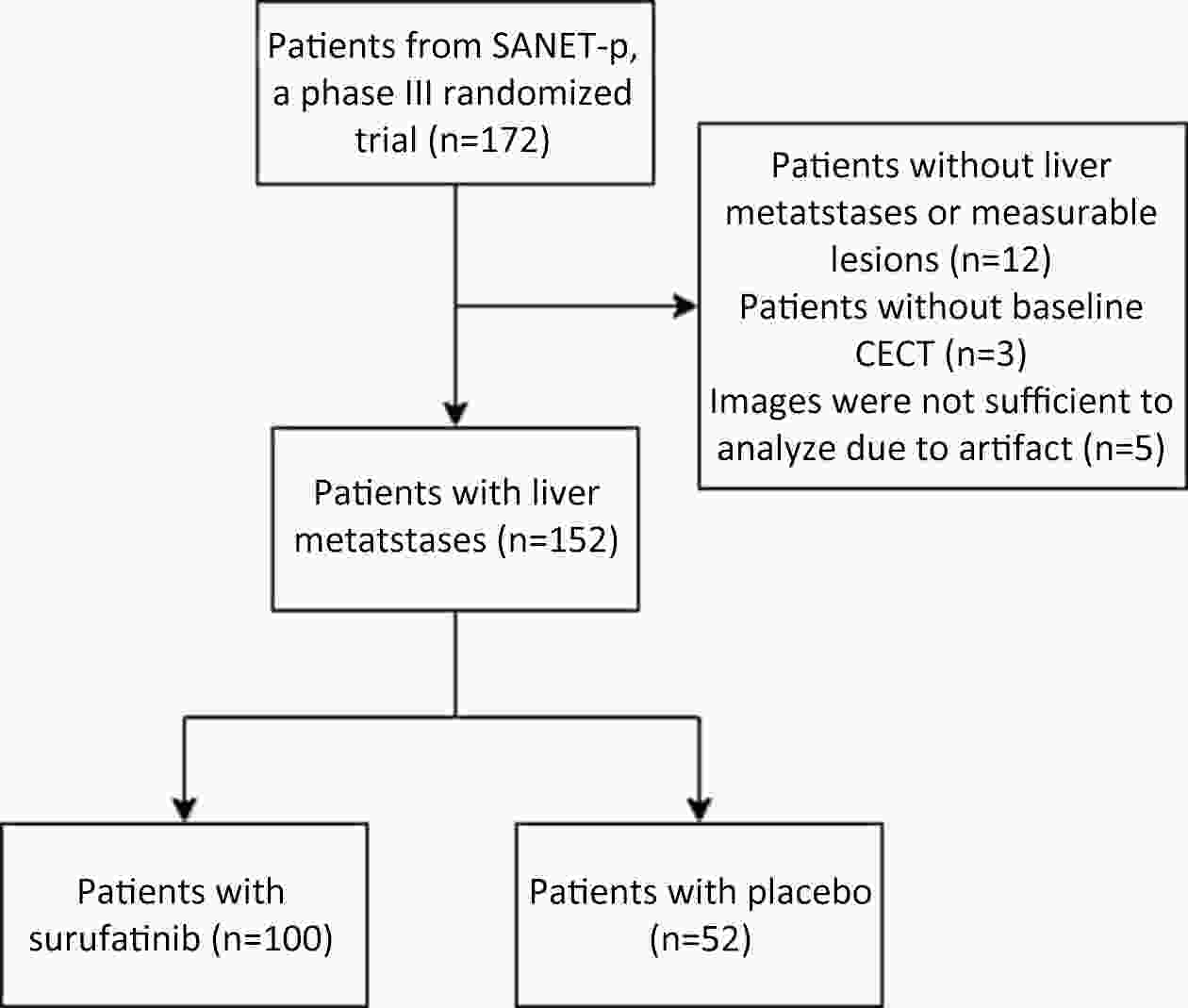
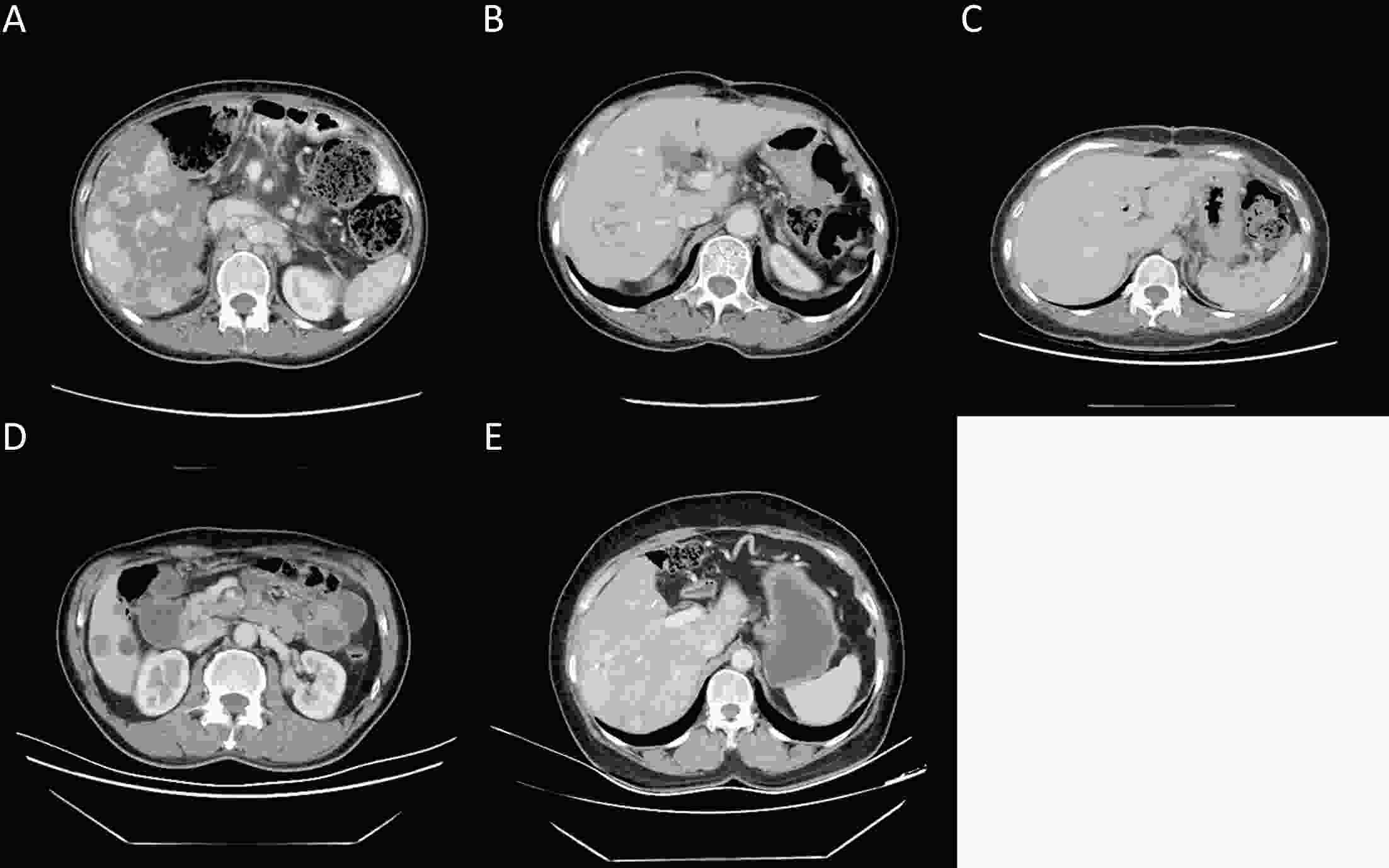
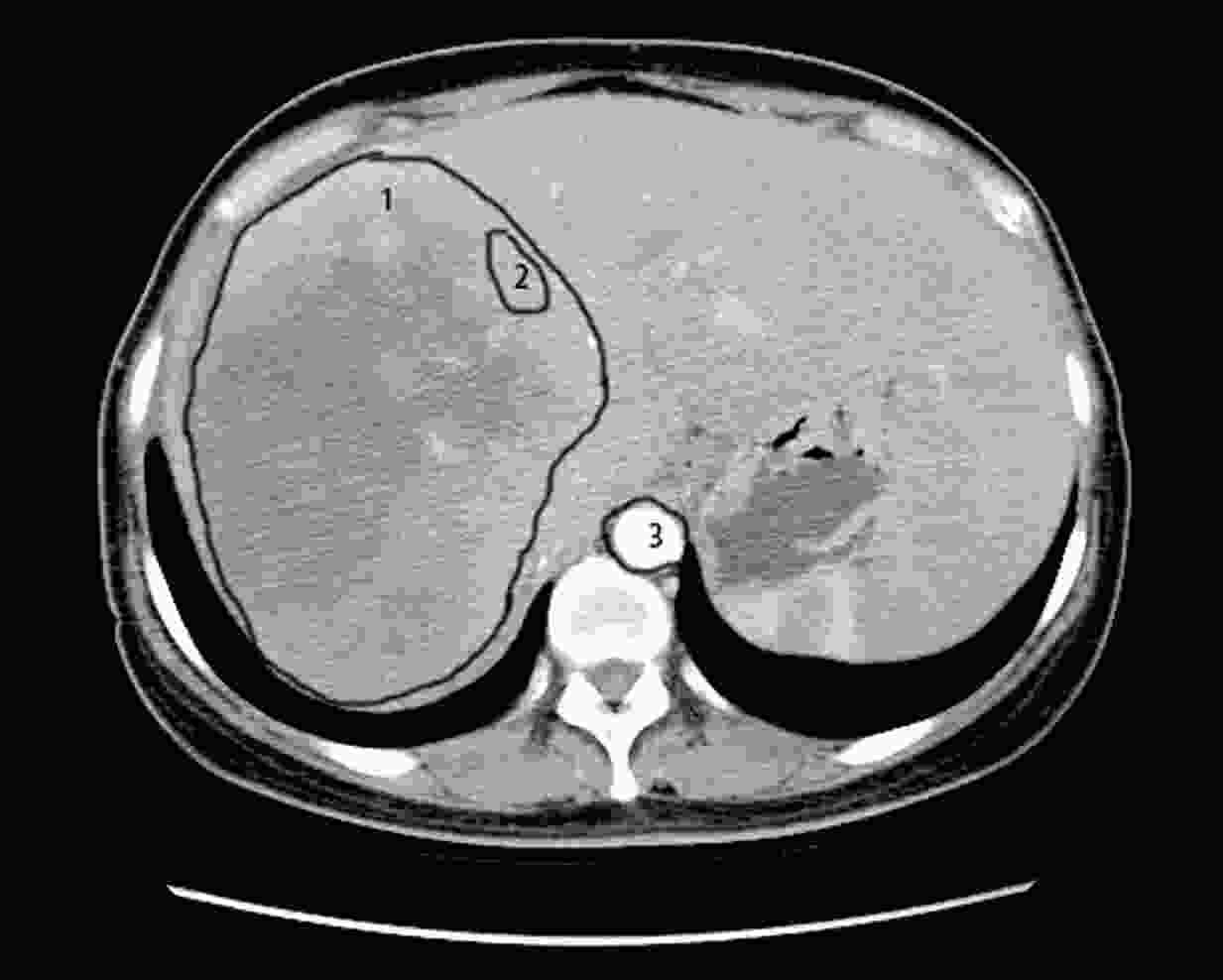
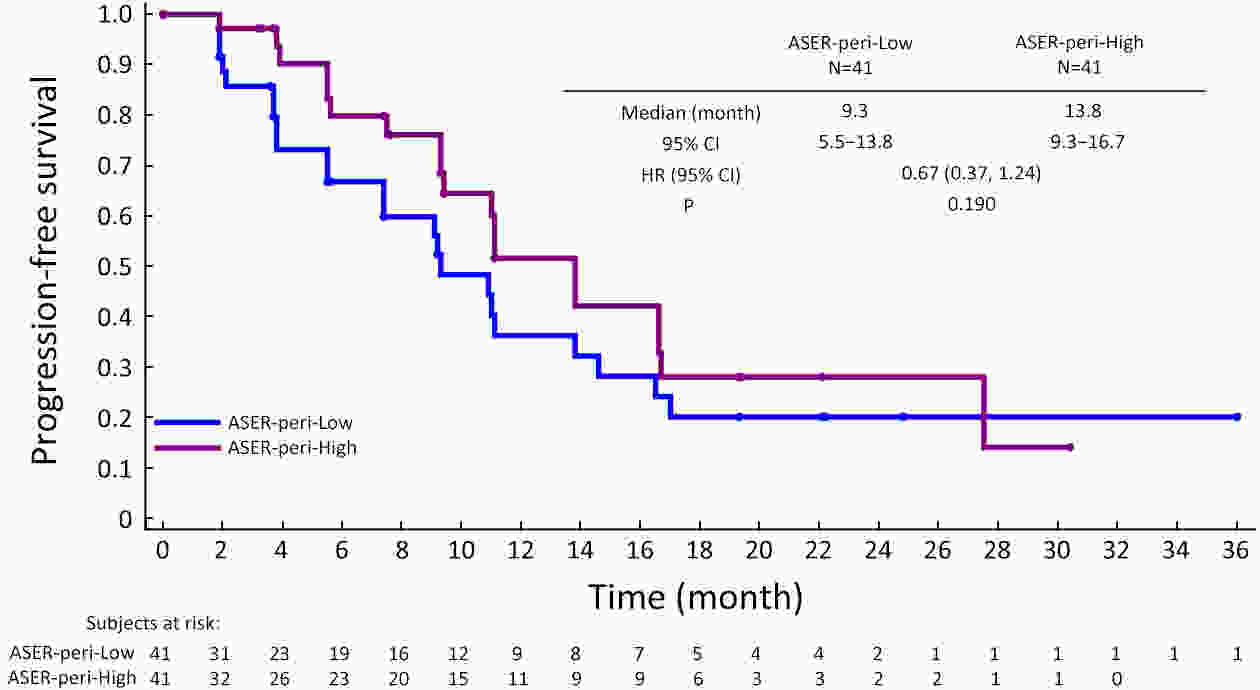
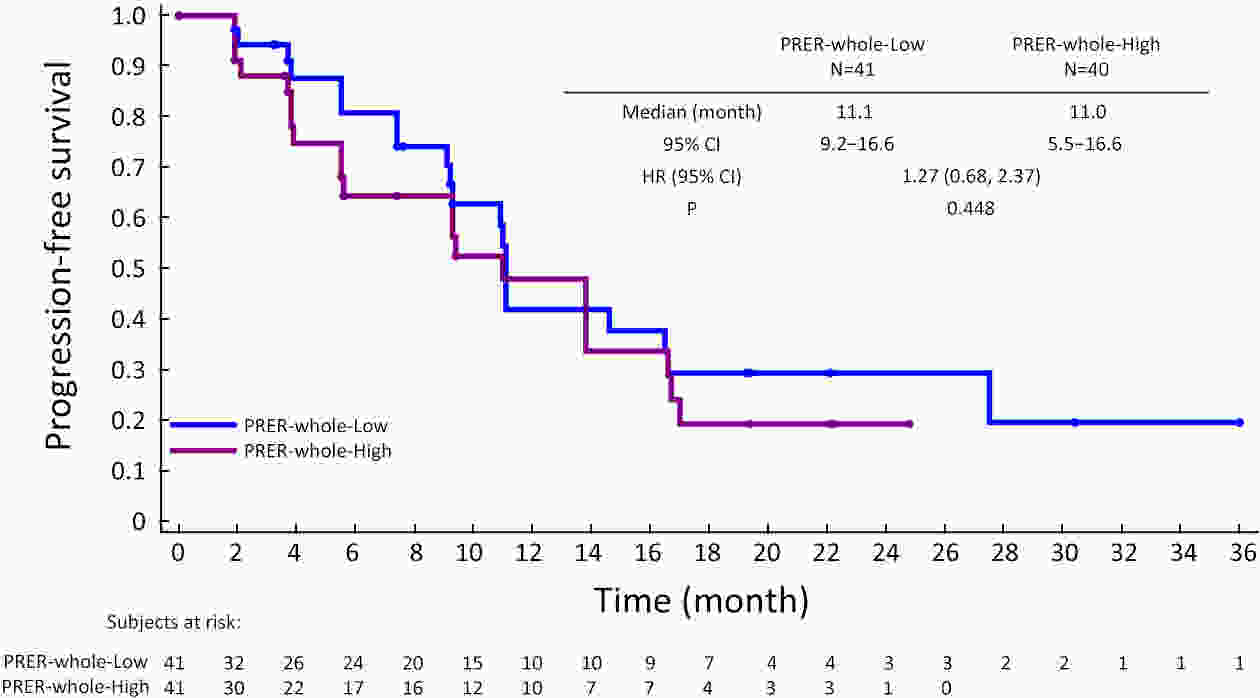
ObjectiveCurrently, pre-treatment prediction of patients with pancreatic neuroendocrine tumors with liver metastases (PNELM) receiving surufatinib treatment was unsatisfying. Our objective was to examine the association between radiological characteristics and efficacy/prognosis. MethodsWe enrolled patients with liver metastases in the phase III, SANET-p trial (NCT02589821) and obtained contrast-enhanced computed tomography (CECT) images. Qualitative and quantitative parameters including hepatic tumor margins, lesion volumes, enhancement pattern, localization types, and enhancement ratios were evaluated. The progression-free survival (PFS) and hazard ratio (HR) were calculated using Cox’s proportional hazard model. Efficacy was analyzed by logistic-regression models. ResultsAmong 152 patients who had baseline CECT assessments and were included in this analysis, the surufatinib group showed statistically superior efficacy in terms of median PFS compared to placebo across various qualitative and quantitative parameters. In the multivariable analysis of patients receiving surufatinib (N=100), those with higher arterial phase standardized enhancement ratio-peri-lesion (ASER-peri) exhibited longer PFS [HR=0.039; 95% confidence interval (95% CI): 0.003−0.483; P=0.012]. Furthermore, patients with a high enhancement pattern experienced an improvement in the objective response ratio [31.3% vs. 14.7%, odds ratio (OR)=3.488; 95% CI: 1.024−11.875; P=0.046], and well-defined tumor margins were associated with a higher disease control rate (DCR) (89.3% vs. 68.2%, OR=4.535; 95% CI: 1.285−16.011; P=0.019) compared to poorly-defined margins. ConclusionsThese pre-treatment radiological features, namely high ASER-peri, high enhancement pattern, and well-defined tumor margins, have the potential to serve as predictive markers of efficacy in patients with PNELM receiving surufatinib.
2023, 35(5): 536-549.
