2024 Vol.36(1)
Display Mode: |
2024, 36(1): 1-16.
doi: 10.21147/j.issn.1000-9604.2024.01.01
Abstract:
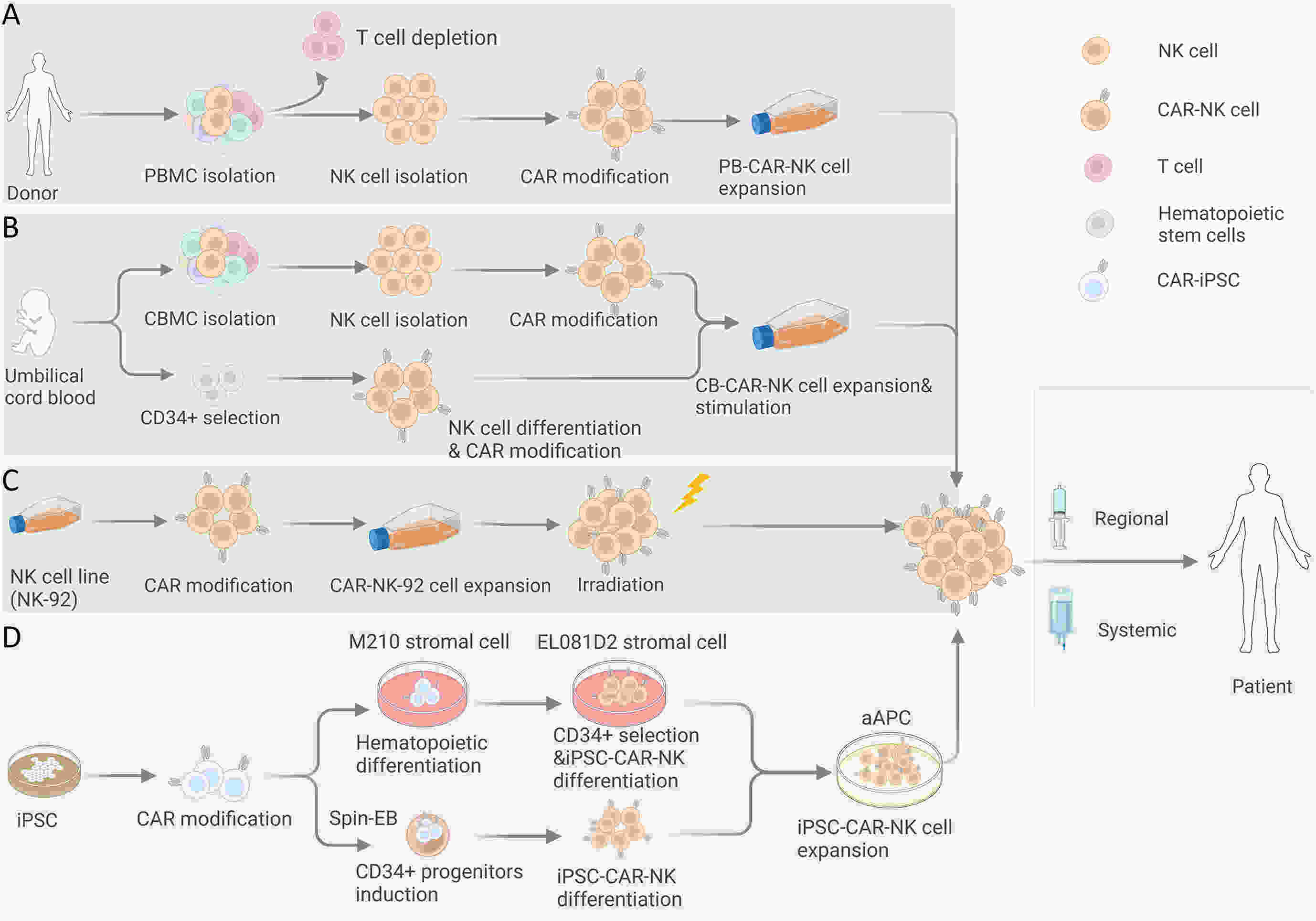
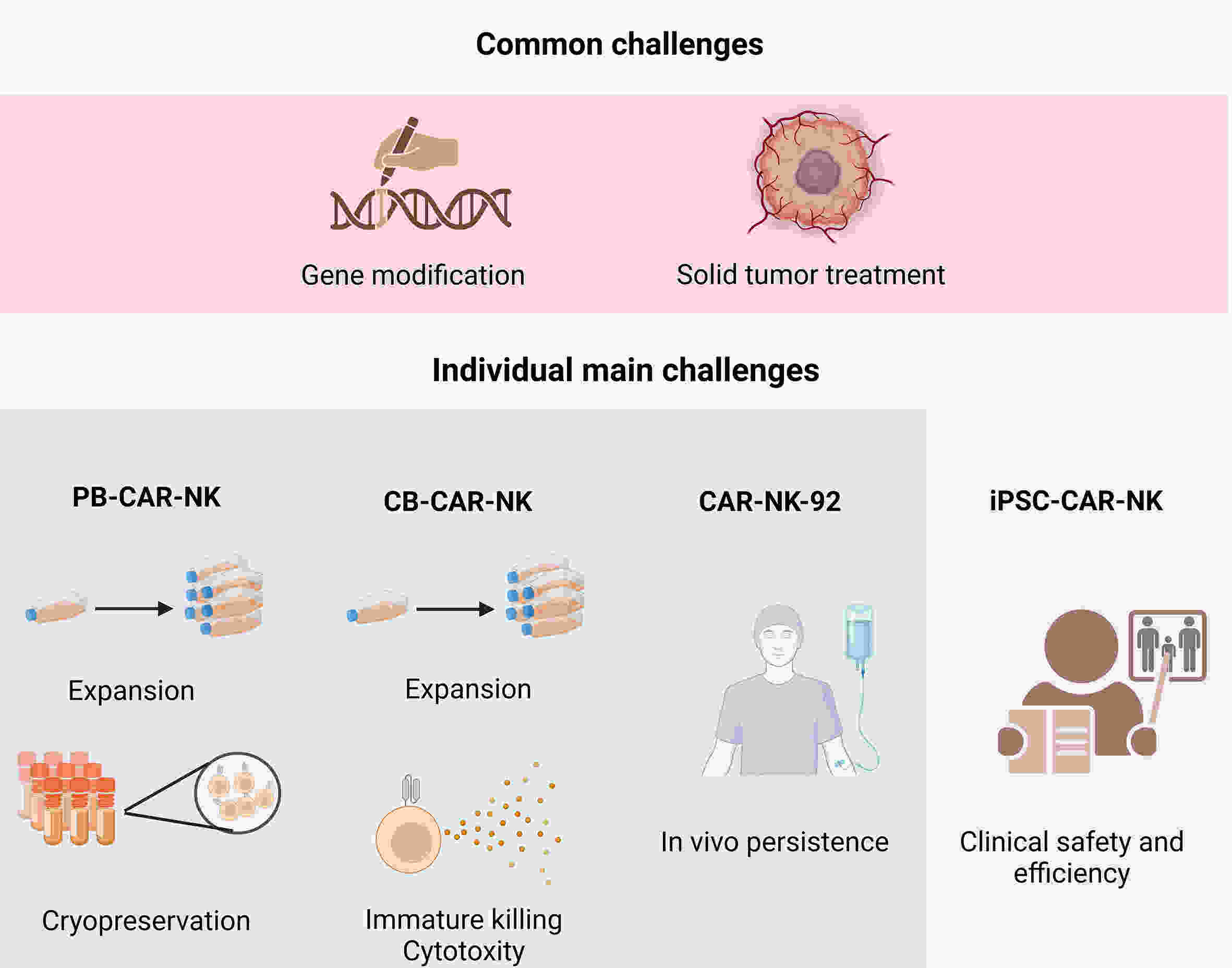
Chimeric antigen receptor-natural killer (CAR-NK) cells have emerged as another prominent player in the realm of tumor immunotherapy following CAR-T cells. The unique features of CAR-NK cells make it possible to compensate for deficiencies in CAR-T therapy, such as the complexity of the manufacturing process, clinical adverse events, and solid tumor challenges. To date, CAR-NK products from different allogeneic sources have exhibited remarkable anti-tumor effects on preclinical studies and have gradually been applied in clinical practice. However, each source has advantages and disadvantages. Selecting a suitable source may help maximize CAR-NK cell efficacy and increase the feasibility of clinical transformation. Therefore, this review discusses the development and challenges of CAR-NK cells from different sources to provide a reference for future exploration.
Chimeric antigen receptor-natural killer (CAR-NK) cells have emerged as another prominent player in the realm of tumor immunotherapy following CAR-T cells. The unique features of CAR-NK cells make it possible to compensate for deficiencies in CAR-T therapy, such as the complexity of the manufacturing process, clinical adverse events, and solid tumor challenges. To date, CAR-NK products from different allogeneic sources have exhibited remarkable anti-tumor effects on preclinical studies and have gradually been applied in clinical practice. However, each source has advantages and disadvantages. Selecting a suitable source may help maximize CAR-NK cell efficacy and increase the feasibility of clinical transformation. Therefore, this review discusses the development and challenges of CAR-NK cells from different sources to provide a reference for future exploration.
2024, 36(1): 17-24.
doi: 10.21147/j.issn.1000-9604.2024.01.02
Abstract:
The prevalence of colorectal cancer (CRC) is increasing annually and metastasis is the principal cause of death in patients with CRC, with the liver being the most frequently affected site. Many studies have shown a strong interplay between the gut flora, particularly Fusobacterium nucleatum (F. nucleatum), Escherichia coli, and Bacteroides fragilis, and the development of gut tumors. Some strains can induce gut inflammation and produce toxins that directly harm gut epithelial cells, ultimately accelerating the onset and progression of CRC. However, little clinical evidence exists on the specific interplay between the gut microflora and colorectal cancer liver metastasis (CRLM). Some research showed the existence of viable F. nucleatum in distant metastasis of CRC. Subsequently, gut microbiota products, such as lipopolysaccharides, sodium butyrate, and protein cathepsin K, were also found to affect the development of CRC. This article summarizes the mechanism and research status of the interplay between gut microflora and CRLM, discusses the importance of gut microflora in the treatment of CRLM, and proposes a new approach to understanding the mechanism of CRLM and potential treatments for the microbiome. It is anticipated that the gut microbiota will be a formidable therapeutic and prophylactic tool for treating and preventing CRLM.
The prevalence of colorectal cancer (CRC) is increasing annually and metastasis is the principal cause of death in patients with CRC, with the liver being the most frequently affected site. Many studies have shown a strong interplay between the gut flora, particularly Fusobacterium nucleatum (F. nucleatum), Escherichia coli, and Bacteroides fragilis, and the development of gut tumors. Some strains can induce gut inflammation and produce toxins that directly harm gut epithelial cells, ultimately accelerating the onset and progression of CRC. However, little clinical evidence exists on the specific interplay between the gut microflora and colorectal cancer liver metastasis (CRLM). Some research showed the existence of viable F. nucleatum in distant metastasis of CRC. Subsequently, gut microbiota products, such as lipopolysaccharides, sodium butyrate, and protein cathepsin K, were also found to affect the development of CRC. This article summarizes the mechanism and research status of the interplay between gut microflora and CRLM, discusses the importance of gut microflora in the treatment of CRLM, and proposes a new approach to understanding the mechanism of CRLM and potential treatments for the microbiome. It is anticipated that the gut microbiota will be a formidable therapeutic and prophylactic tool for treating and preventing CRLM.
2024, 36(1): 25-35.
doi: 10.21147/j.issn.1000-9604.2024.01.03
Abstract:
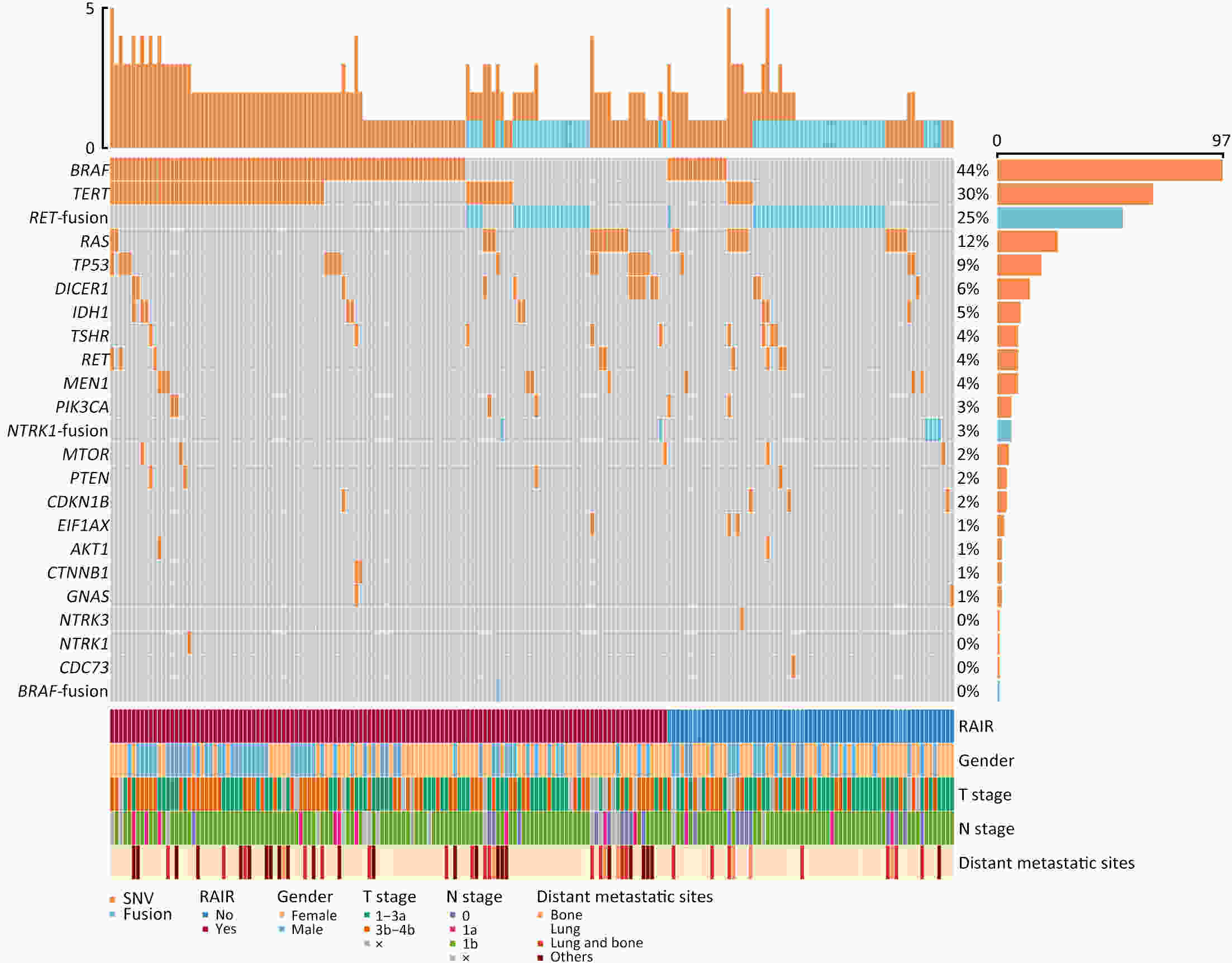
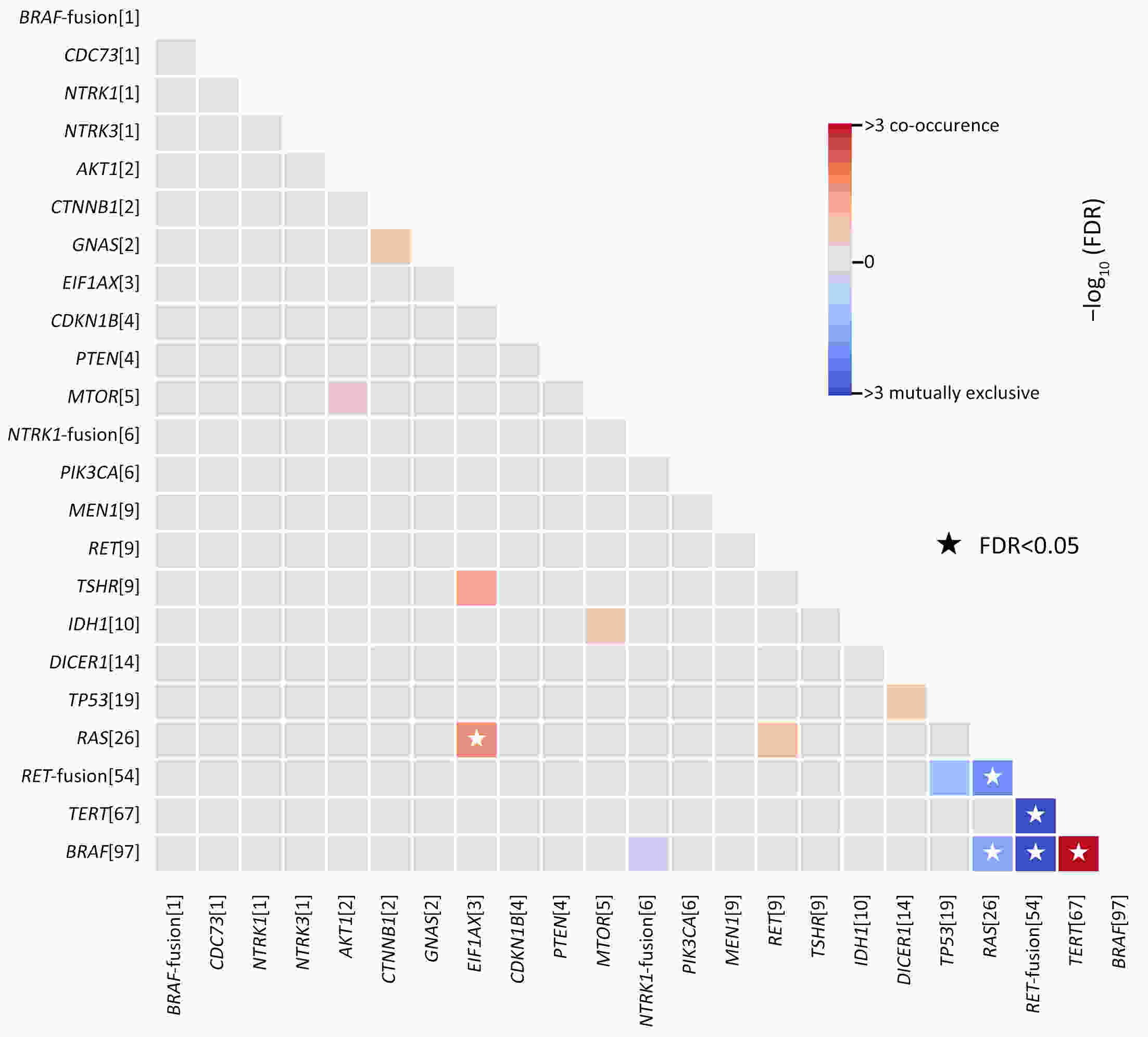
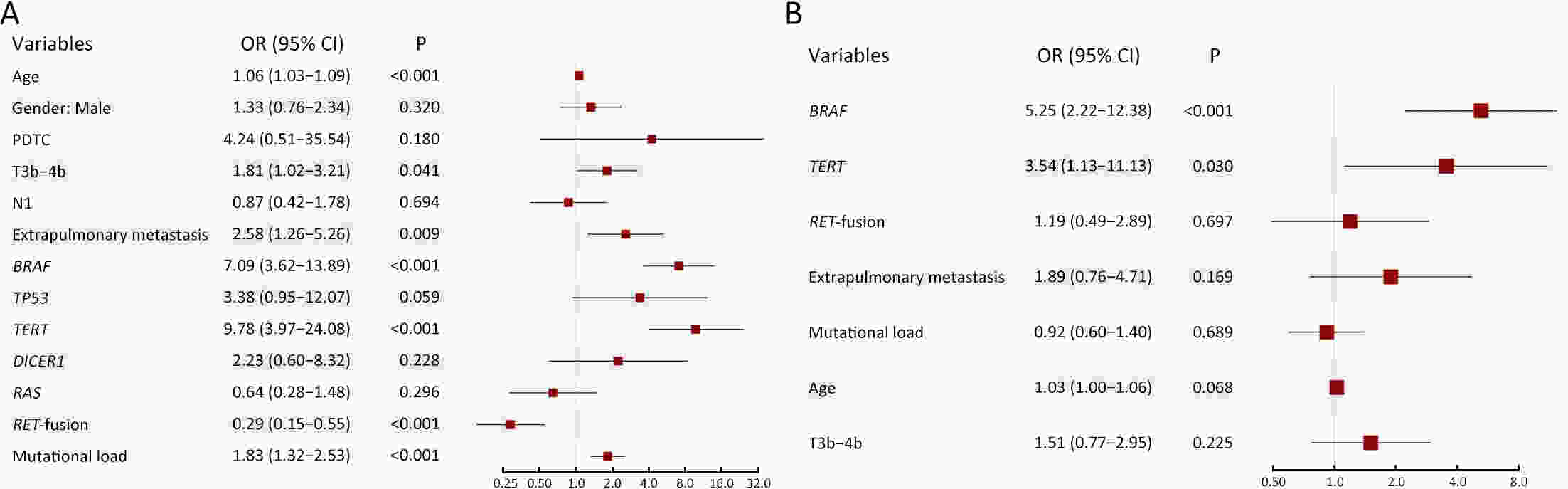
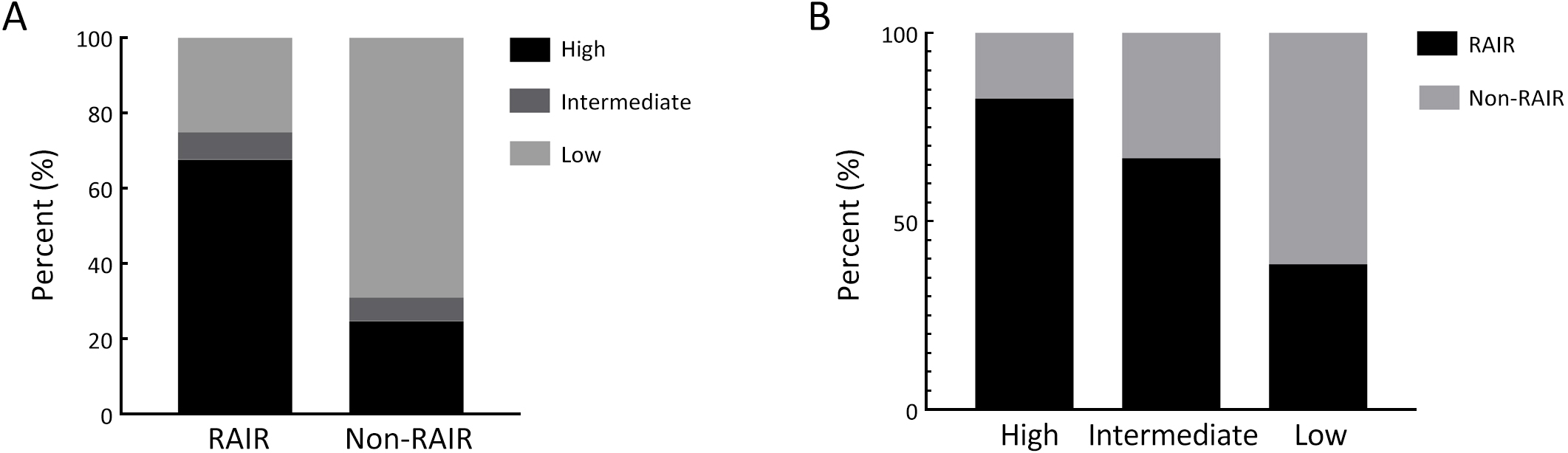
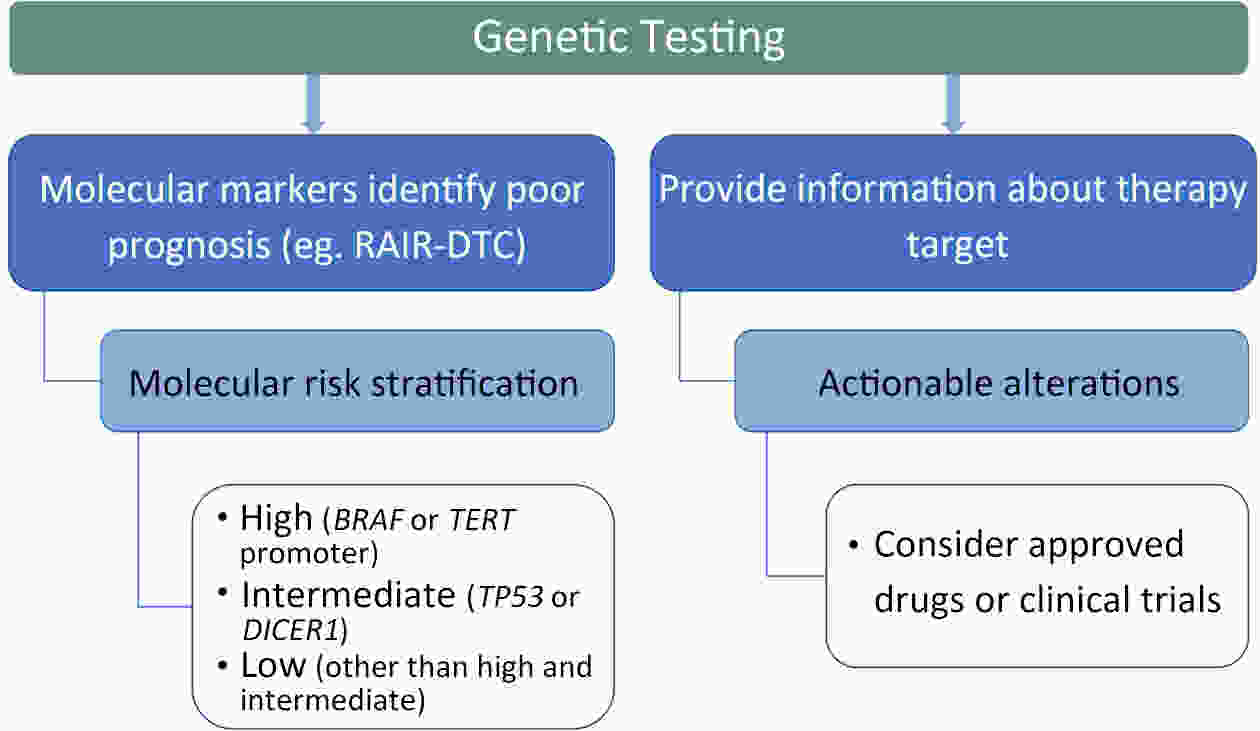
ObjectivePatients with radioactive iodine-refractory differentiated thyroid cancer (RAIR-DTC) are often diagnosed with delay and constrained to limited treatment options. The correlation between RAI refractoriness and the underlying genetic characteristics has not been extensively studied. MethodsAdult patients with distant metastatic DTC were enrolled and assigned to undergo next-generation sequencing of a customized 26-gene panel (ThyroLead). Patients were classified into RAIR-DTC or non-RAIR groups to determine the differences in clinicopathological and molecular characteristics. Molecular risk stratification (MRS) was constructed based on the association between molecular alterations identified and RAI refractoriness, and the results were classified as high, intermediate or low MRS. ResultsA total of 220 patients with distant metastases were included, 63.2% of whom were identified as RAIR-DTC. Genetic alterations were identified in 90% of all the patients, with BRAF (59.7% vs. 17.3%), TERT promoter (43.9% vs. 7.4%), and TP53 mutations (11.5% vs. 3.7%) being more prevalent in the RAIR-DTC group than in the non-RAIR group, except for RET fusions (15.8% vs. 39.5%), which had the opposite pattern. BRAF and TERT promoter are independent predictors of RAIR-DTC, accounting for 67.6% of patients with RAIR-DTC. MRS was strongly associated with RAI refractoriness (P<0.001), with an odds ratio (OR) of high to low MRS of 7.52 [95% confidence interval (95% CI), 3.96−14.28; P<0.001] and an OR of intermediate to low MRS of 3.20 (95% CI, 1.01−10.14; P=0.041). ConclusionsMolecular alterations were associated with RAI refractoriness, with BRAF and TERT promoter mutations being the predominant contributors, followed by TP53 and DICER1 mutations. MRS might serve as a valuable tool for both prognosticating clinical outcomes and directing precision-based therapeutic interventions.
2024, 36(1): 36-45.
doi: 10.21147/j.issn.1000-9604.2024.01.04
Abstract:
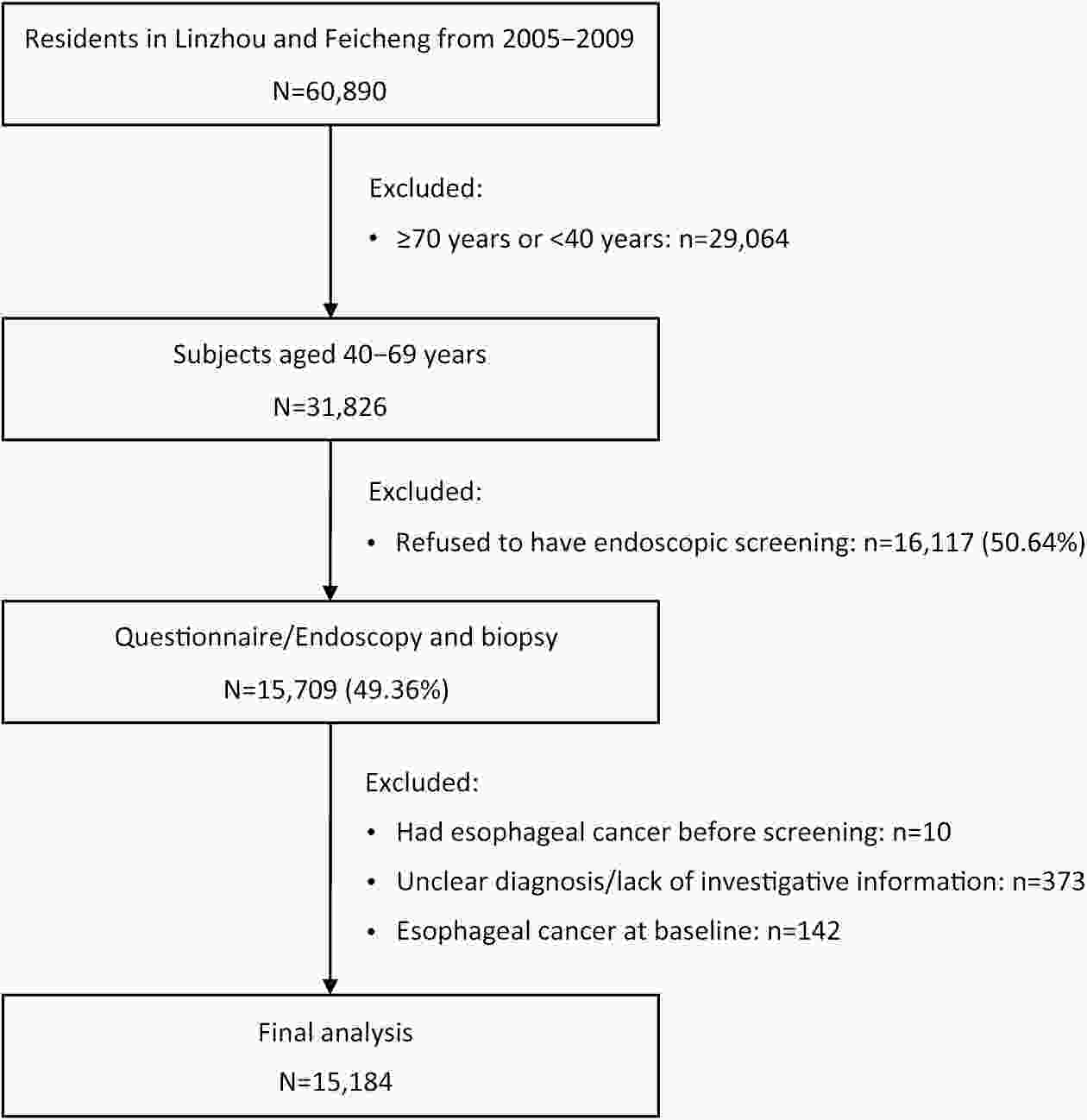
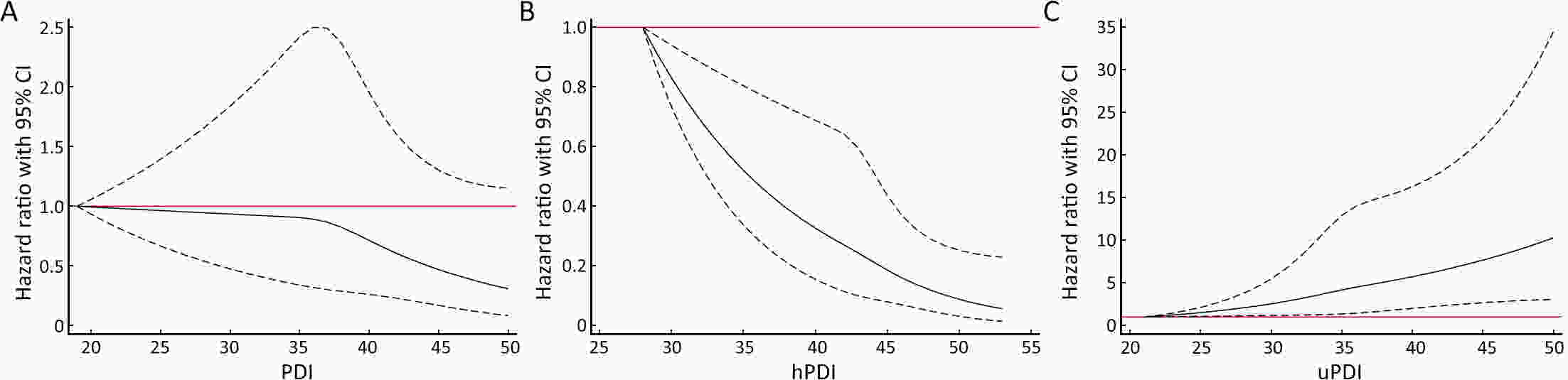
ObjectivePlant-based diets have multiple health benefits for cancers; however, little is known about the association between plant-based dietary patterns and esophageal cancer (EC).This study presents an investigation of the prospective associations among three predefined indices of plant-based dietary patterns and the risk of EC. MethodsWe performed endoscopic screening for 15,709 participants aged 40−69 years from two high-risk areas of China from January 2005 to December 2009 and followed the cohort until December 31, 2022. The overall plant-based diet index (PDI), healthful plant-based diet index (hPDI), and unhealthful plant-based diet index (uPDI), were calculated using survey responses to assess dietary patterns. We applied Cox proportional hazard regression to estimate the multivariable hazard ratios (HRs) and 95% confidence intervals (95% CIs) of EC across 3 plant-based diet indices and further stratified the analysis by subgroups. ResultsThe final study sample included 15,184 participants in the cohort. During a follow-up of 219,365 person-years, 176 patients with EC were identified. When the highest quartile was compared with the lowest quartile, the pooled multivariable-adjusted HR of EC was 0.50 (95% CI, 0.32−0.77) for hPDI. In addition, the HR per 10-point increase in the hPDI score was 0.42 (95% CI, 0.27−0.66) for ECs. Conversely, uPDI was positively associated with the risk of EC, and the HR was 1.80 (95% CI, 1.16−2.82). The HR per 10-point increase in the uPDI score was 1.90 (95% CI, 1.26−2.88) for ECs. The associations between these scores and the risk of EC were consistent in most subgroups. These results remained robust in sensitivity analyses. ConclusionsA healthy plant-based dietary pattern was associated with a reduced risk of EC. Emphasizing the healthiness and quality of plant-based diets may be important for preventing the development of EC.