doi: 10.21147/j.issn.1000-9604.2023.05.10
Abstract:
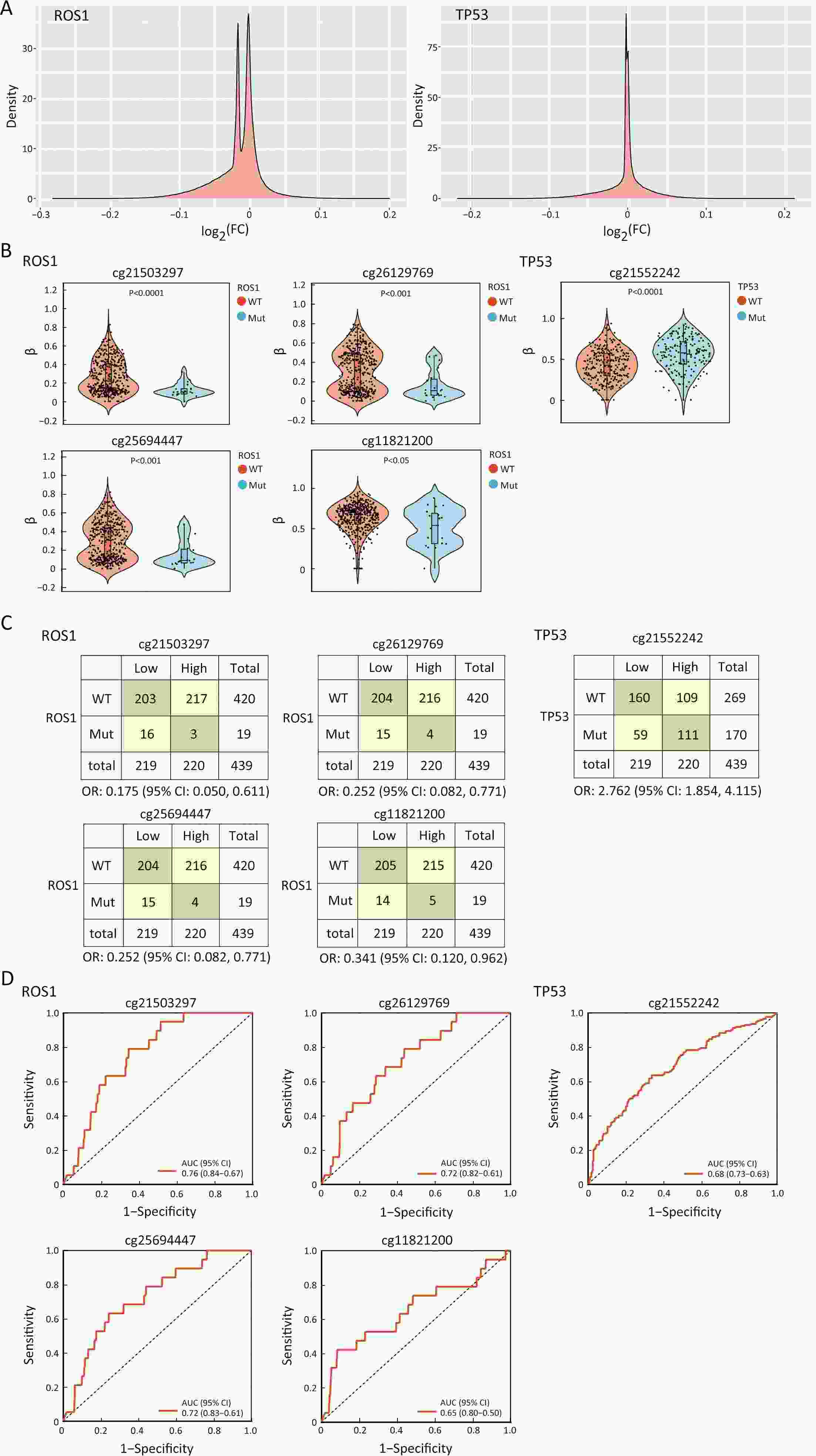
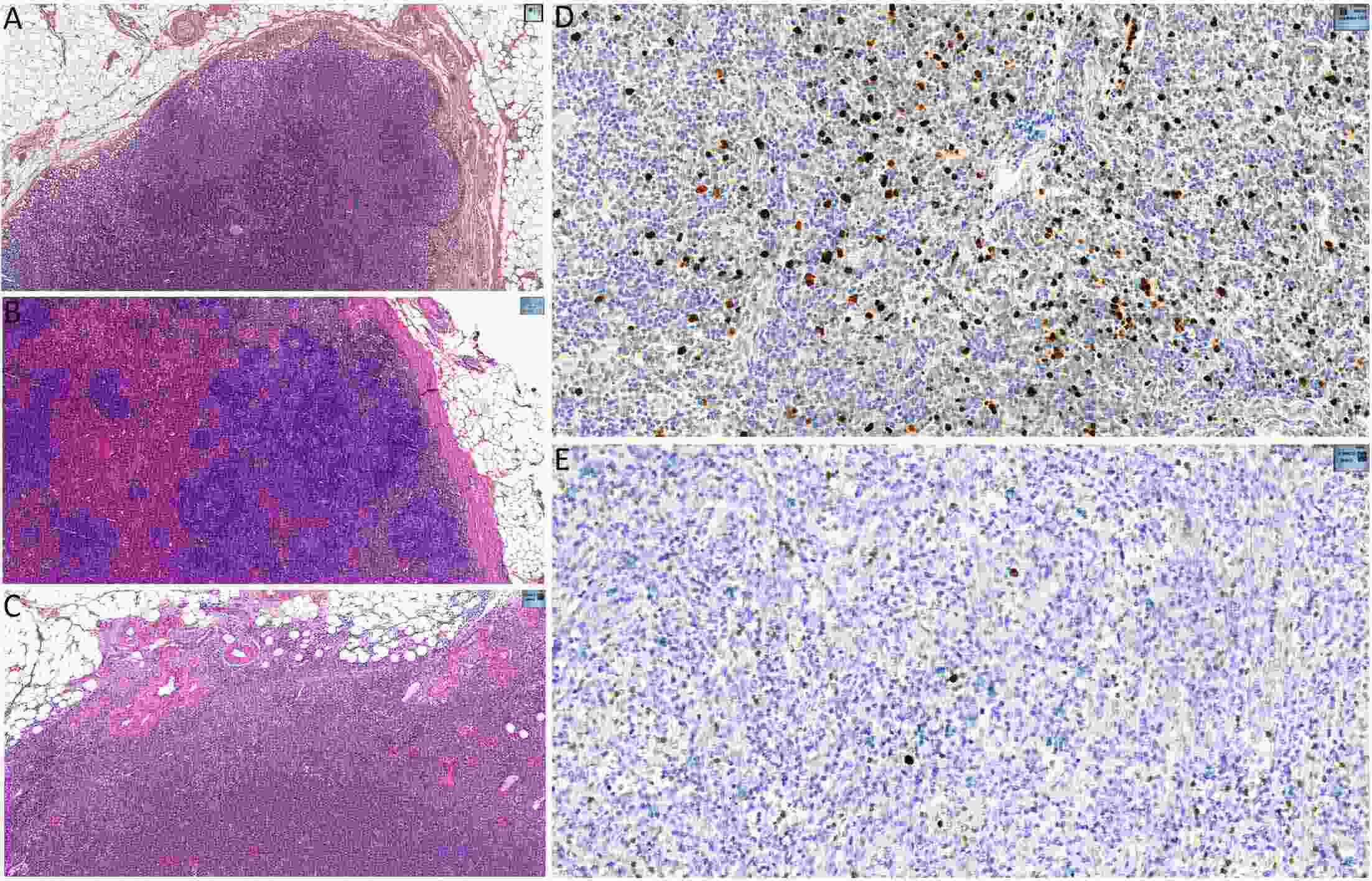
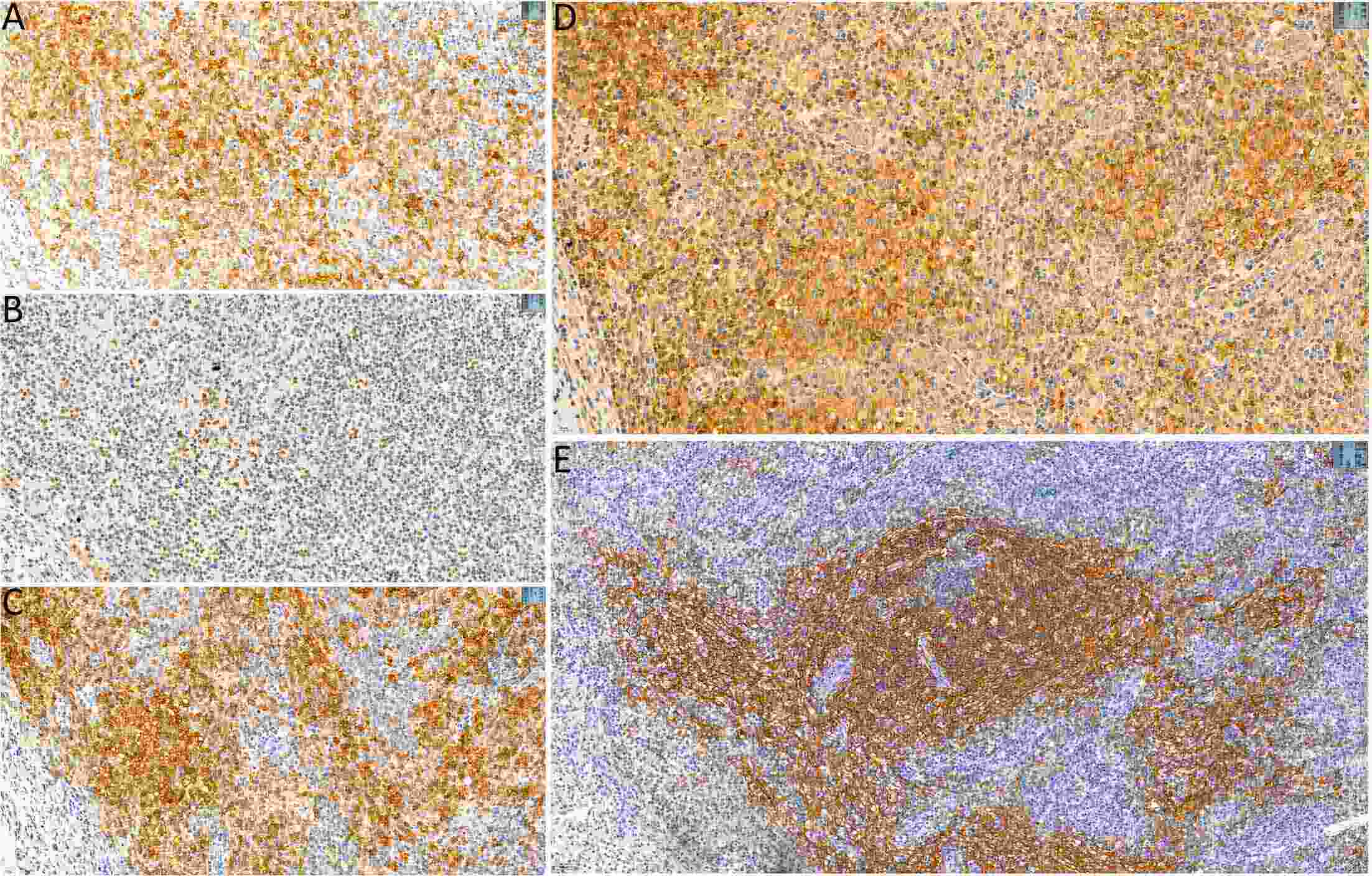
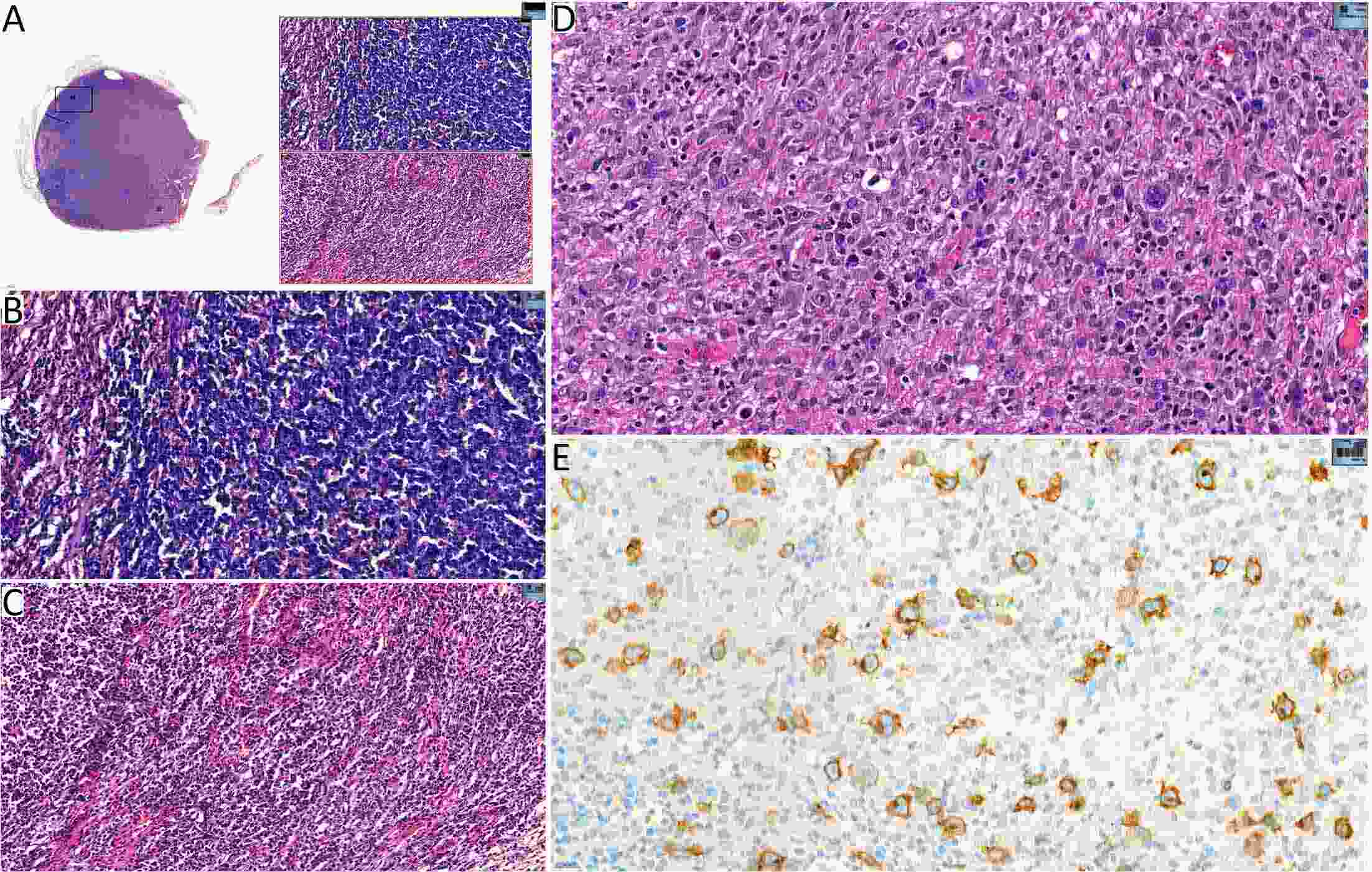
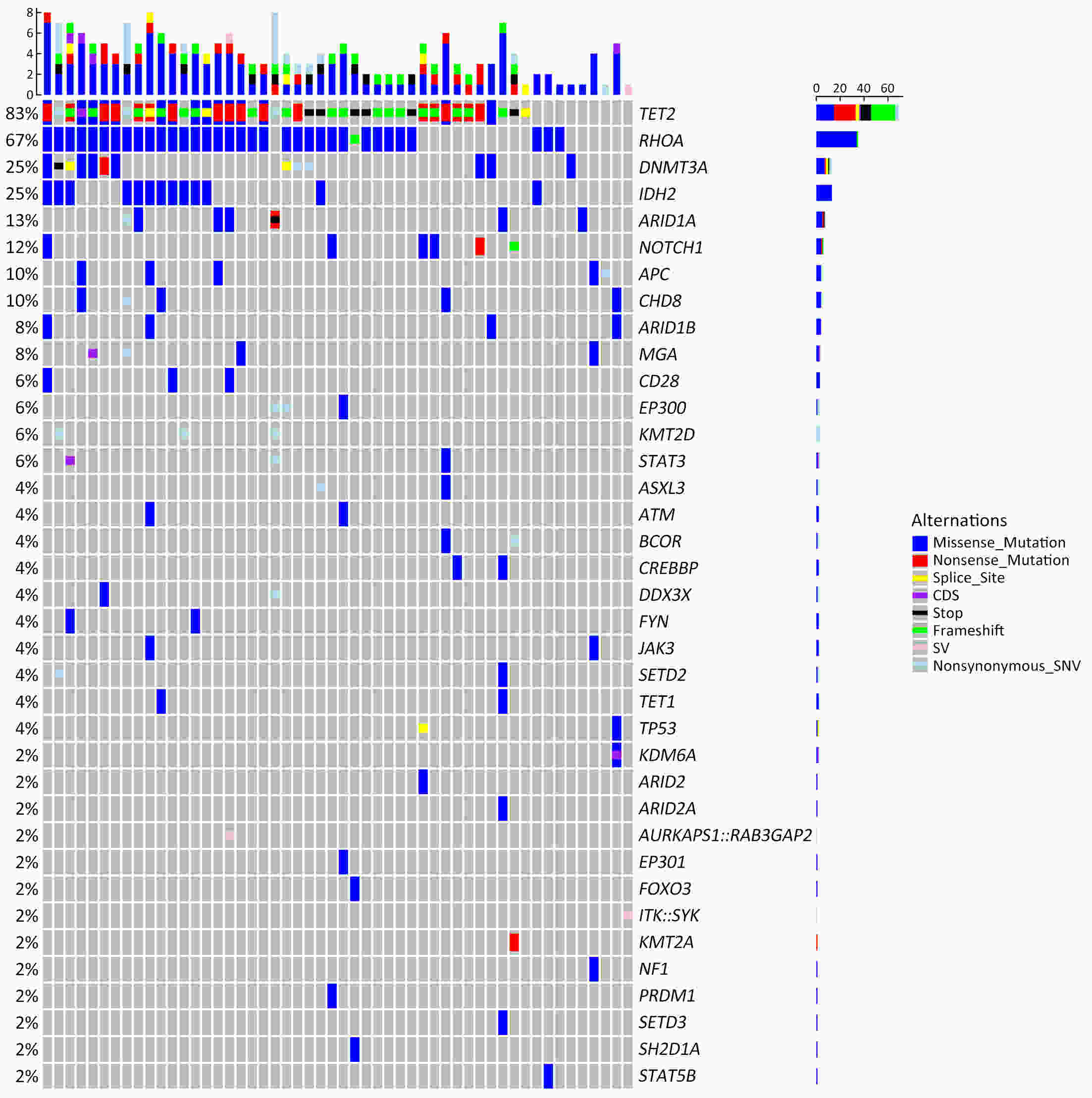
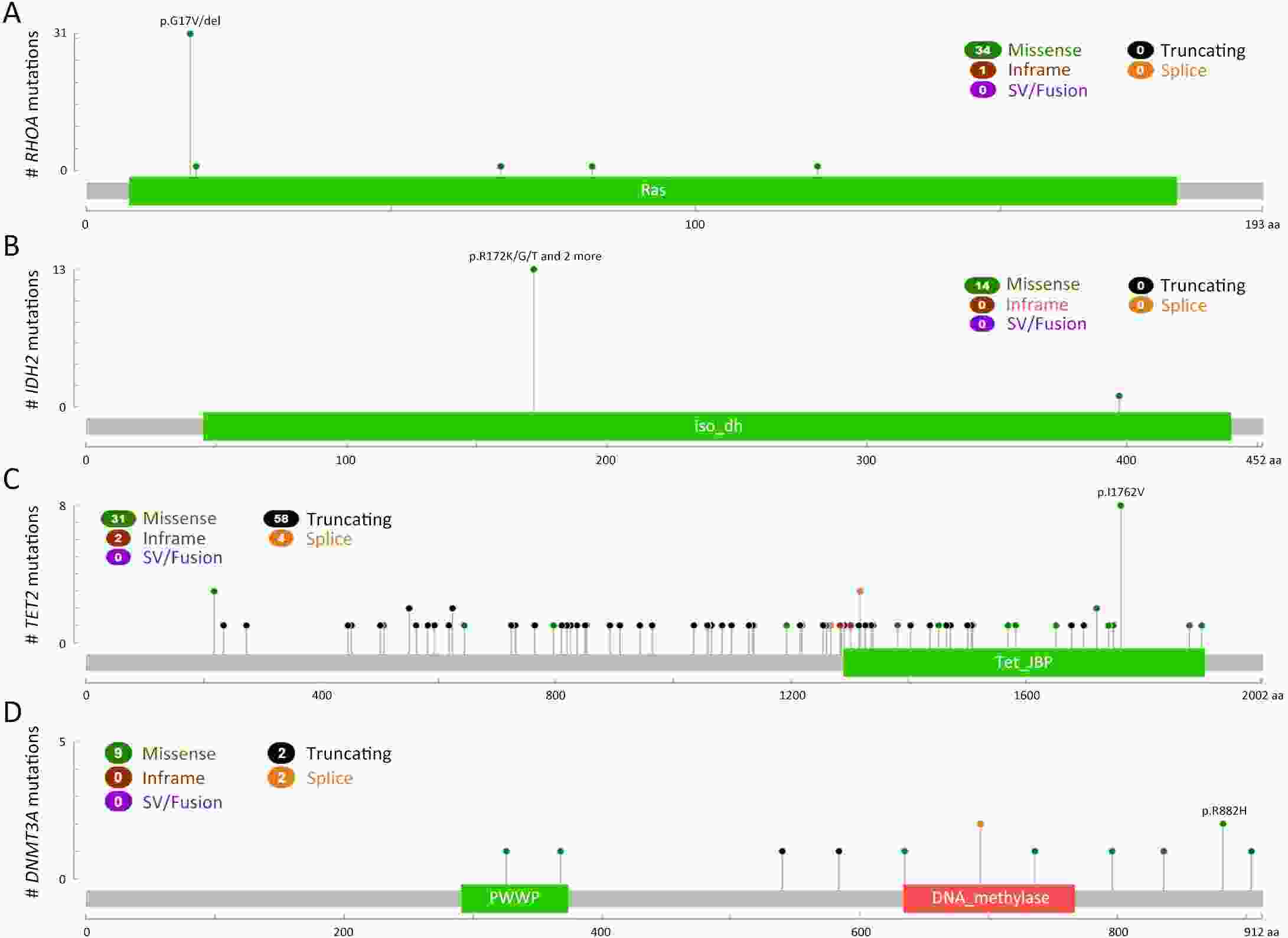
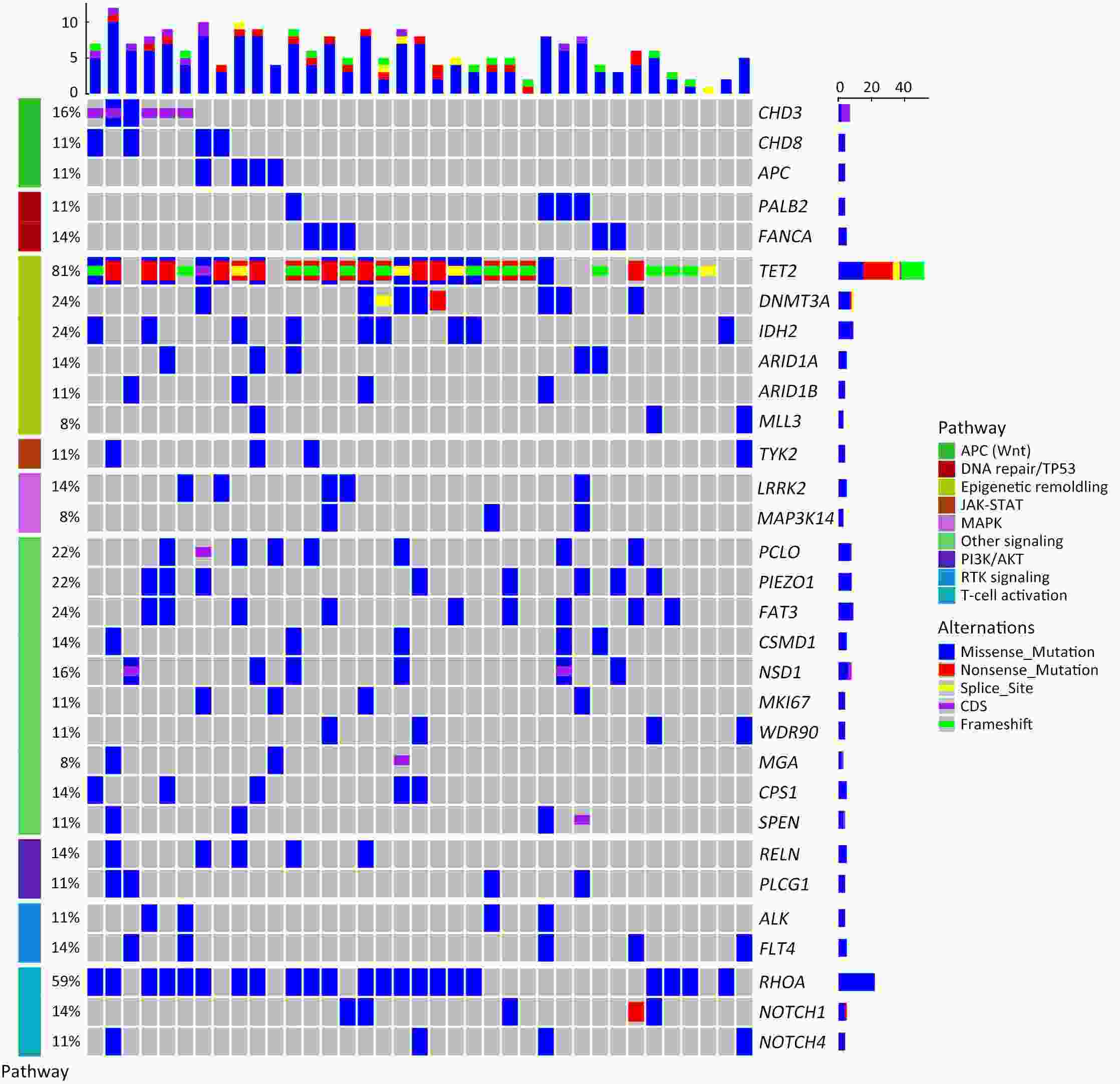
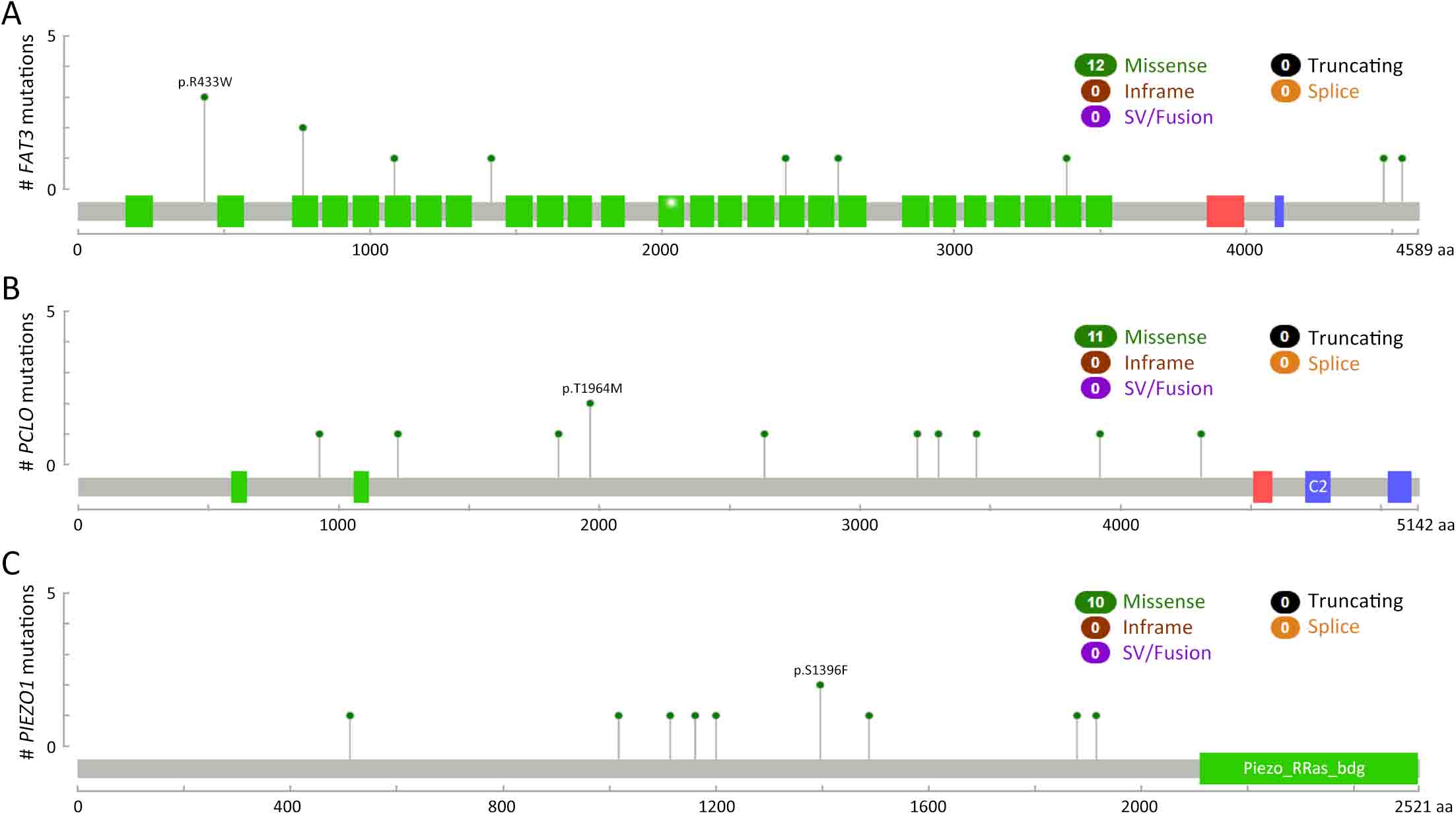
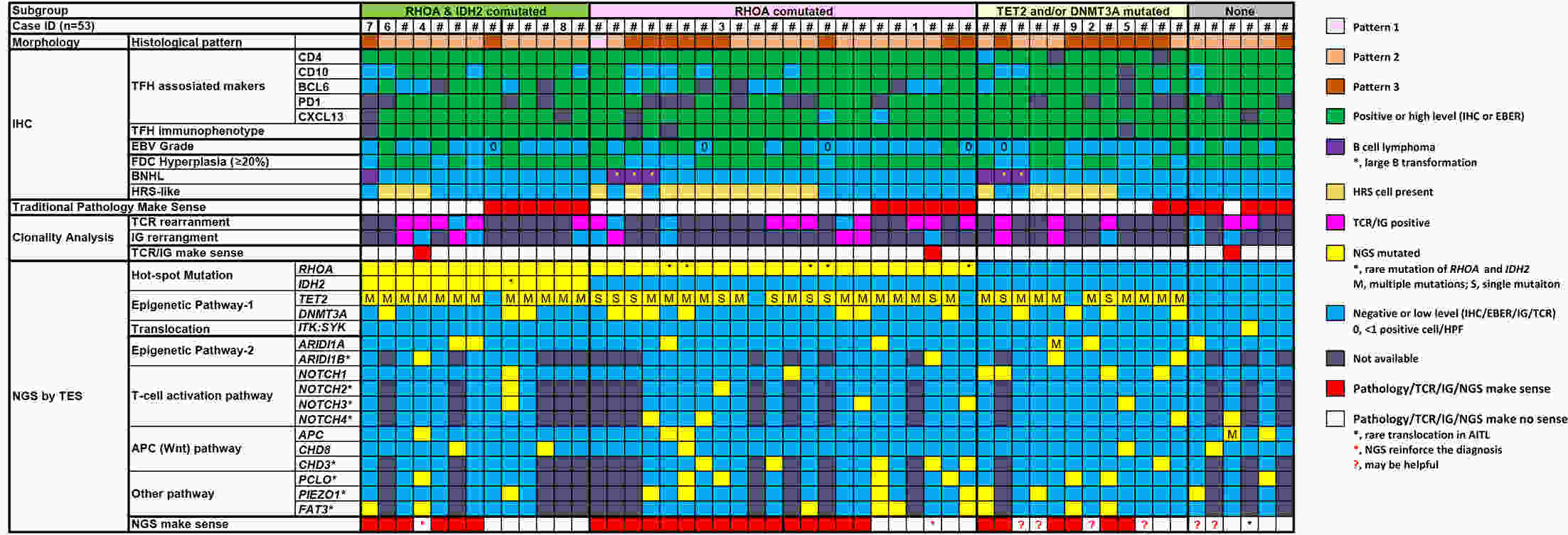
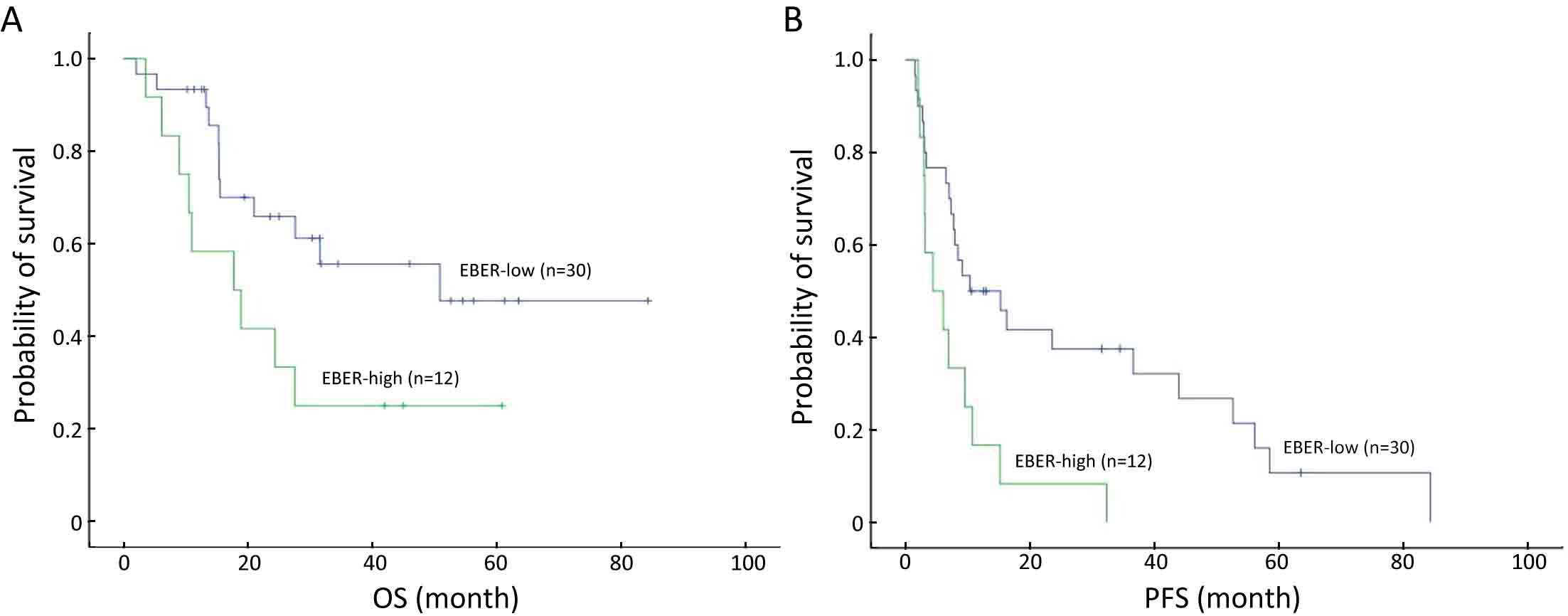
ObjectiveTo explore the application of genetic abnormalities in the diagnosis of angioimmunoblastic T-cell lymphoma (AITL) and the reliable pathological prognostic factors. MethodsThis study included 53 AITL cases, which were reviewed for morphological patterns, immunophenotypes, presence of