2024, 36(1): 46-54.
doi: 10.21147/j.issn.1000-9604.2024.01.05
Abstract:
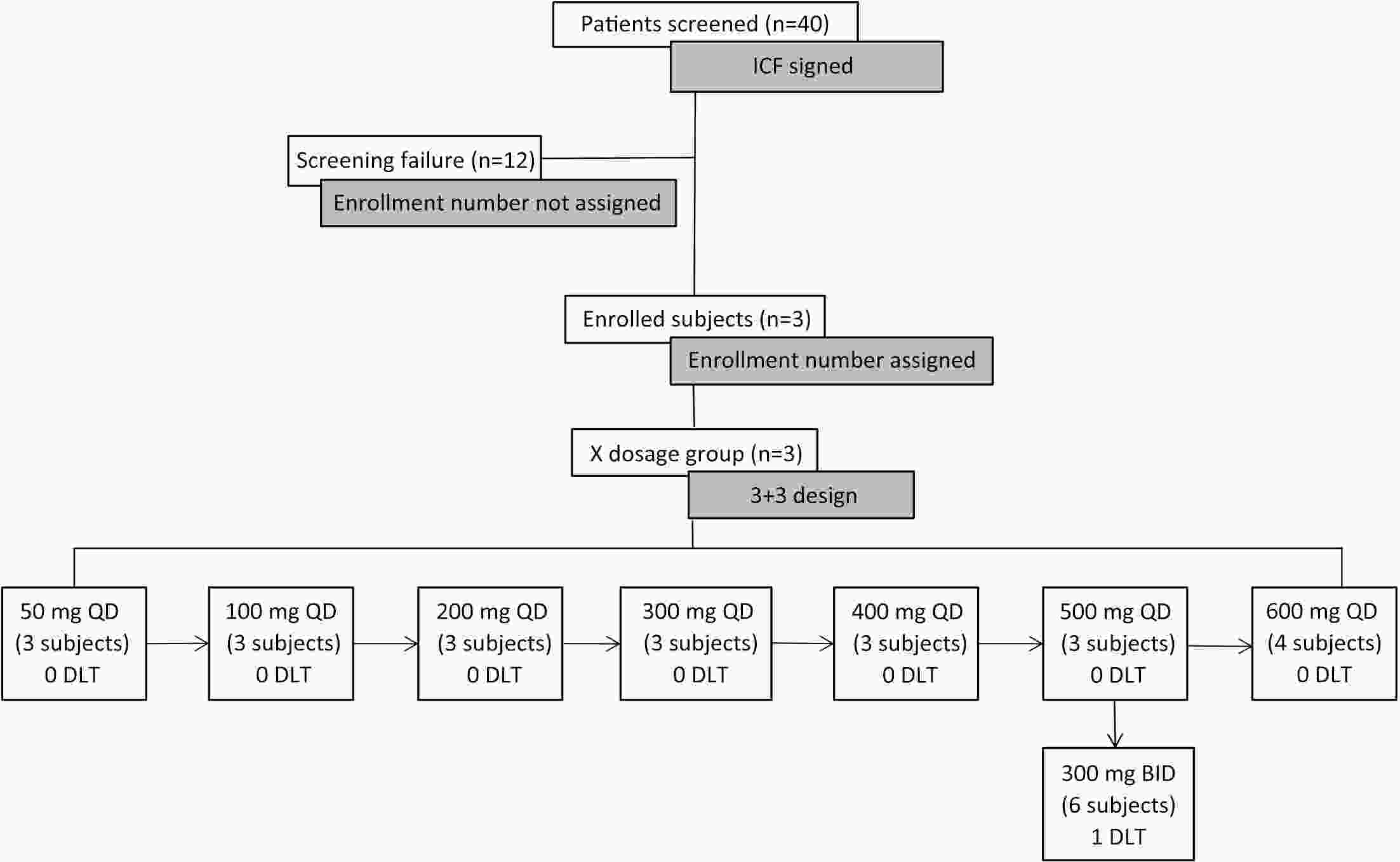
ObjectiveHemay022 is a novel small-molecule and an irreversible tyrosine kinase inhibitor with the target of epidermal growth factor receptor (EGFR)/human epidermal growth factor receptor 2 (HER2), which demonstrated anti-tumor activity in preclinical studies. This first-in-human study evaluated the safety, pharmacokinetics, tolerability and preliminary anti-tumor activity of Hemay022 in HER2-positive advanced breast cancer patients. MethodsHeavily pretreated patients with HER2-positive advanced breast cancer were assigned to eight dose cohorts in a 3+3 dose-escalation pattern at doses of 50−600 mg QD and 300 mg BID. Eligible patients were given a single dose of Hemay022 on d 1 in week 0, followed by once daily continuous doses for four weeks in 28-day cycles. Pharmacokinetic samples were obtained on d 1 and d 28. Clinical responses were assessed every eight weeks. ResultsTwenty-eight patients with advanced breast cancer were treated with Hemay022. The most frequently reported drug-related adverse events were diarrhoea (85.7%), vomiting (28.6%), nausea (25.0%) and decreased appetite (17.9%). No grade 4 drug-related adverse events were reported. At 50−600 mg doses, steady state areas under the concentration-time curve and peak concentrations increased with doses. One patient achieved complete response (CR), and three achieved partial response (PR). The objective response rate (ORR) and disease control rate (DCR) were 14.3% and 46.4% in 28 patients, respectively. The median progression-free survival (PFS) was 3.98 months. ConclusionsHemay022 at the dose of 500 mg once daily was well tolerated. The pharmacokinetic properties and encouraging anti-tumor activities of Hemay022 in advanced breast cancer patients warranted further evaluation of Hemay022 for treating breast cancer patients in the current phase III trial (No. NCT05122494).
2024, 36(1): 55-65.
doi: 10.21147/j.issn.1000-9604.2024.01.06
Abstract:
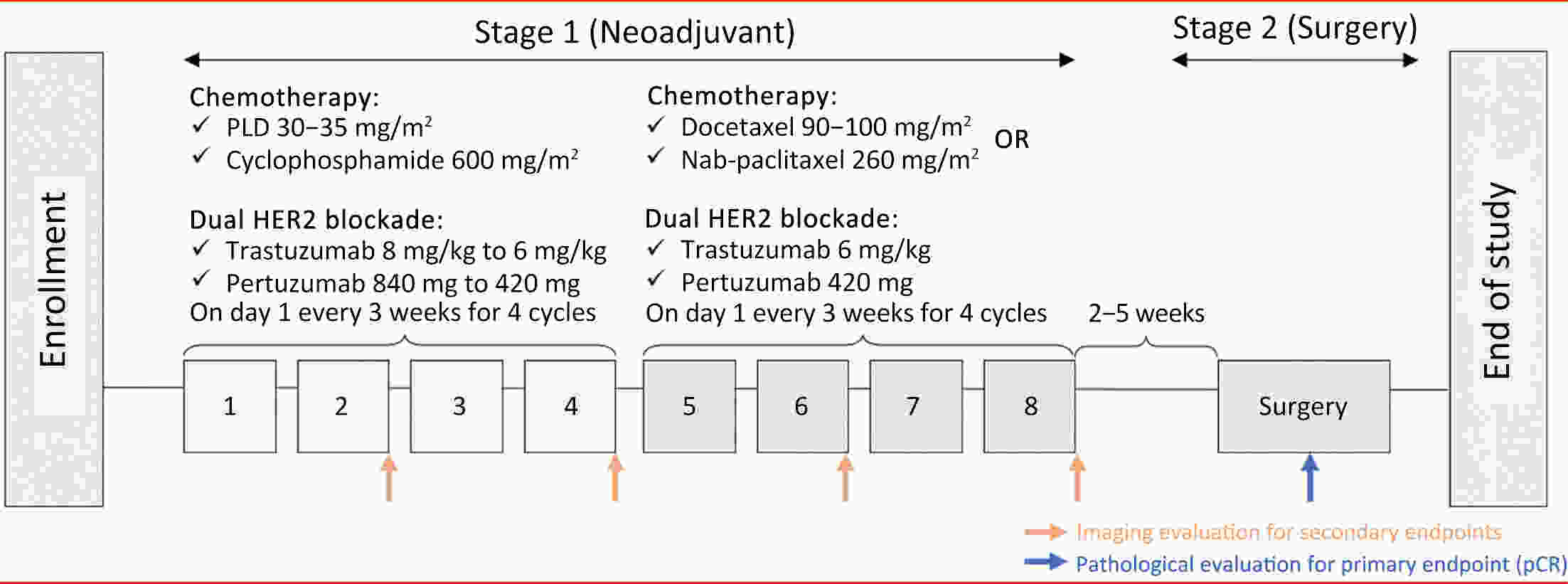
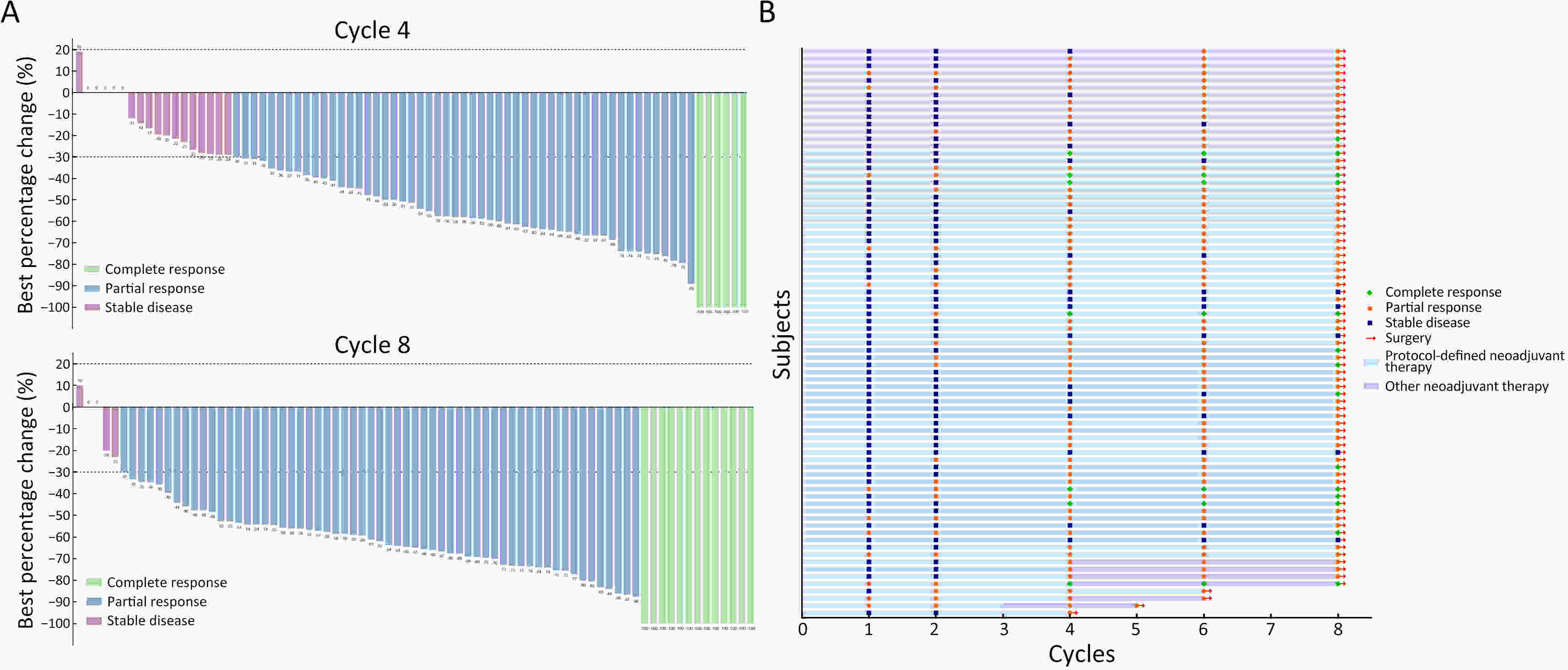
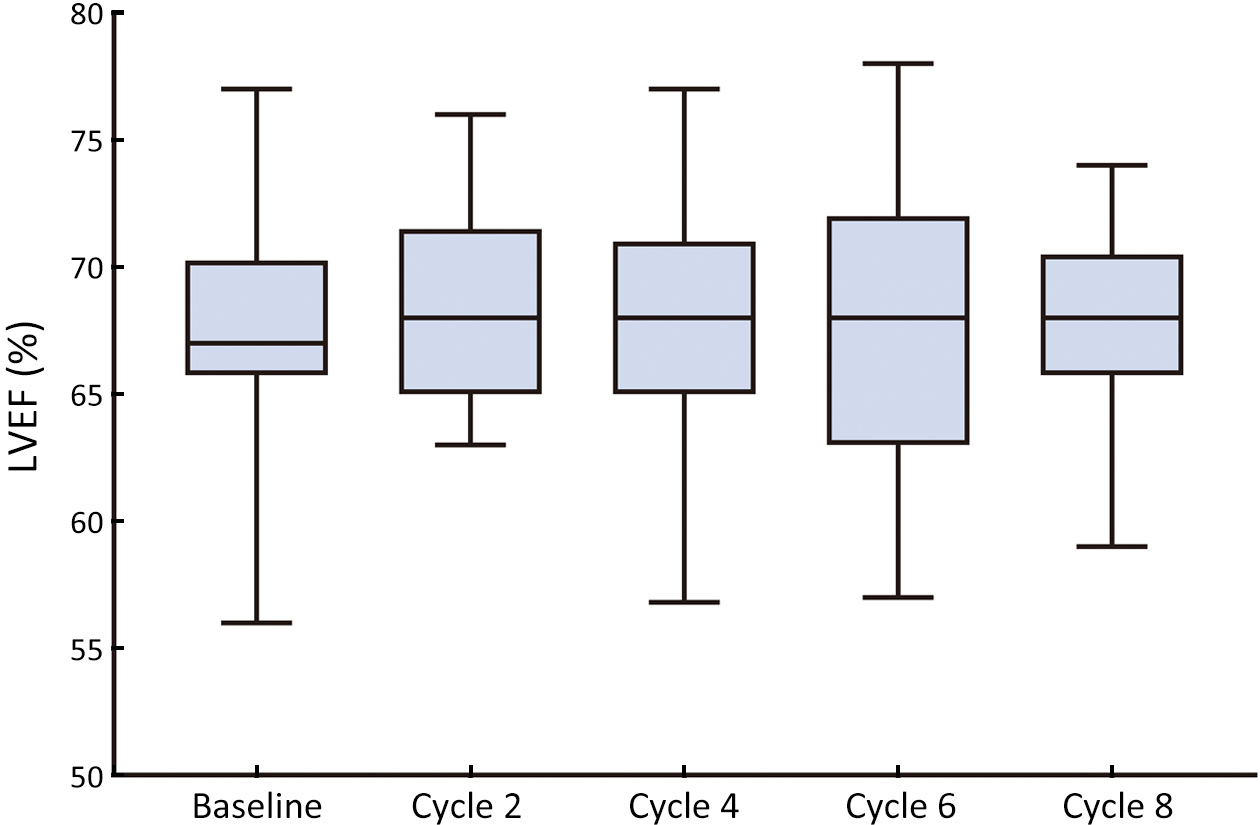
ObjectiveDespite cardiotoxicity overlap, the trastuzumab/pertuzumab and anthracycline combination remains crucial due to significant benefits. Pegylated liposomal doxorubicin (PLD), a less cardiotoxic anthracycline, was evaluated for efficacy and cardiac safety when combined with cyclophosphamide and followed by taxanes with trastuzumab/pertuzumab in human epidermal growth factor receptor-2 (HER2)-positive early breast cancer (BC). MethodsIn this multicenter, phase II study, patients with confirmed HER2-positive early BC received four cycles of PLD (30−35 mg/m2) and cyclophosphamide (600 mg/m2), followed by four cycles of taxanes (docetaxel, 90−100 mg/m2 or nab-paclitaxel, 260 mg/m2), concomitant with eight cycles of trastuzumab (8 mg/kg loading dose, then 6 mg/kg) and pertuzumab (840 mg loading dose, then 420 mg) every 3 weeks. The primary endpoint was total pathological complete response (tpCR, ypT0/is ypN0). Secondary endpoints included breast pCR (bpCR), objective response rate (ORR), disease control rate, rate of breast-conserving surgery (BCS), and safety (with a focus on cardiotoxicity). ResultsBetween May 27, 2020 and May 11, 2022, 78 patients were treated with surgery, 42 (53.8%) of whom had BCS. After neoadjuvant therapy, 47 [60.3%, 95% confidence interval (95% CI), 48.5%−71.2%] patients achieved tpCR, and 49 (62.8%) achieved bpCR. ORRs were 76.9% (95% CI, 66.0%−85.7%) and 93.6% (95% CI, 85.7%−97.9%) after 4-cycle and 8-cycle neoadjuvant therapy, respectively. Nine (11.5%) patients experienced asymptomatic left ventricular ejection fraction (LVEF) reductions of ≥10% from baseline, all with a minimum value of >55%. No treatment-related abnormal cardiac function changes were observed in mean N-terminal pro-BNP (NT-proBNP), troponin I, or high-sensitivity troponin. ConclusionsThis dual HER2-blockade with sequential polychemotherapy showed promising activity with rapid tumor regression in HER2-positive BC. Importantly, this regimen showed an acceptable safety profile, especially a low risk of cardiac events, suggesting it as an attractive treatment approach with a favorable risk-benefit balance.
2024, 36(1): 66-77.
doi: 10.21147/j.issn.1000-9604.2024.01.07
Abstract:
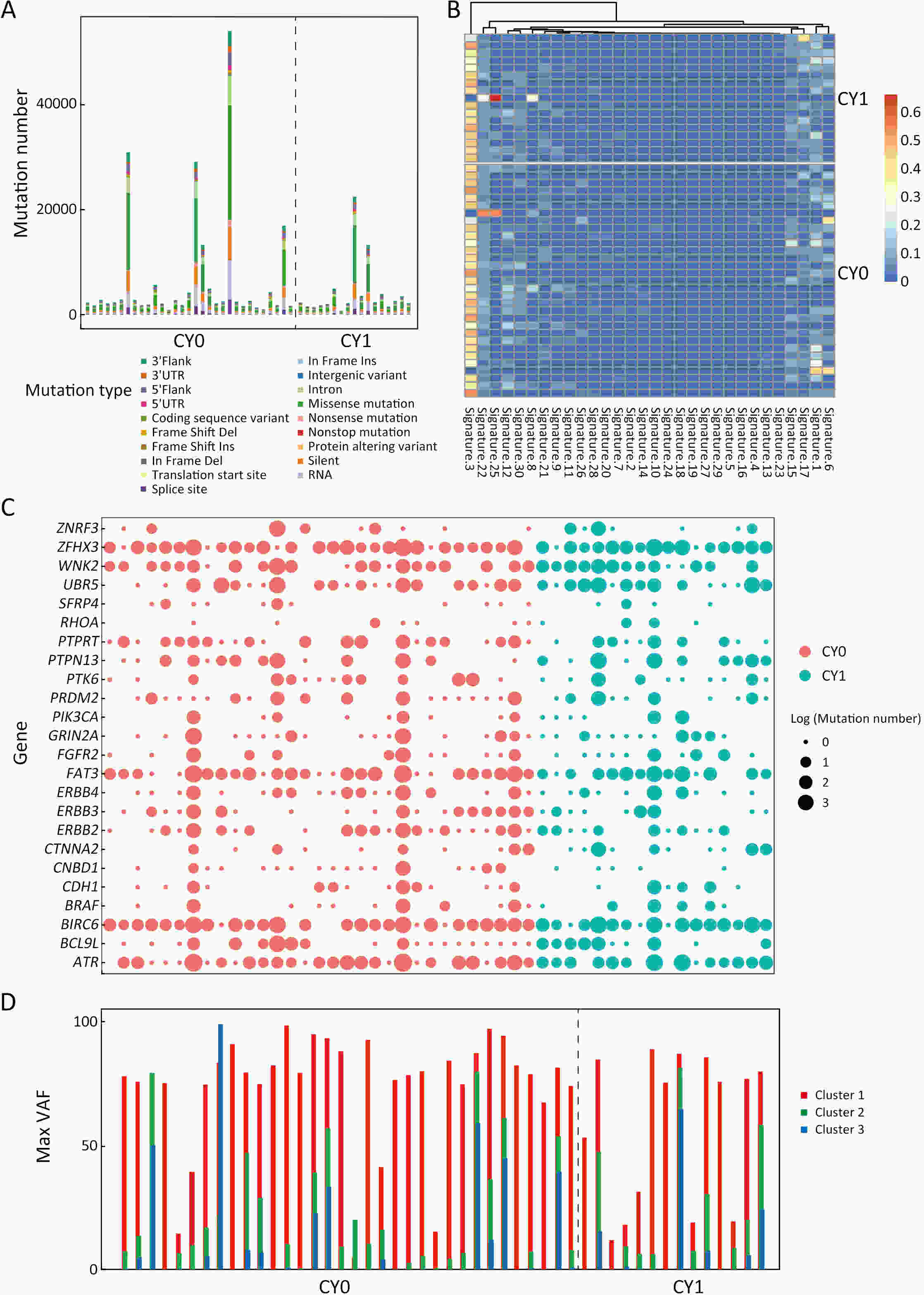
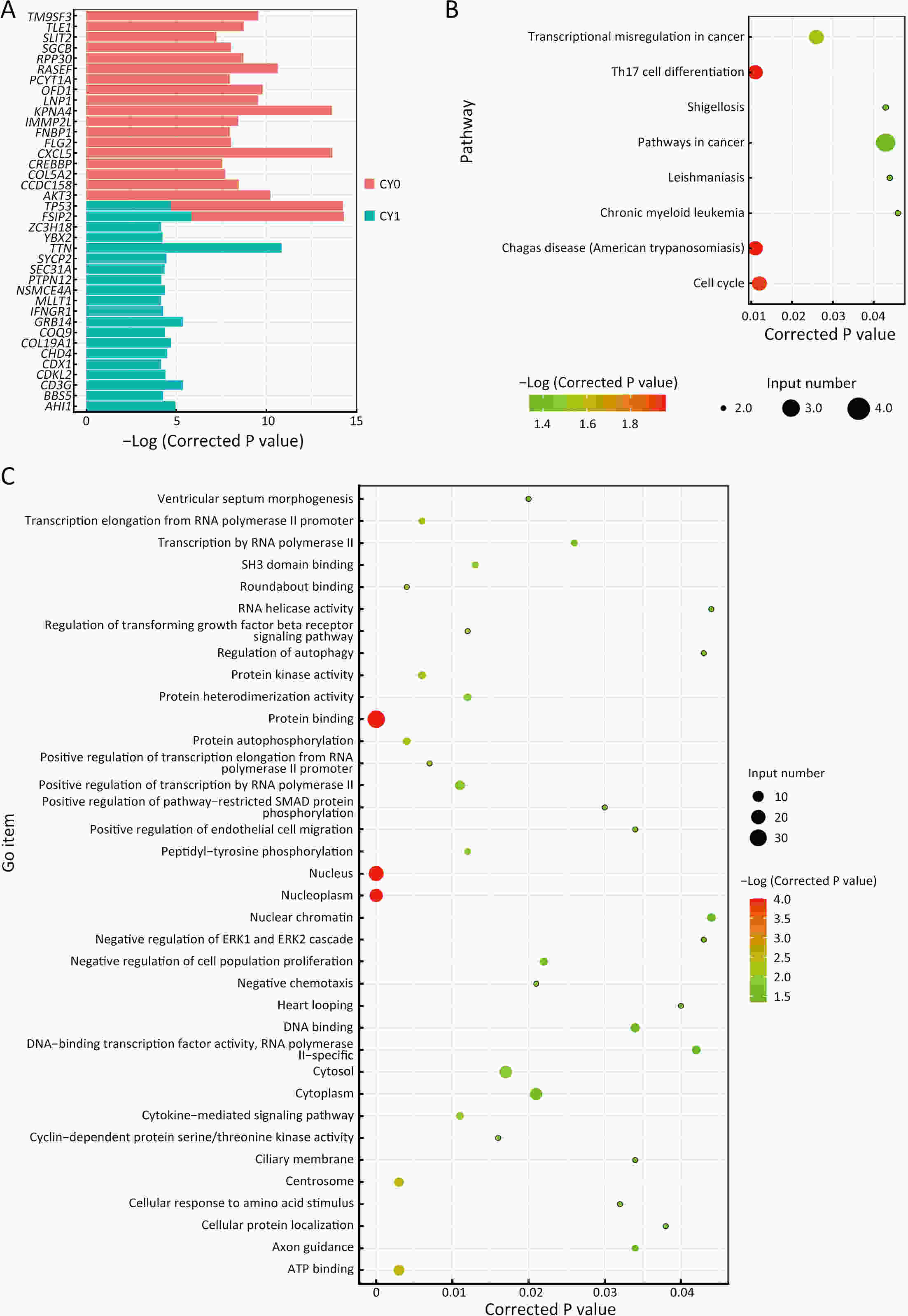
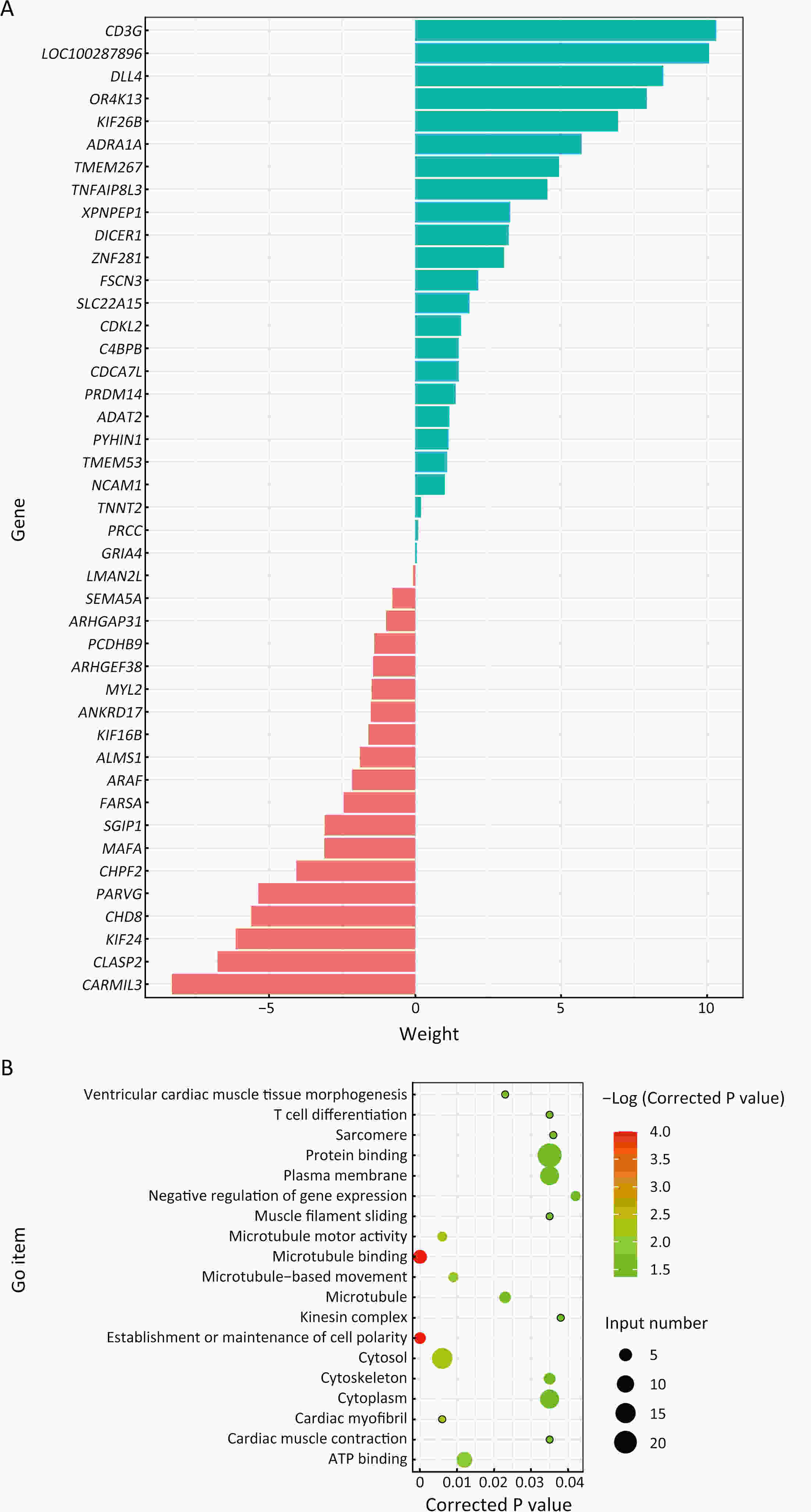
ObjectivePositive peritoneal lavege cytology (CY1) gastric cancer is featured by dismal prognosis, with high risks of peritoneal metastasis. However, there is a lack of evidence on pathogenic mechanism and signature of CY1 and there is a continuous debate on CY1 therapy. Therefore, exploring the mechanism of CY1 is crucial for treatment strategies and targets for CY1 gastric cancer. MethodsIn order to figure out specific driver genes and marker genes of CY1 gastric cancer, and ultimately offer clues for potential marker and risk assessment of CY1, 17 cytology-positive gastric cancer patients and 31 matched cytology-negative gastric cancer patients were enrolled in this study. The enrollment criteria were based on the results of diagnostic laparoscopy staging and cytology inspection of exfoliated cells. Whole exome sequencing was then performed on tumor samples to evaluate genomic characterization of cytology-positive gastric cancer. ResultsLeast absolute shrinkage and selection operator (LASSO) algorithm identified 43 cytology-positive marker genes, while MutSigCV identified 42 cytology-positive specific driver genes. CD3G and CDKL2 were both driver and marker genes of CY1. Regarding mutational signatures, driver gene mutation and tumor subclone architecture, no significant differences were observed between CY1 and negative peritoneal lavege cytology (CY0). ConclusionsThere might not be distinct differences between CY1 and CY0, and CY1 might represent the progression of CY0 gastric cancer rather than constituting an independent subtype. This genomic analysis will thus provide key molecular insights into CY1, which may have a direct effect on treatment recommendations for CY1 and CY0 patients, and provides opportunities for genome-guided clinical trials and drug development.