Hodgkin and Reed-Sternberg (HRS)-like cells, and co-occurrence of B cell proliferation. The Epstein-Barr virus (EBV)-positive cells in tissues were counted, and cases were classified into “EBV encoded RNA (EBER) high-density” group if >50/HPF. Targeted exome sequencing was performed. ResultsMutation data can assist AITL diagnosis: 1) with considerable HRS-like cells (20 cases): RHOA mutated in 14 cases (IDH2 co-mutated in 3 cases, 4 cases with rare RHOA mutation), TET2 was mutated in 5 cases (1 case co-mutated with DNMT3A), and DNMT3A mutated in 1 case; 2) accompanied with B cell lymphoma (7 cases): RHOA mutated in 4 cases (1 case had IDH2 mutation), TET2 mutated in 2 cases and DNMT3A mutated in 1 case; 3) mimic peripheral T cell lymphoma, not otherwise specified (5 cases): RHOA mutated in 2 cases (IDH2 co-mutated in 1 case), TET2 mutated in 3 cases, and DNMT3A mutated in 1 case; 4) pattern 1 (1 case), RHOA and TET2 co-mutated. Besides RHOAG17V (30/35), rare variant included RHOAK18N, RHOAR68H, RHOAC83Y, RHOAD120G and RHOAG17del, IDH2R172 co-mutated with IDH2M397V in one case. There were recurrent mutations of FAT3, PCLO and PIEZO1 and genes of epigenetic remodeling, T-cell activation, APC and PI3K/AKT pathway. EBER high-density independently indicated adverse overall survival and progression-free survival (P=0.046 and P=0.008, Kaplan-Meier/log-rank). ConclusionsOver half AITL cases might be confused in diagnosis for certain conditions without mutation data. Targeted exome sequencing with a comprehensive panel is crucial to detect both hot-spot and rare mutation variants for RHOA and IDH2 and other recurrent mutated genes in addition to TET2 and DNMT3A. EBER high-density independently indicated adverse survival.
2023, 35(5): 550-562.
doi: 10.21147/j.issn.1000-9604.2023.05.11
Abstract:
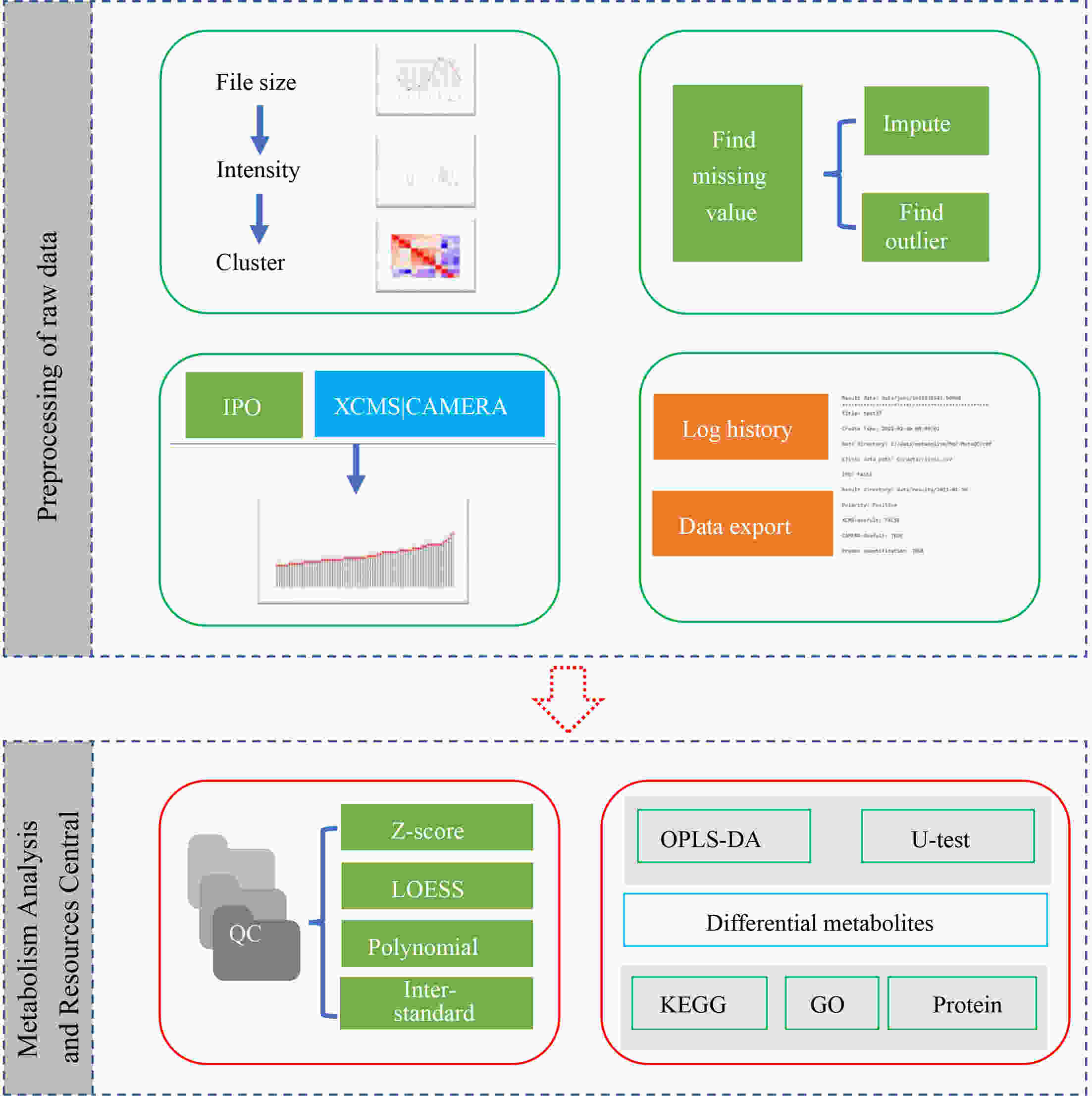
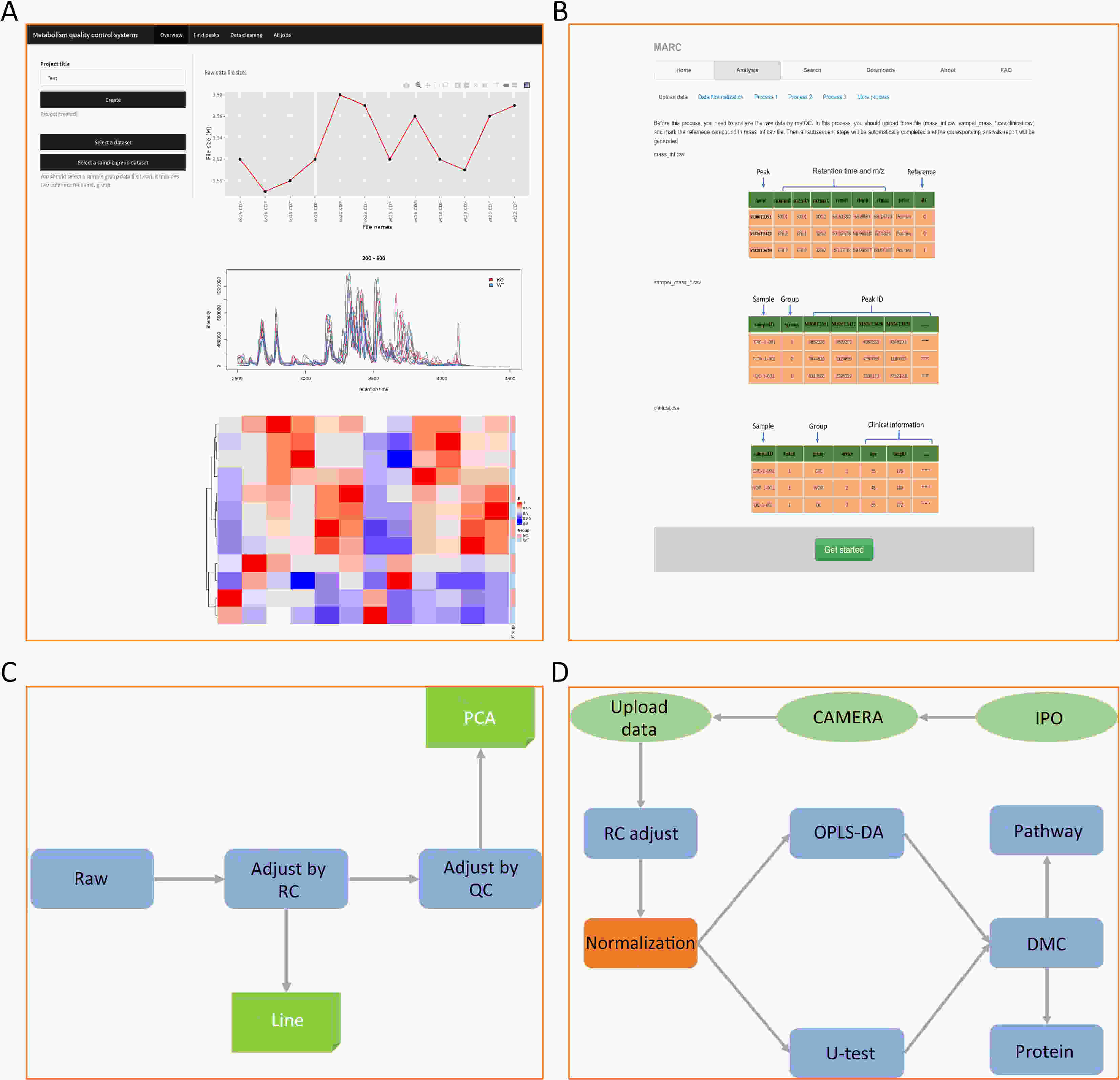
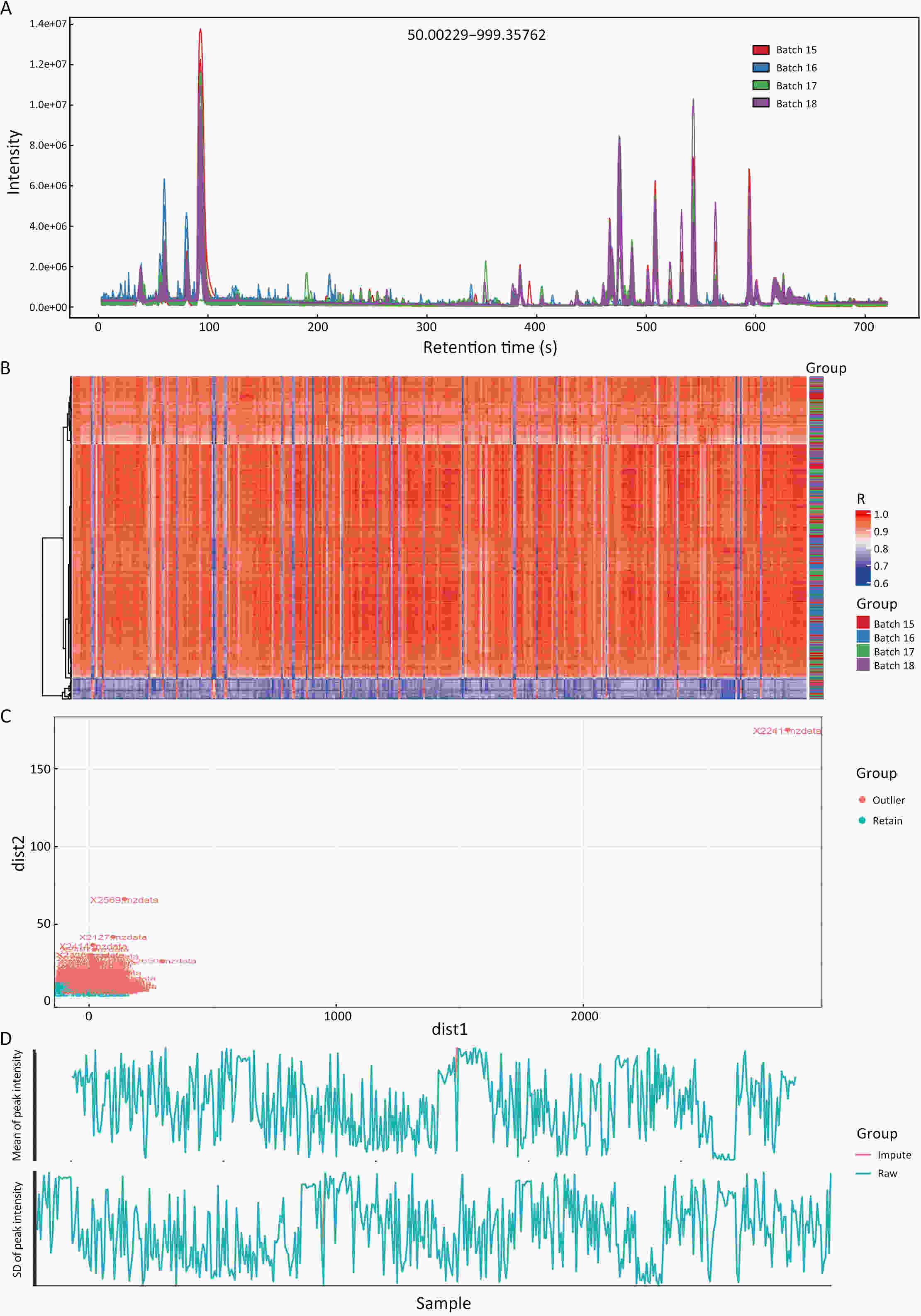
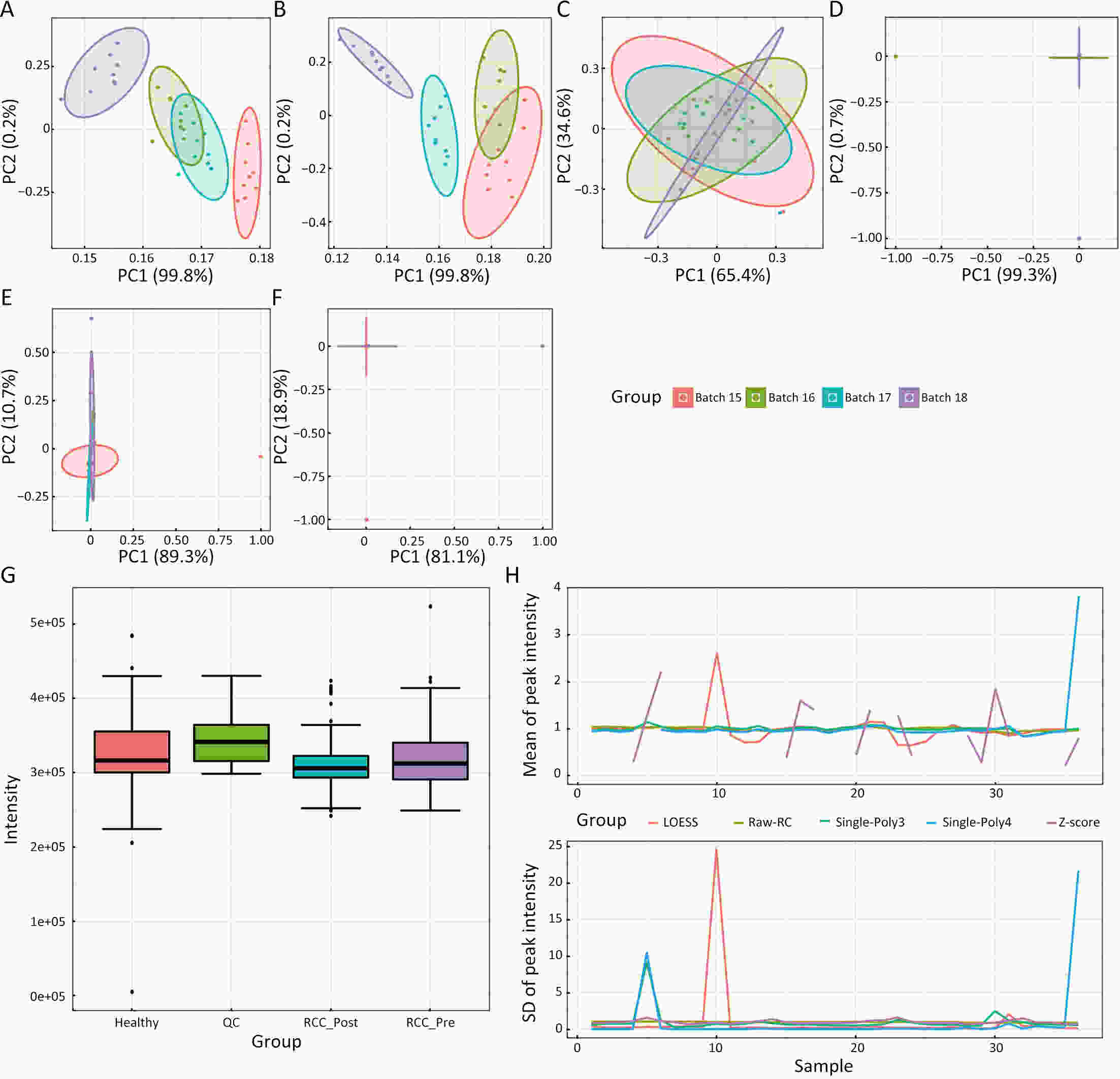
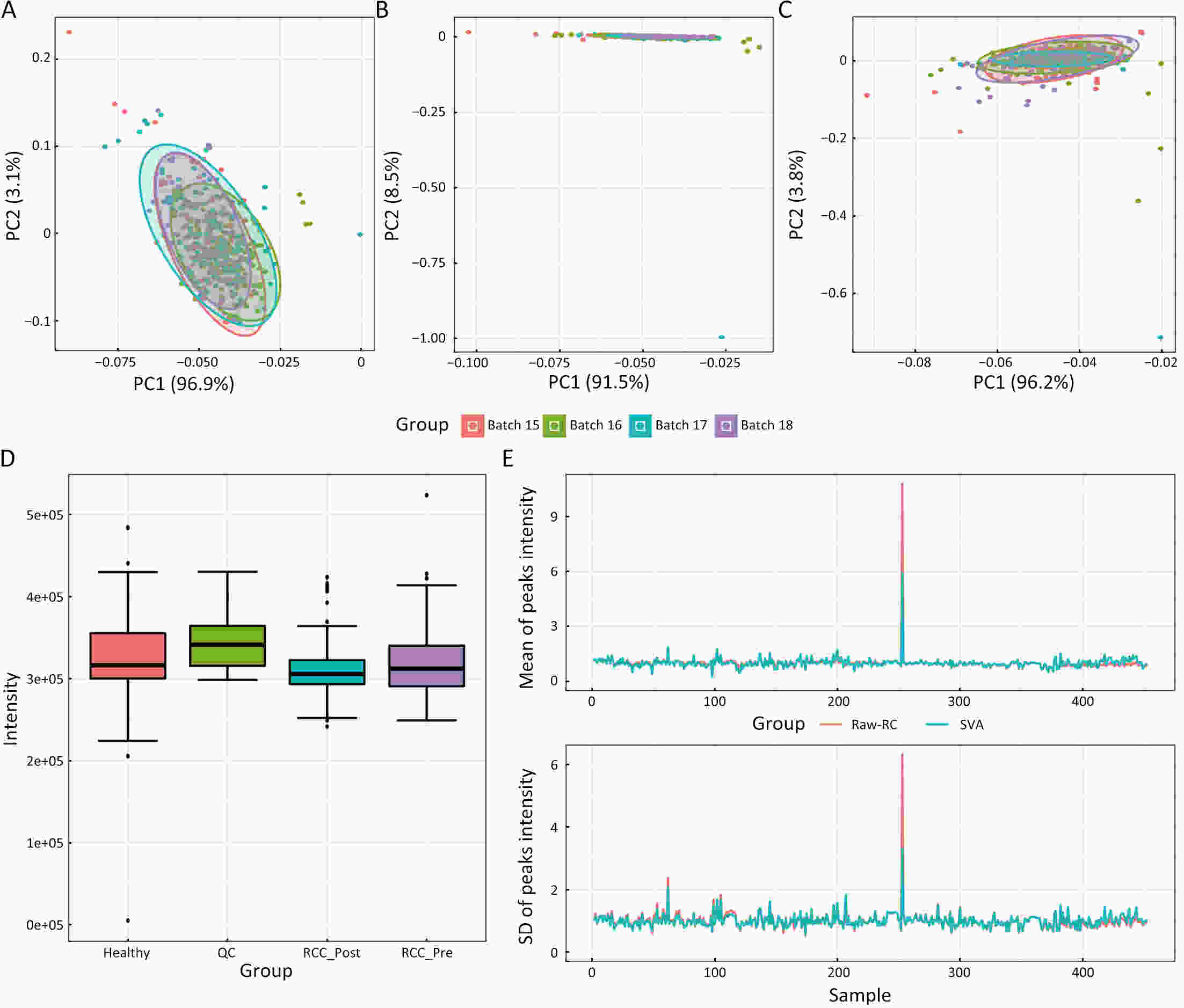
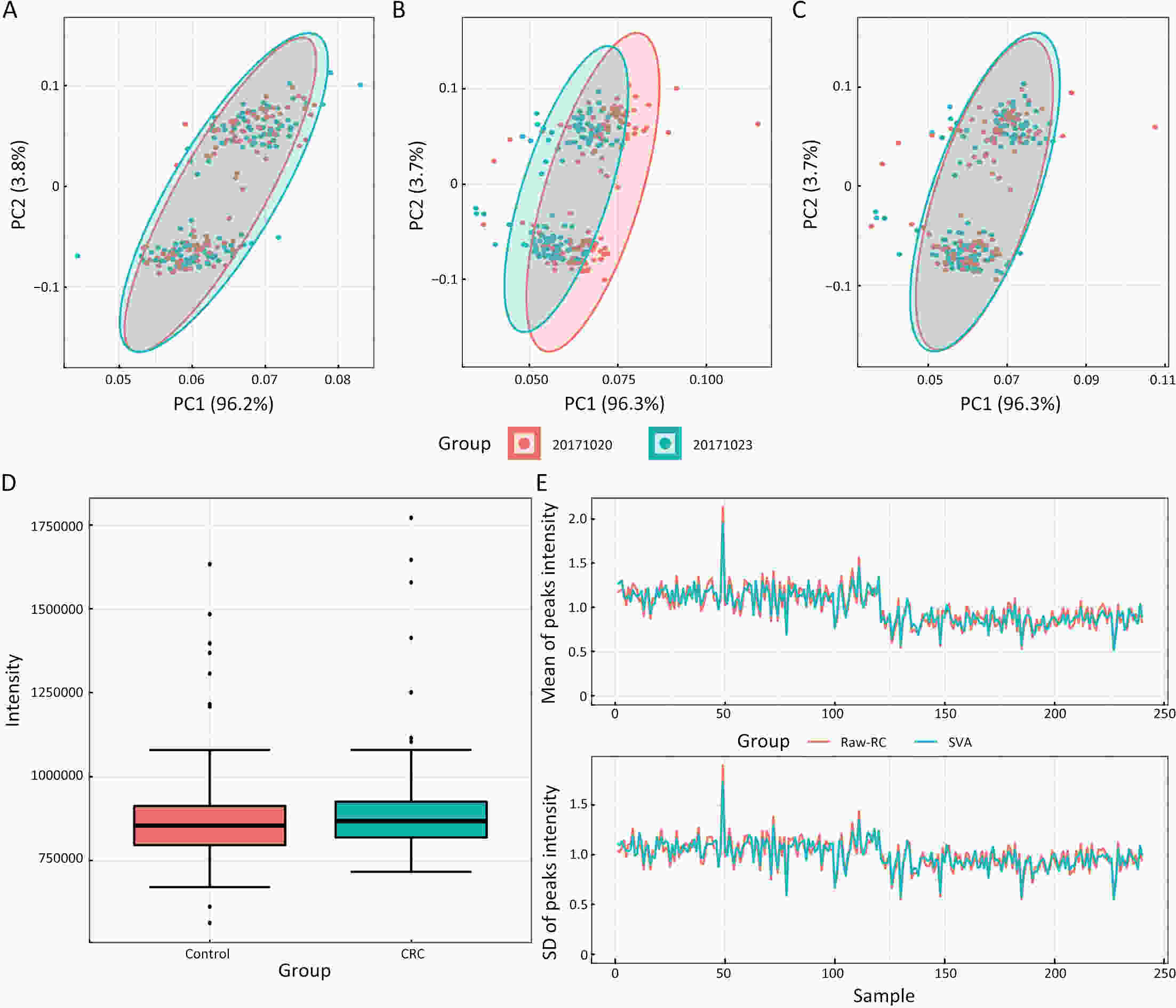
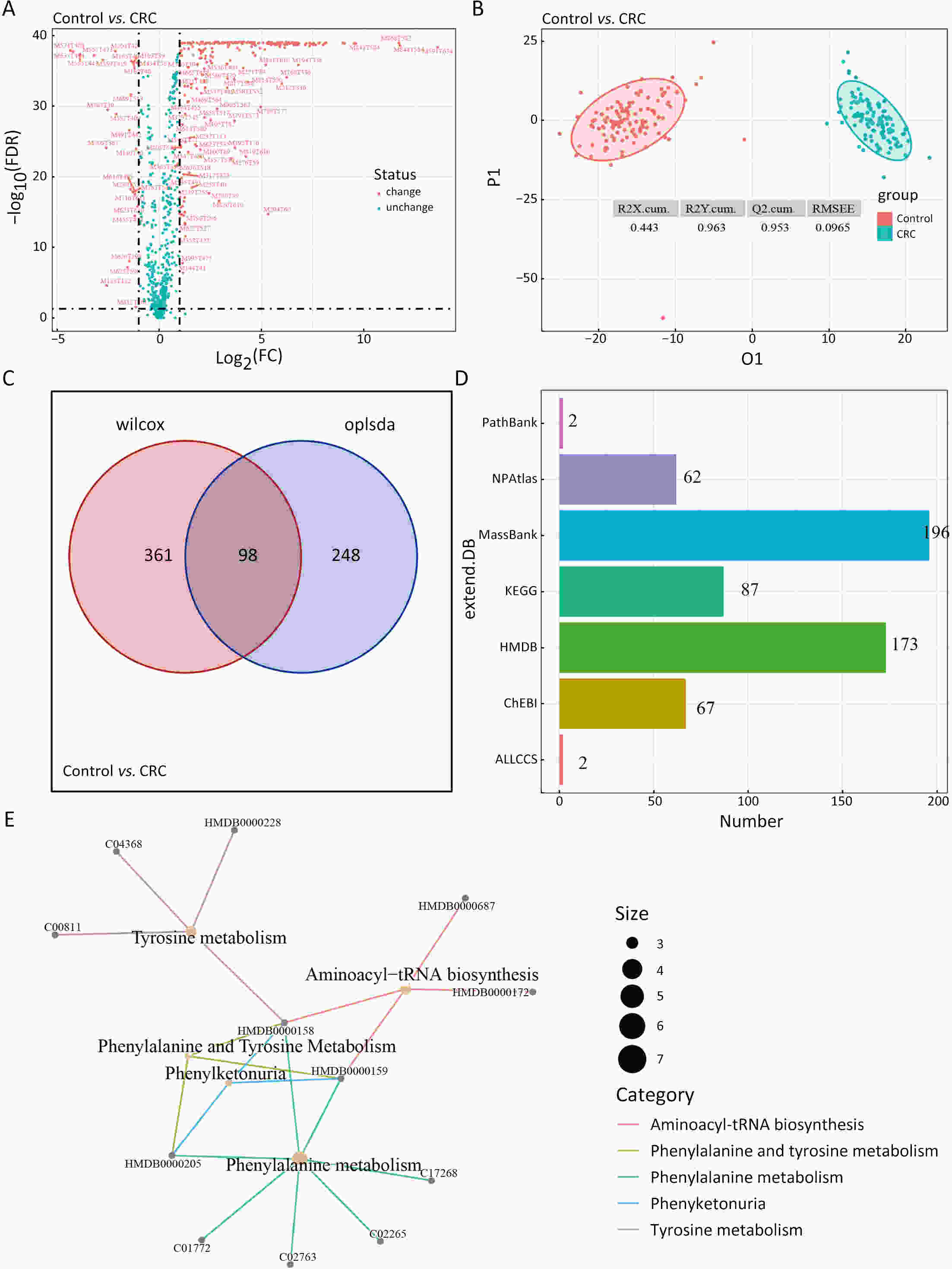
ObjectiveAs an important part of metabolomics analysis, untargeted metabolomics has become a powerful tool in the study of tumor mechanisms and the discovery of metabolic markers with high-throughput spectrometric data which also poses great challenges to data analysis, from the extraction of raw data to the identification of differential metabolites. To date, a large number of analytical tools and processes have been developed and constructed to serve untargeted metabolomics research. The different selection of analytical tools and parameter settings lead to varied results of untargeted metabolomics data. Our goal is to establish an easily operated platform and obtain a repeatable analysis result. MethodsWe used the R language basic environment to construct the preprocessing system of the original data and the LAMP (Linux+Apache+MySQL+PHP) architecture to build a cloud mass spectrum data analysis system. ResultsAn open-source analysis software for untargeted metabolomics data (openNAU) was constructed. It includes the extraction of raw mass data and quality control for the identification of differential metabolic ion peaks. A reference metabolomics database based on public databases was also constructed. ConclusionsA complete analysis system platform for untargeted metabolomics was established. This platform provides a complete template interface for the addition and updating of the analysis process, so we can finish complex analyses of untargeted metabolomics with simple human-computer interactions. The source code can be downloaded from https://github.com/zjuRong/openNAU .

 Abstract
Abstract FullText HTML
FullText HTML PDF 829KB
PDF 829KB