2024, 36(1): 78-89.
doi: 10.21147/j.issn.1000-9604.2024.01.08
Abstract:
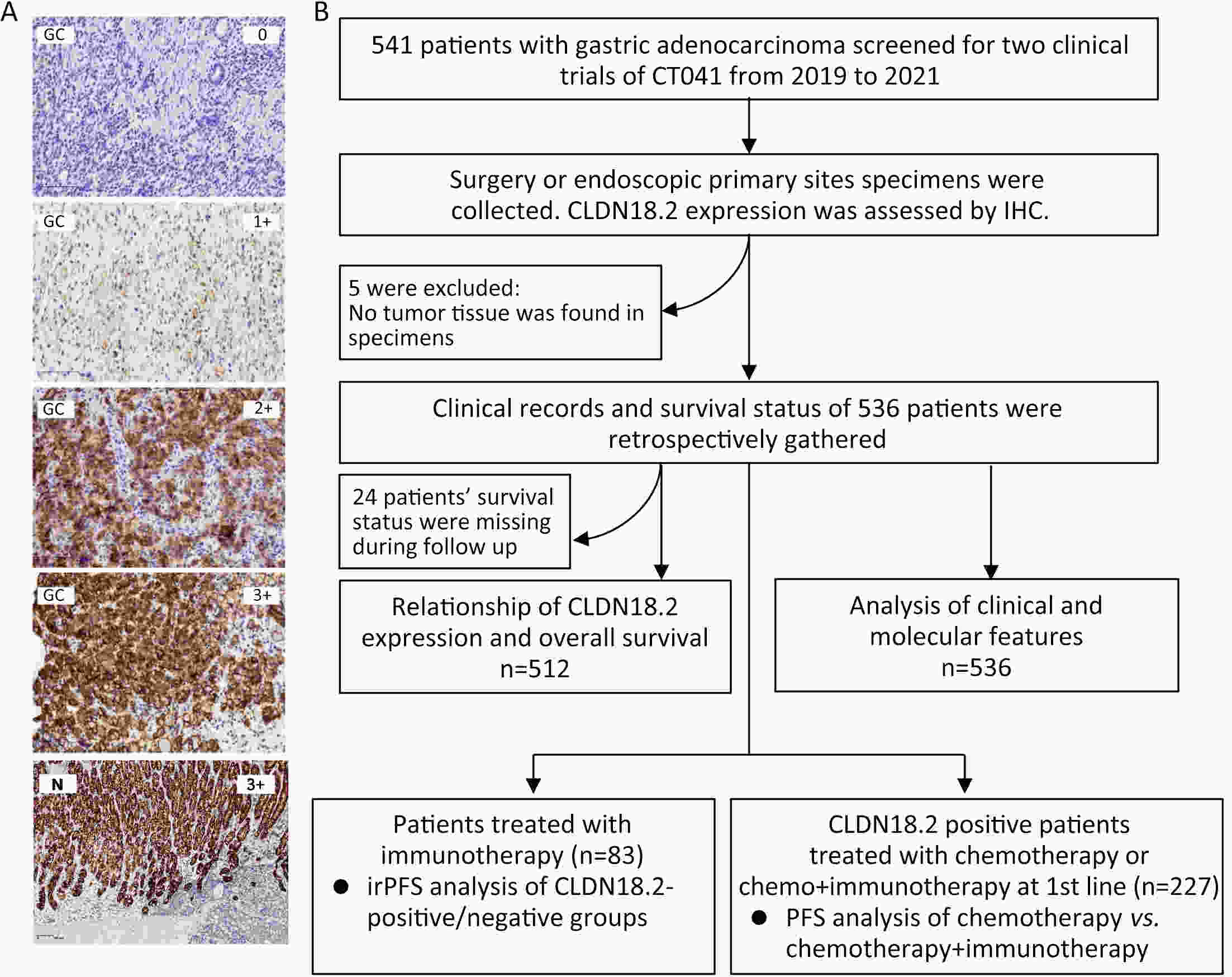
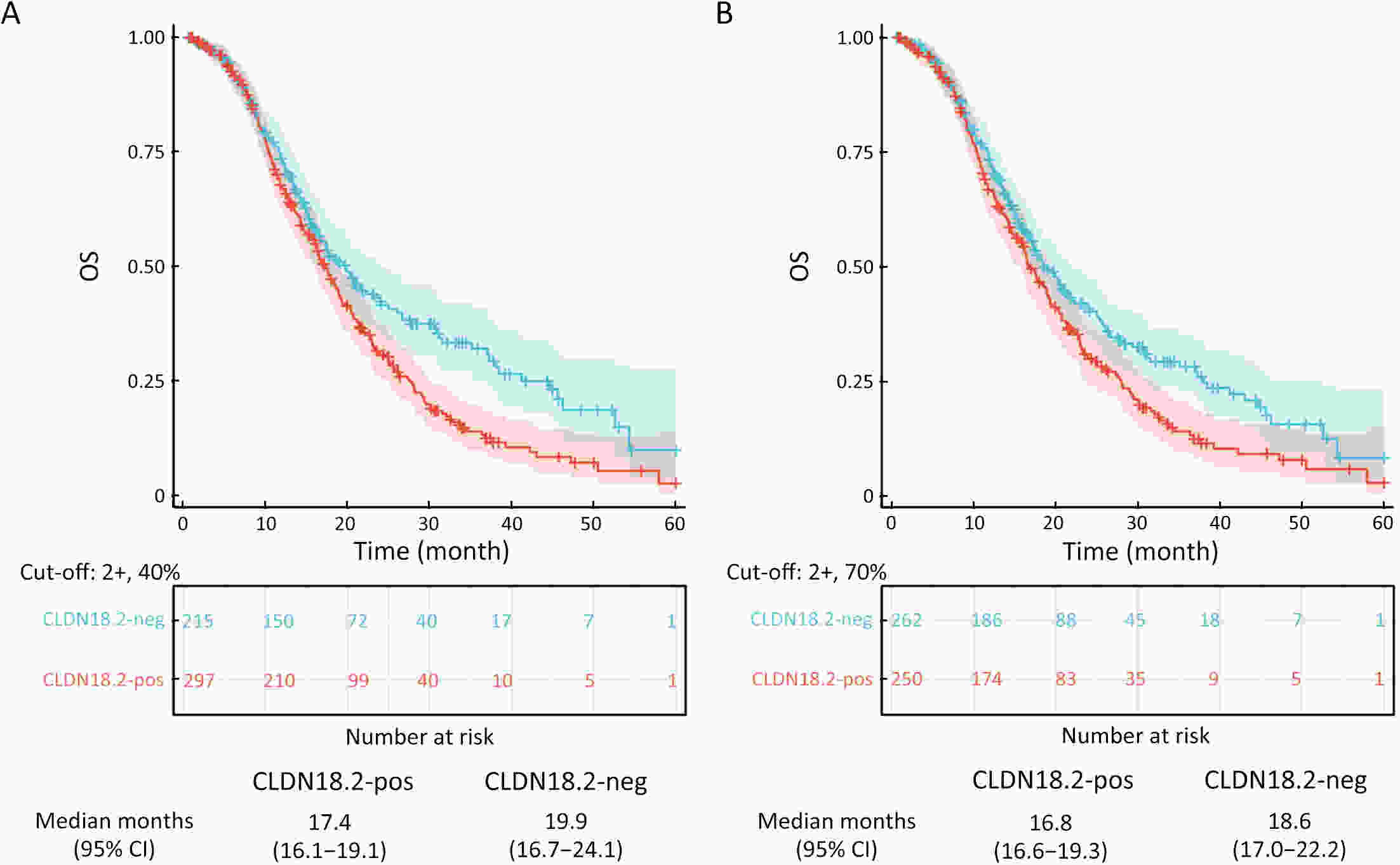
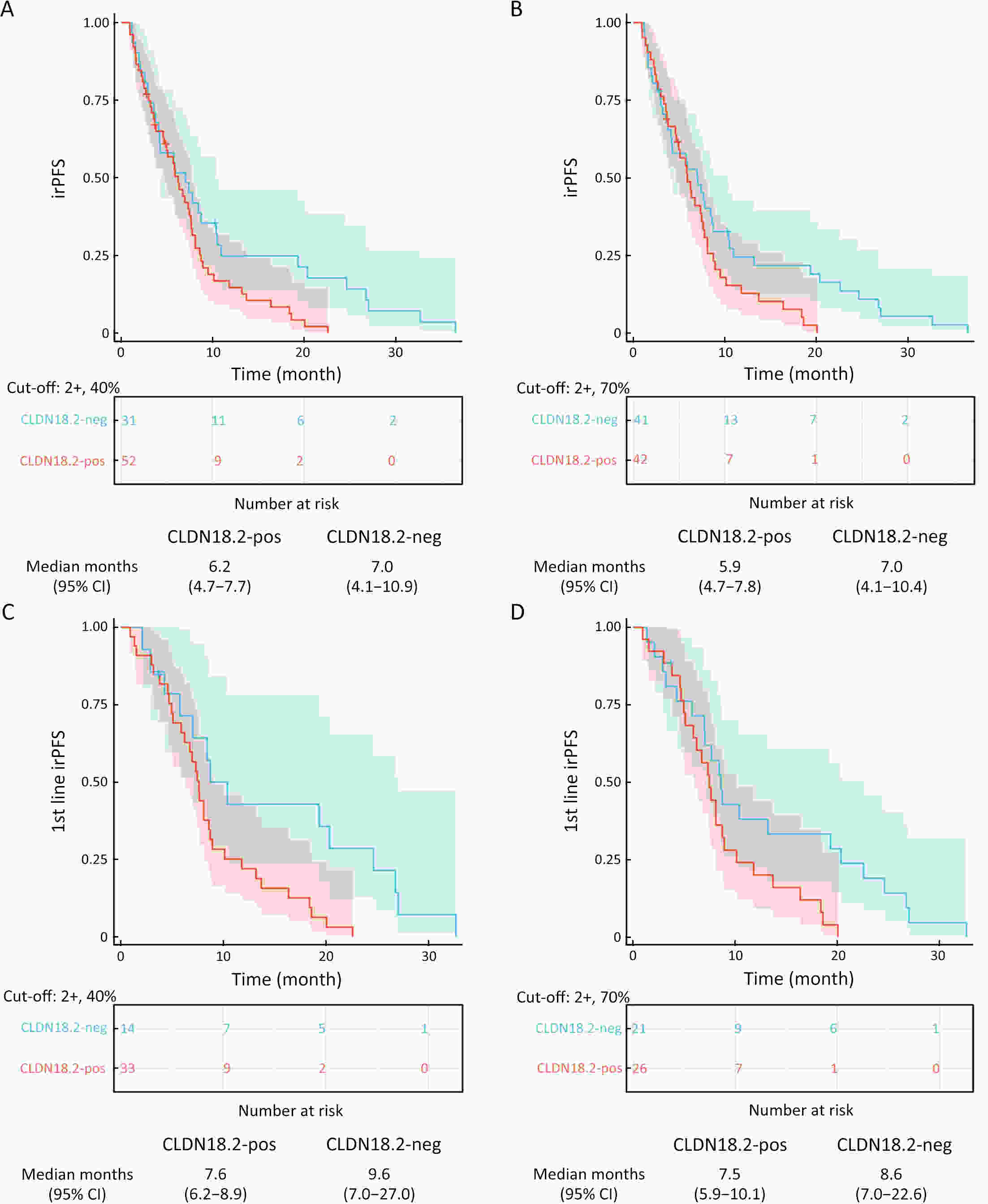
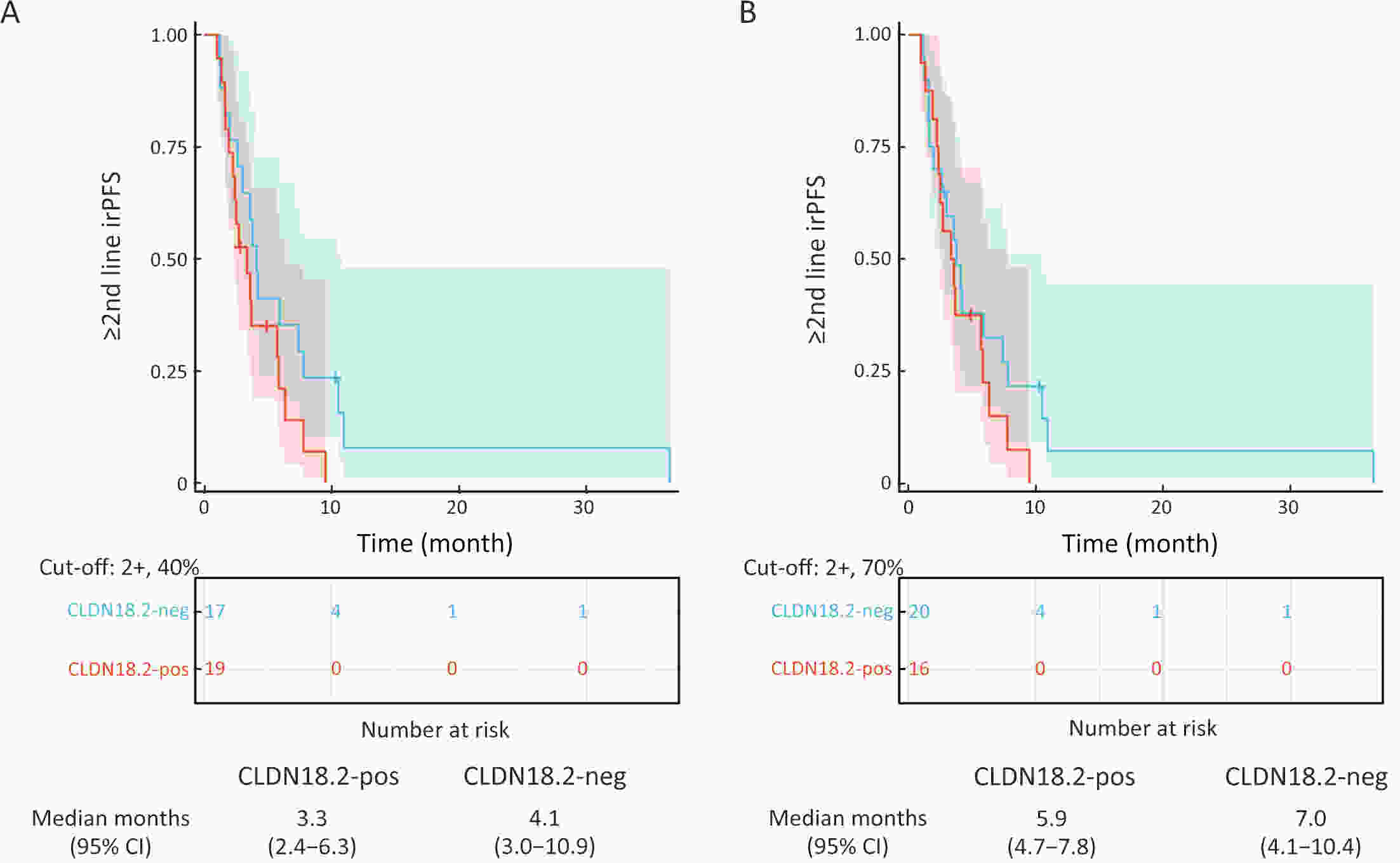
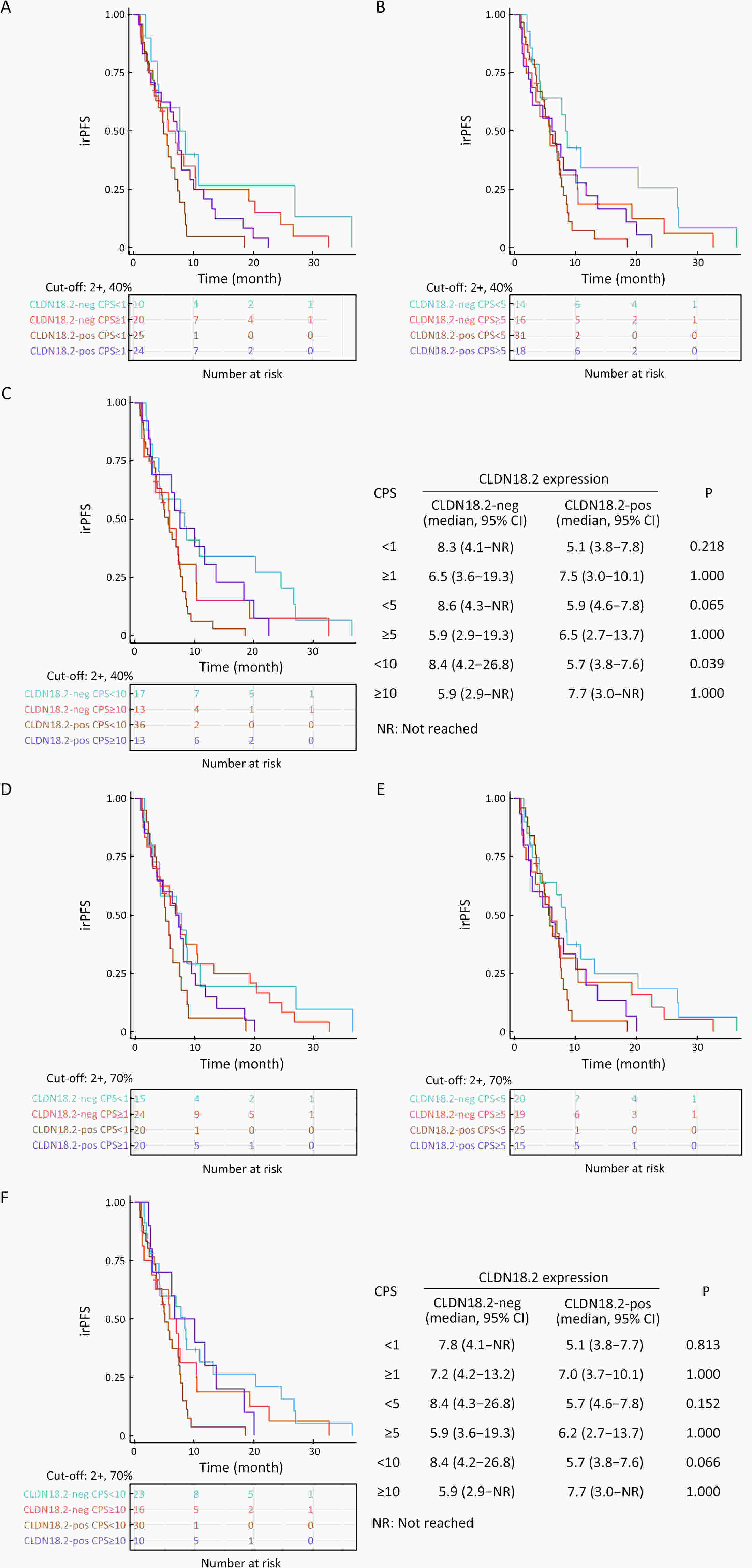
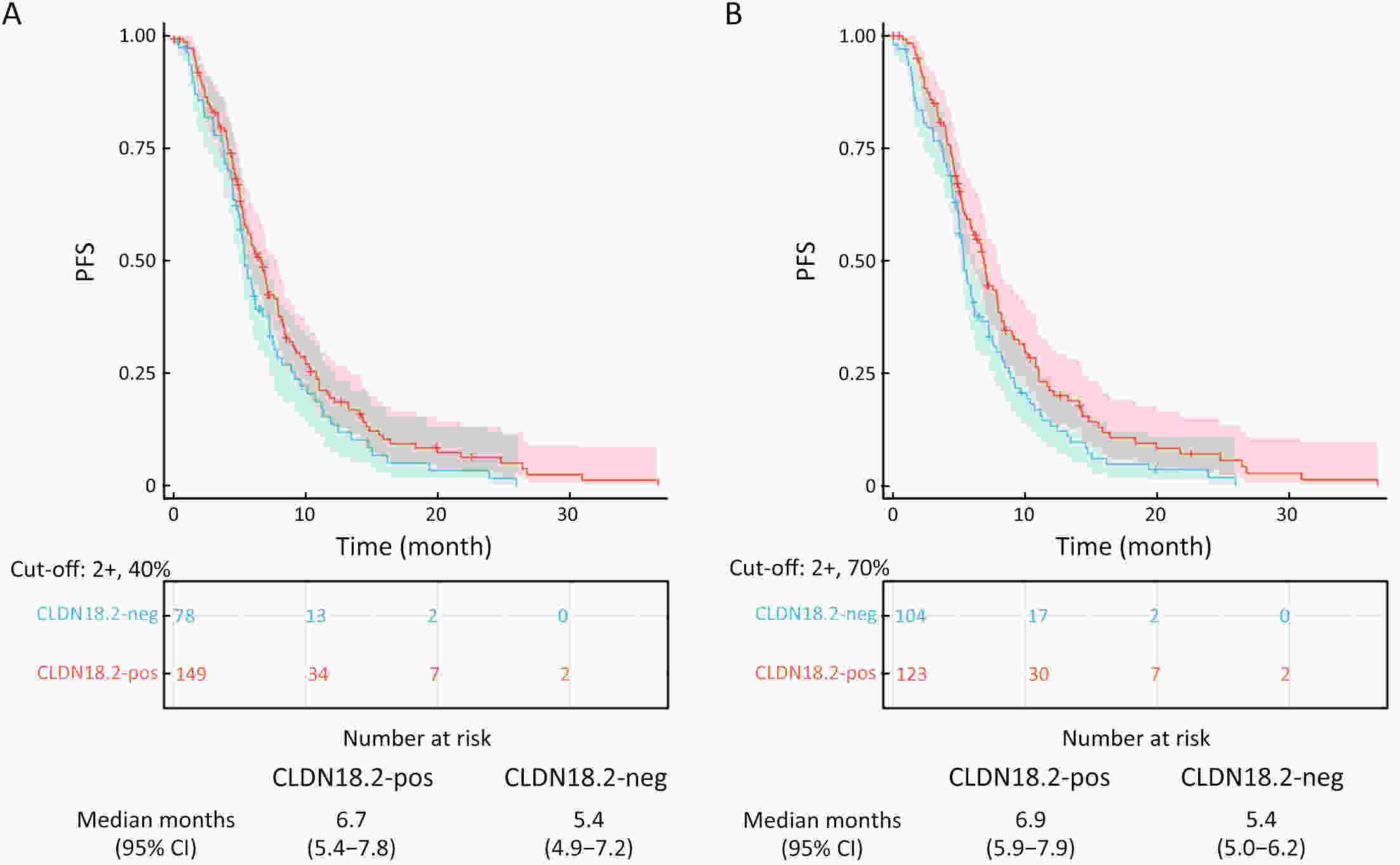
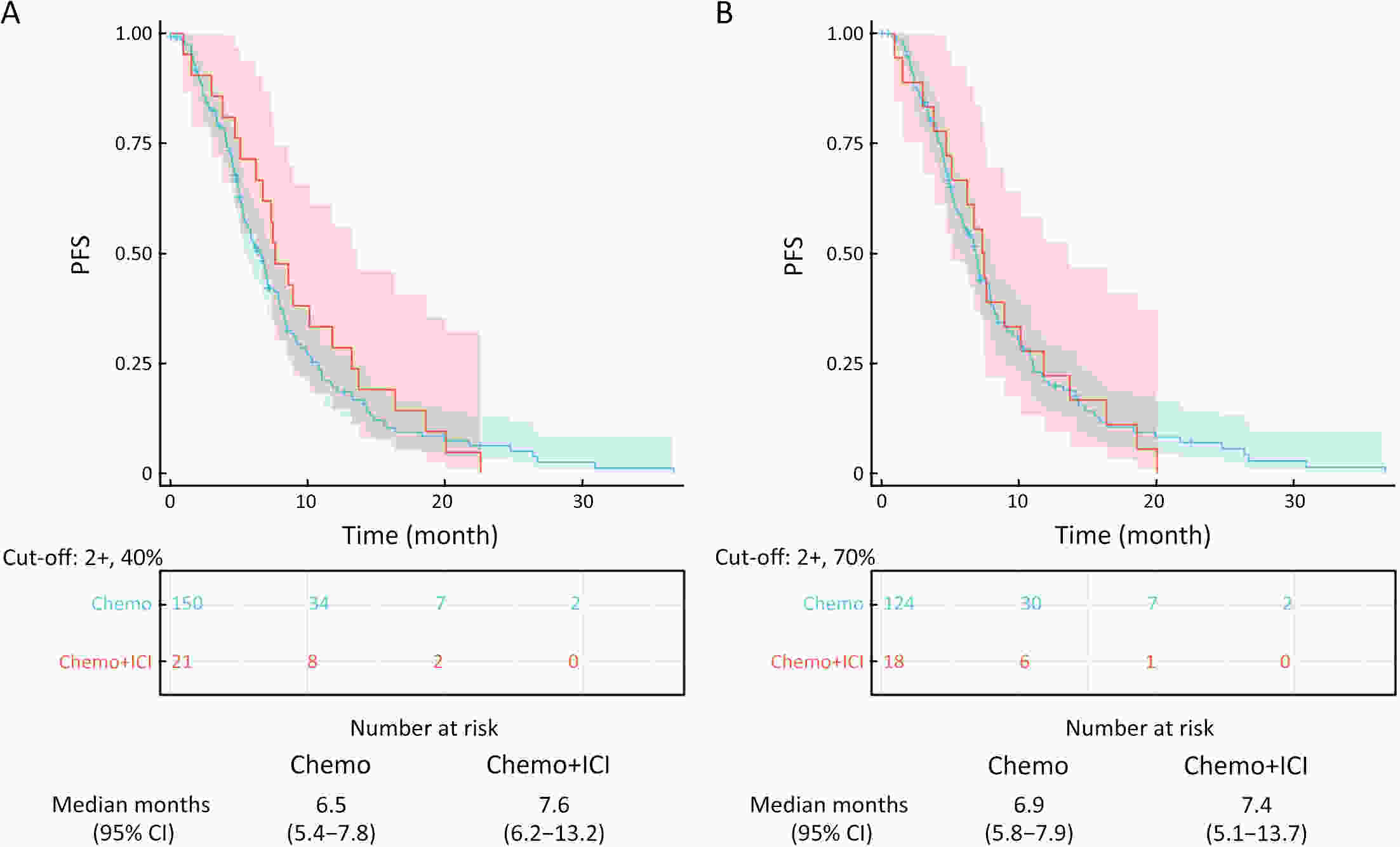
ObjectiveImmunotherapeutic outcomes and clinical characteristics of claudin 18 isoform 2 positive (CLDN18.2-positive) gastric cancer (GC) vary in different clinical studies, making it difficult to optimize anti-CLDN18.2 therapy. We conducted a retrospective analysis to explore the association of CLDN18.2 expression with clinicopathological characteristics and immunotherapeutic outcomes in GC. MethodsA total of 536 advanced GC patients from 2019 to 2021 in the CT041-CG4006 and CT041-ST-01 clinical trials were included in the analysis. CLDN18.2 expression on ≥40% of tumor cells (2+, 40%) and CLDN18.2 expression on ≥70% of tumor cells (2+, 70%) were considered the two levels of positively expressed GC. The clinicopathological characteristics and immunotherapy outcomes of GC patients were analyzed according to CLDN18.2 expression status. ResultsCLDN18.2 was expressed in 57.6% (cut-off: 2+, 40%) and 48.9% (cut-off: 2+, 70%) of patients. Programmed death-ligand 1 (PD-L1) and CLDN18.2 were co-expressed in 19.8% [combined positive score (CPS)≥1, CLDN18.2 (cut-off: 2+, 40%)] and 17.2% [CPS≥5, CLDN18.2 (cut-off: 2+, 70%)] of patients. CLDN18.2 expression positively correlated with younger age, female sex, non-gastroesophageal junction (non-GEJ), and diffuse phenotype (P<0.001). HER2 and PD-L1 expression were significantly lower in CLDN18.2-positive GC (both P<0.05). Uterine adnexa metastasis (P<0.001) was more frequent and liver metastasis (P<0.001) was less common in CLDN18.2-positive GC. Overall survival and immunotherapy-related progression-free survival (irPFS) were inferior in the CLDN18.2-positive group. ConclusionsCLDN18.2-positive GC is associated with poor prognosis and worse immunotherapeutic outcomes. The combination of anti-CLDN18.2 therapy, anti-PD-L1/PD-1 therapy, and chemotherapy for GC requires further investigation.
2024, 36(1): 90-102.
doi: 10.21147/j.issn.1000-9604.2024.01.09
Abstract:
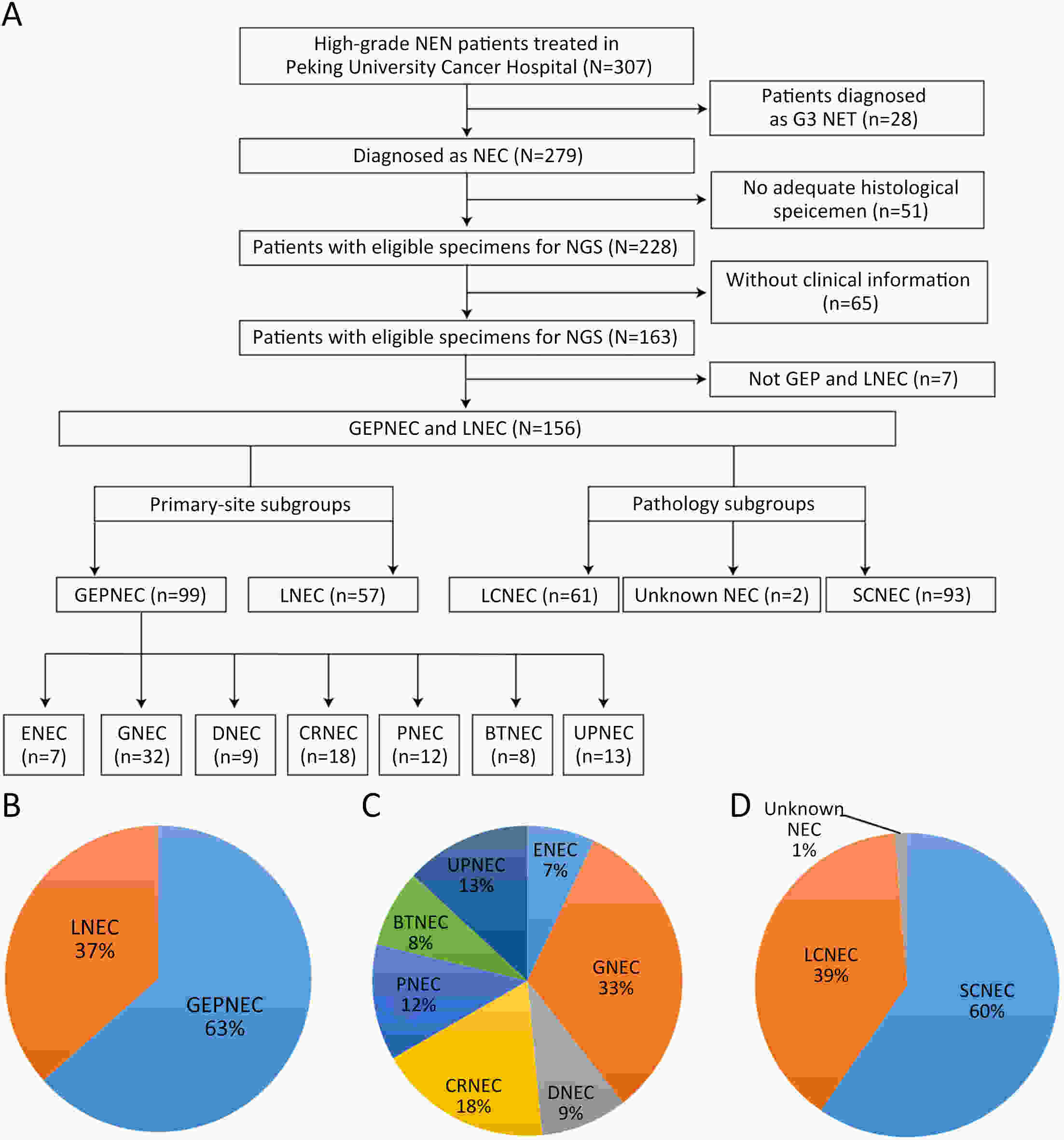
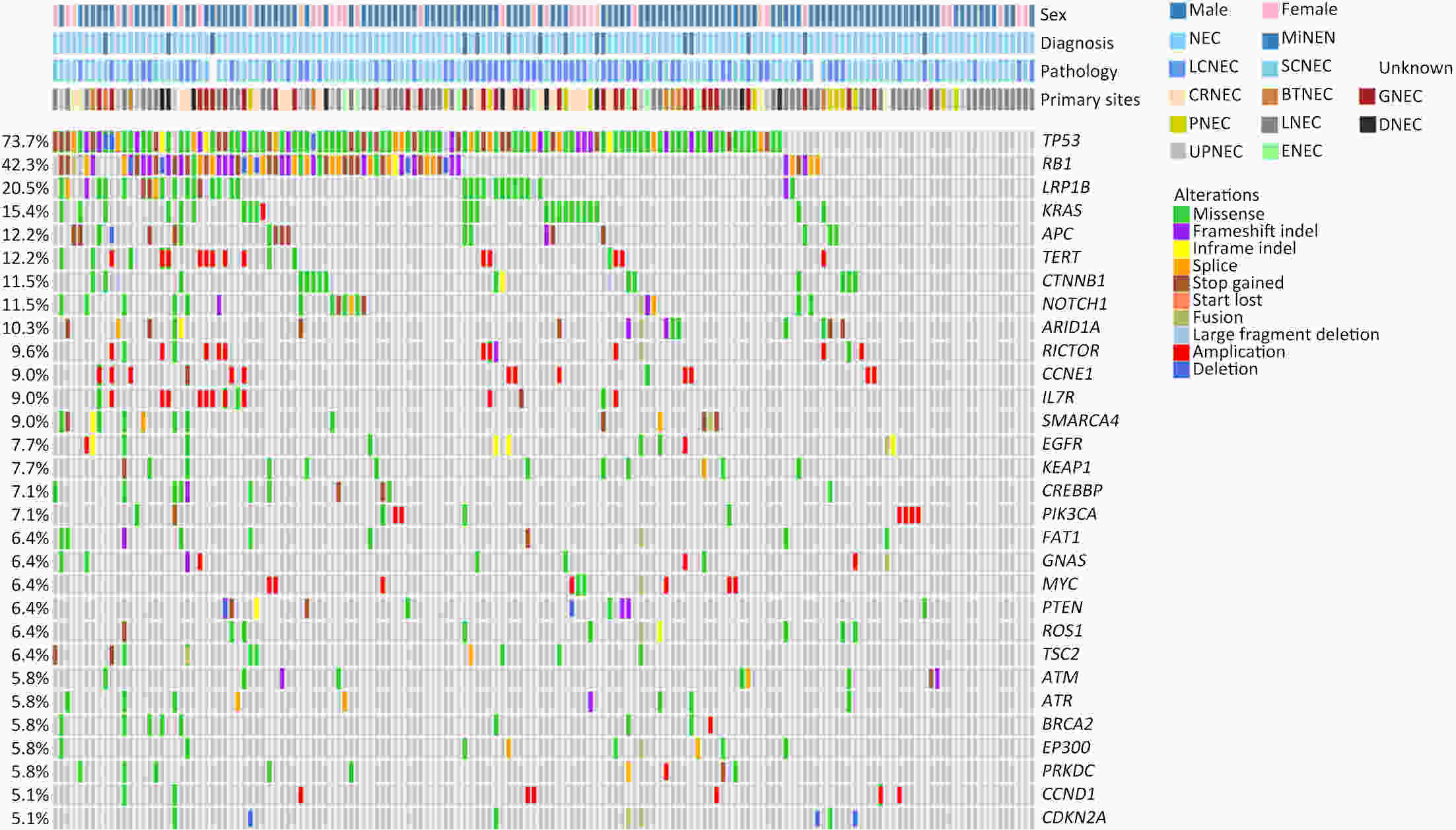
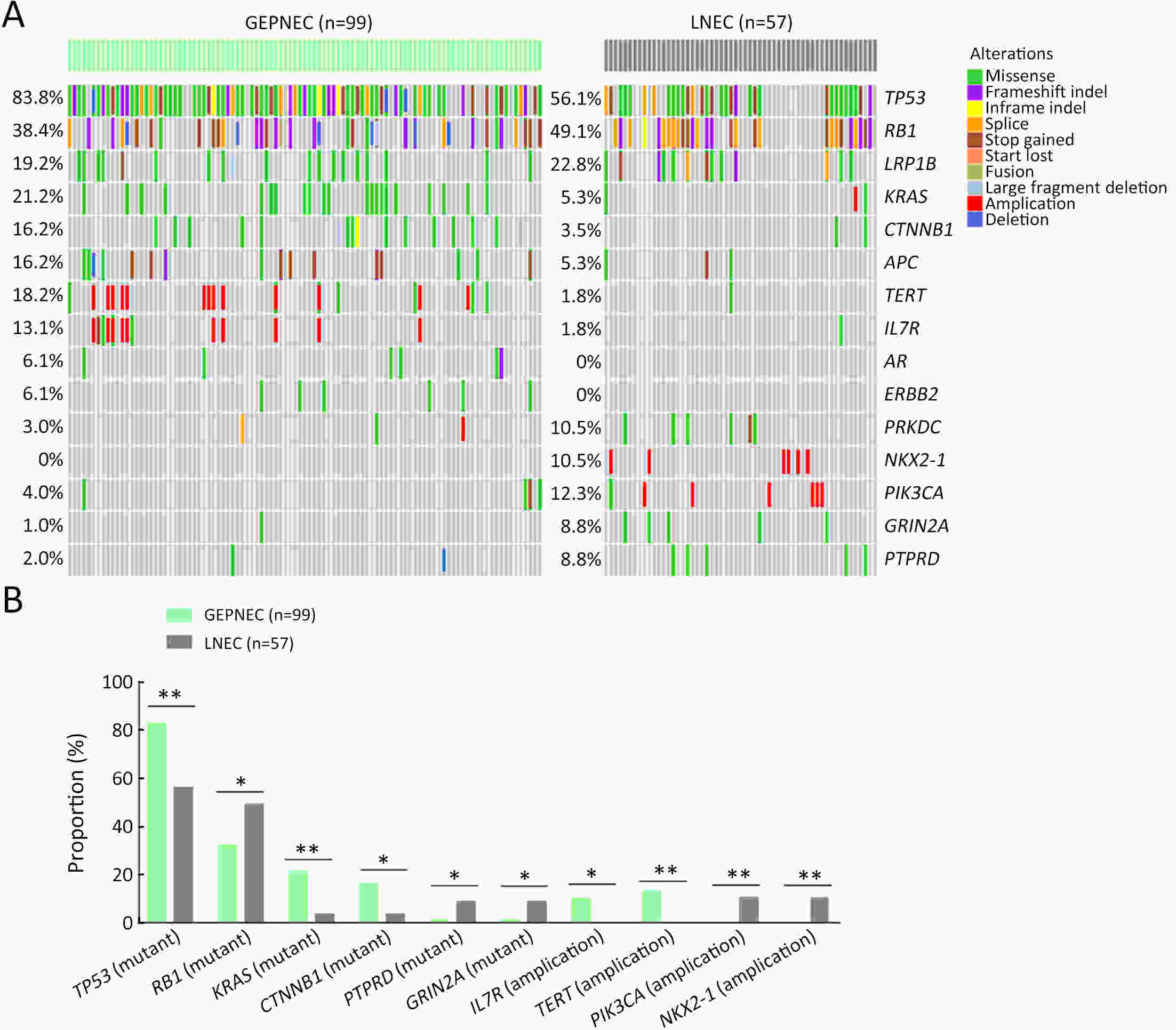
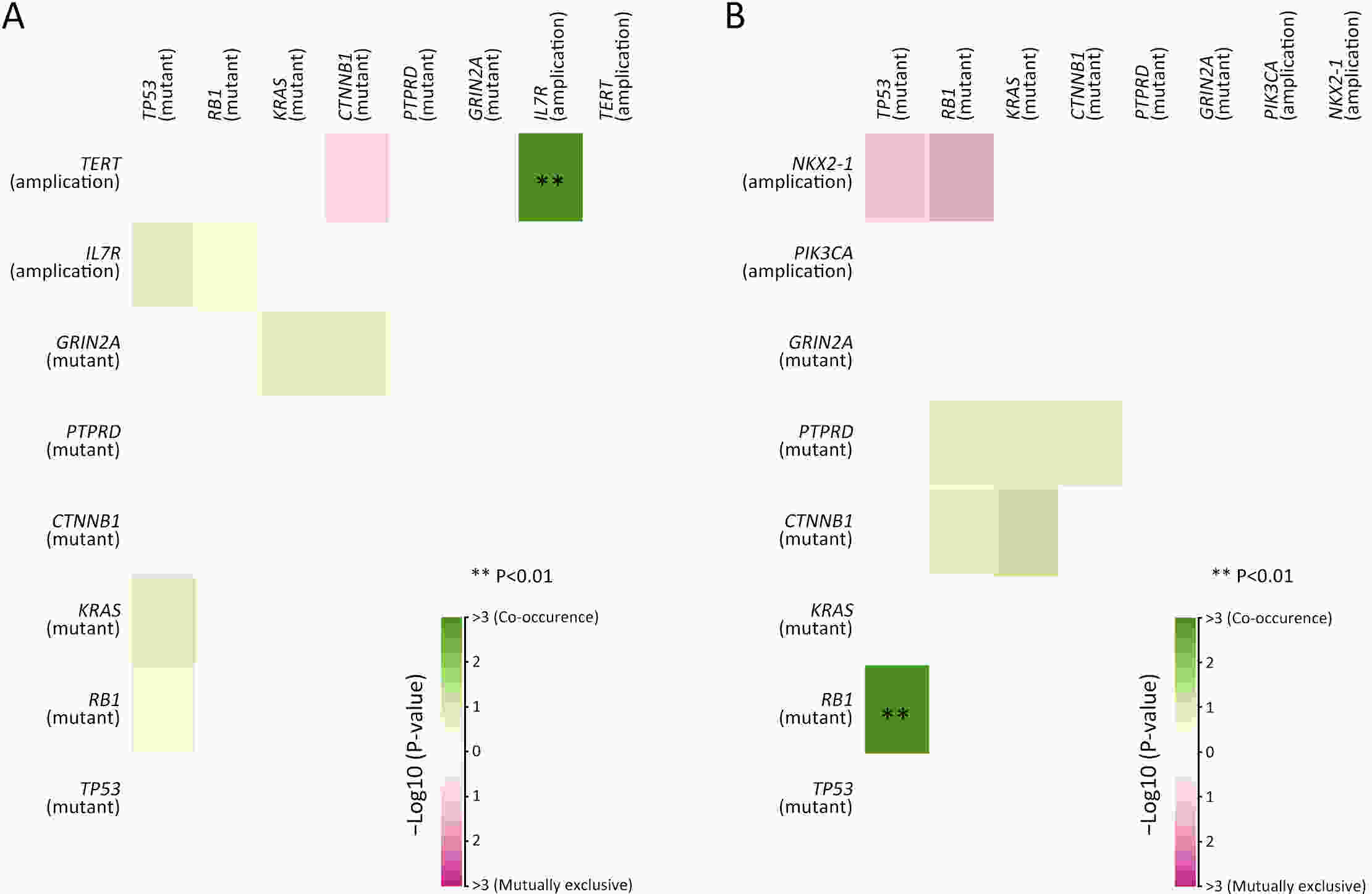
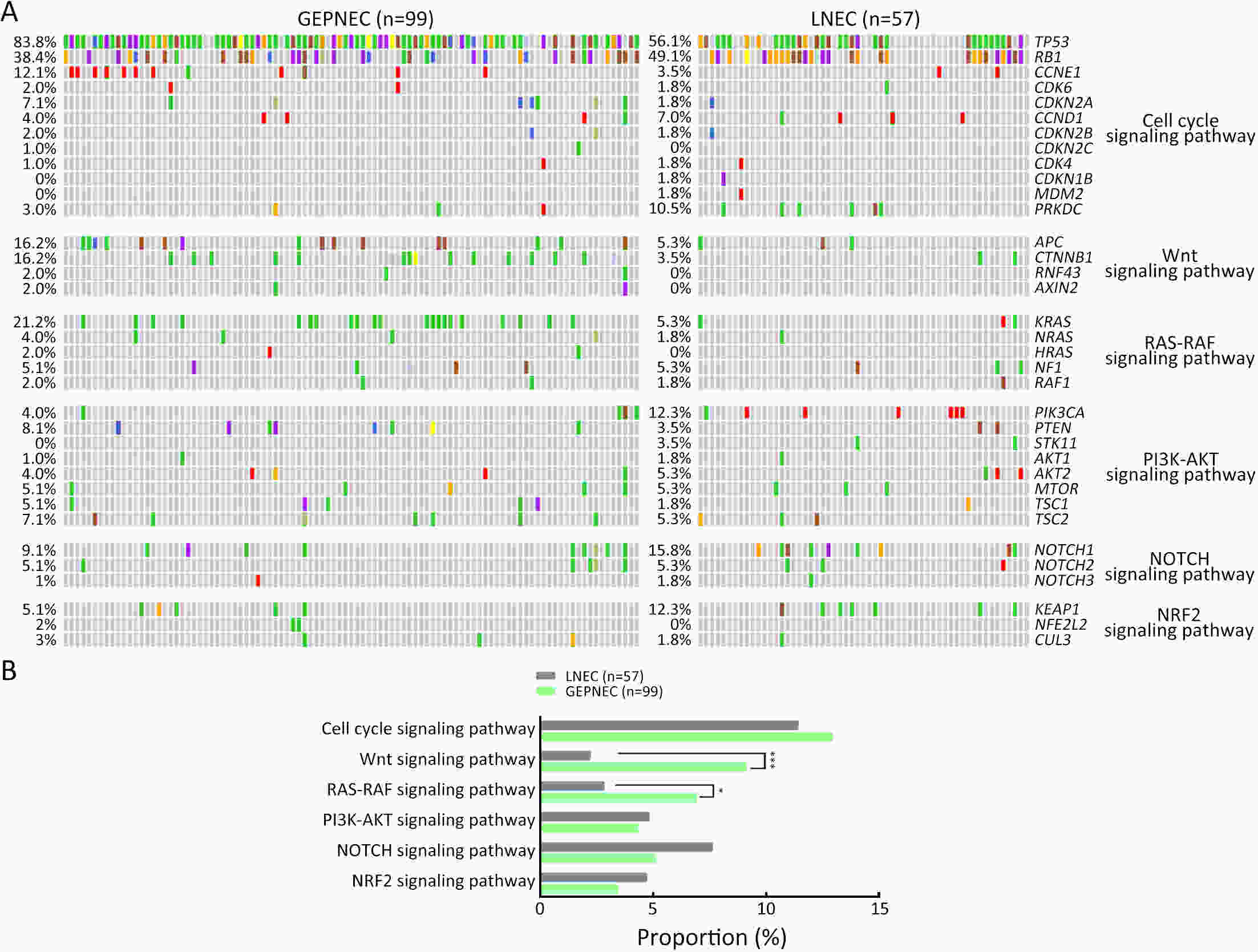
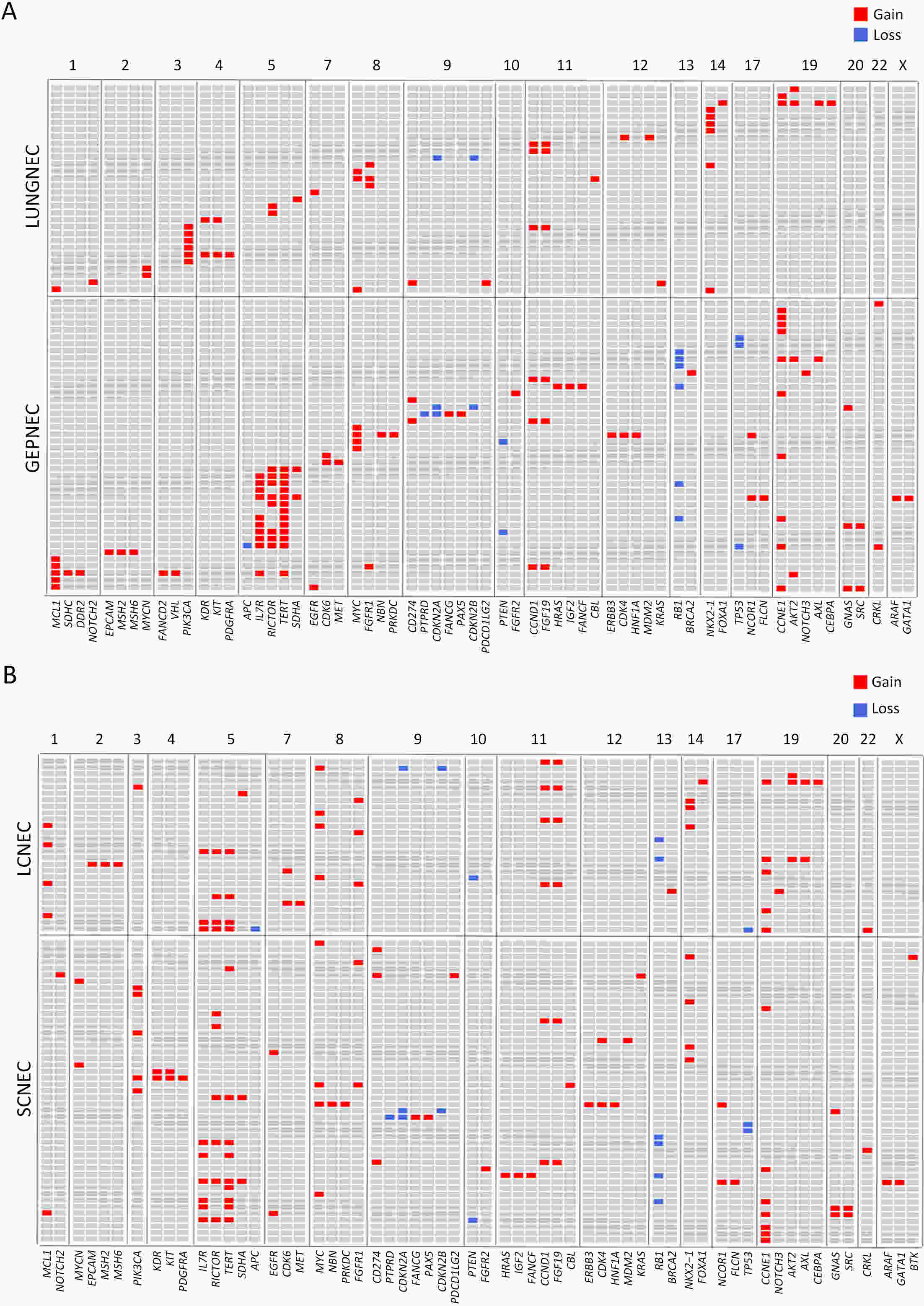
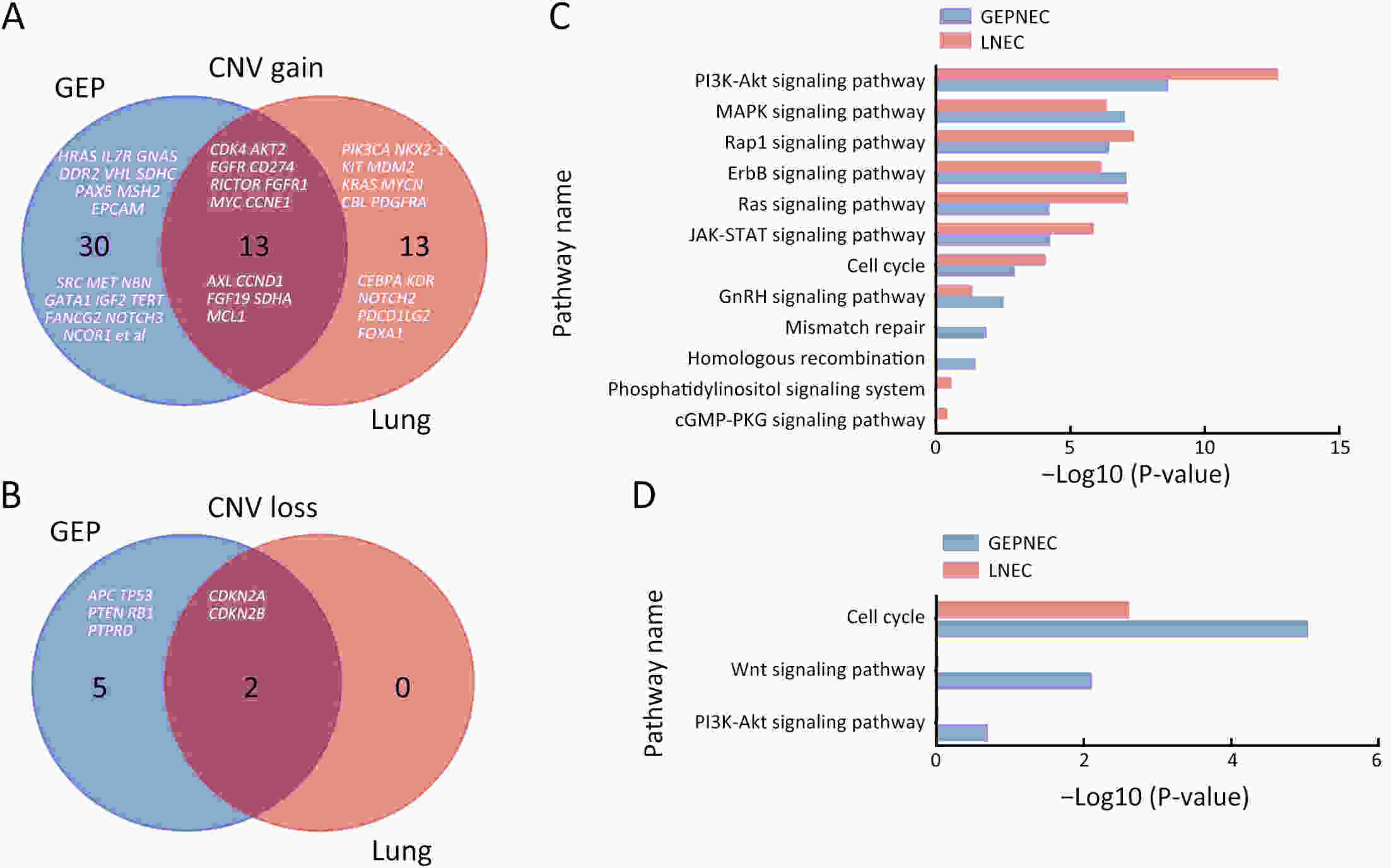
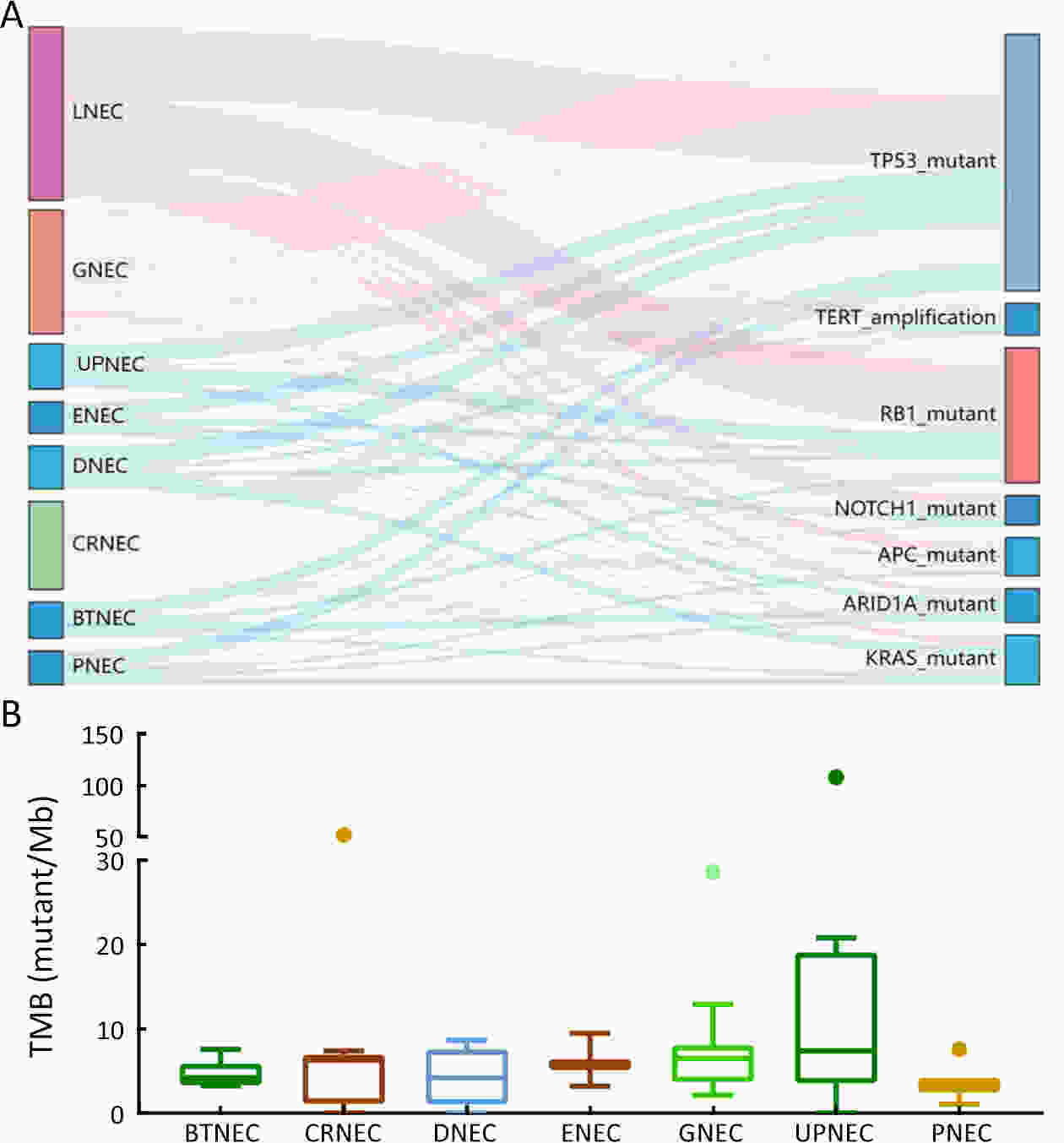
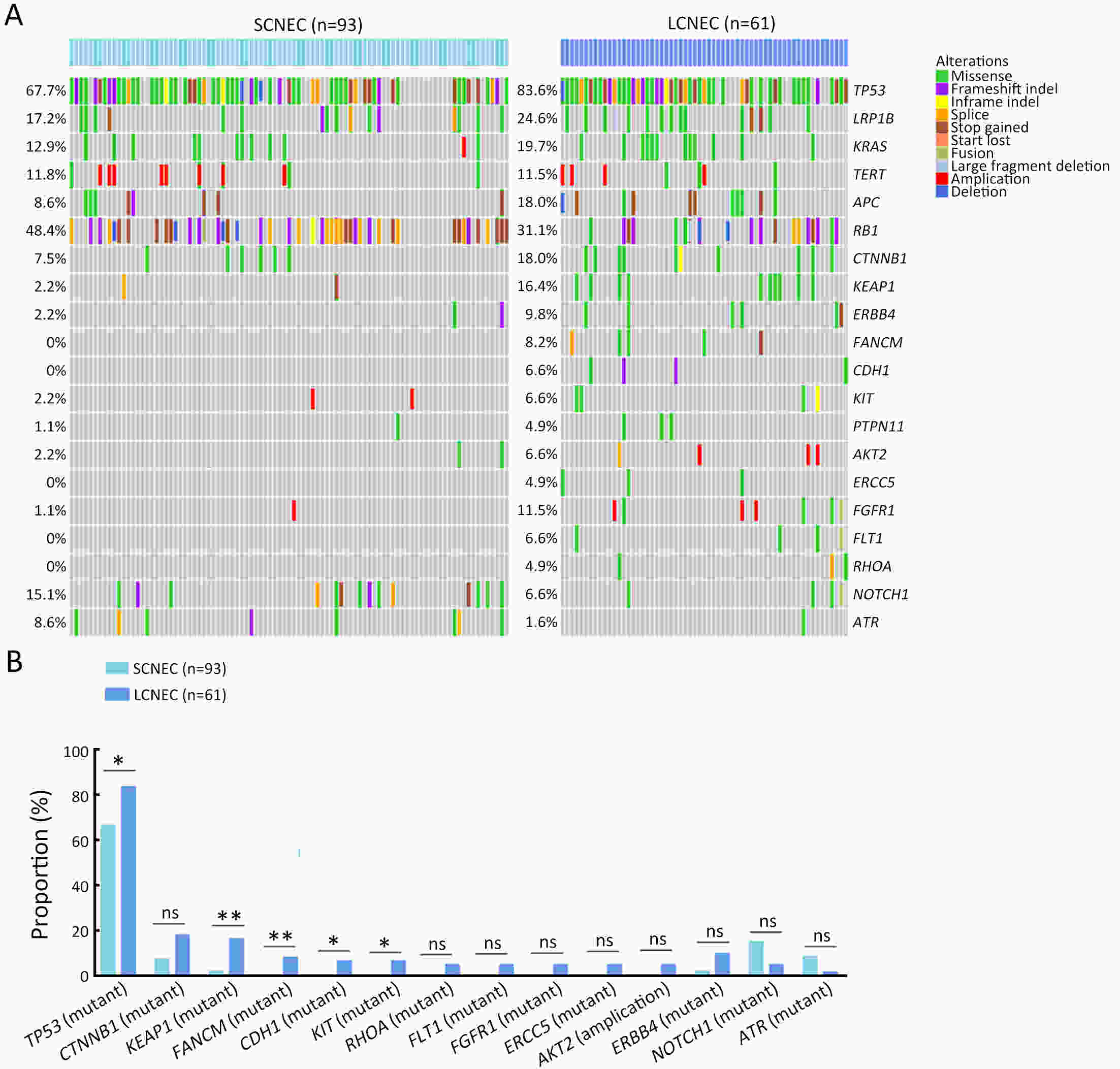
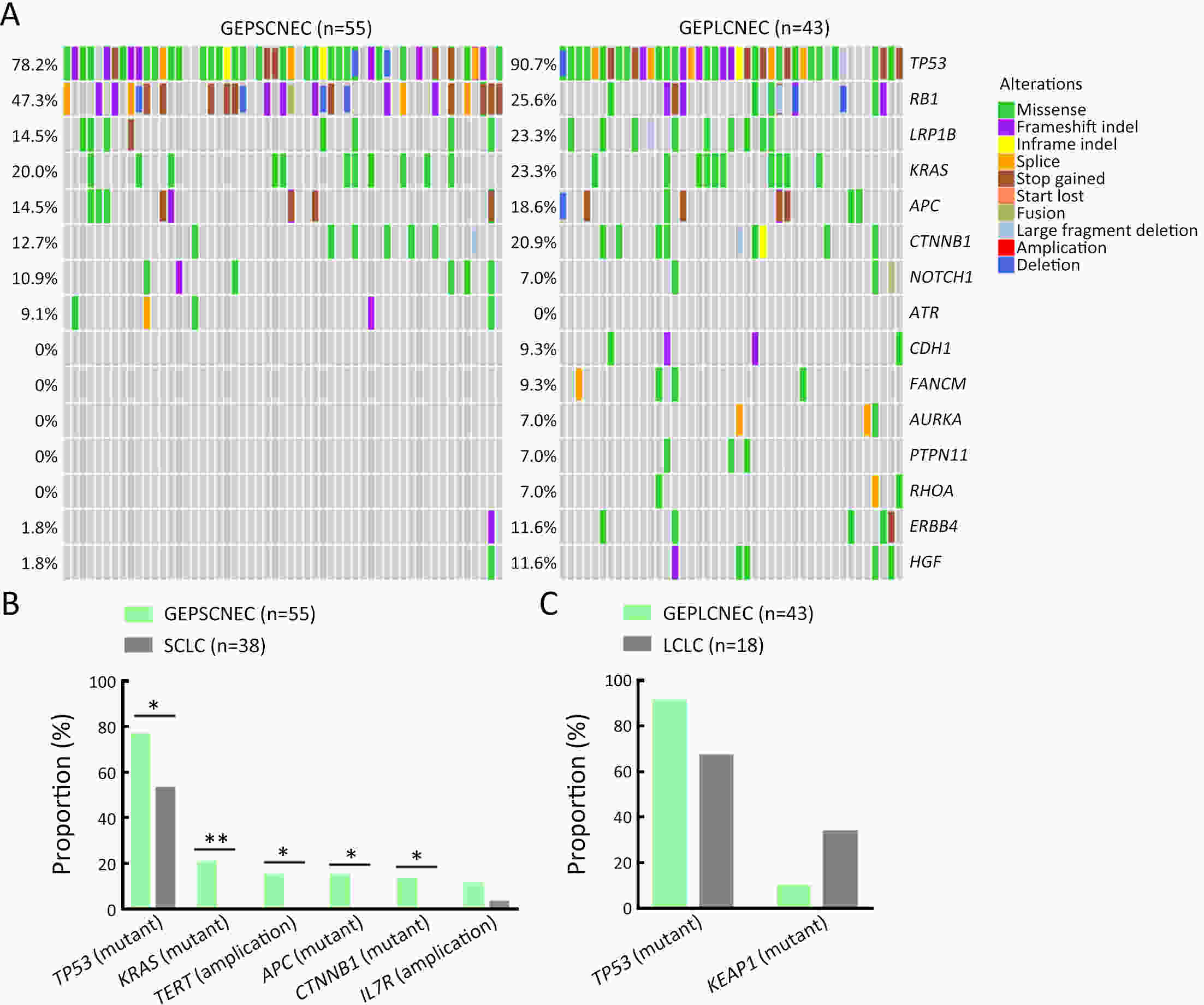
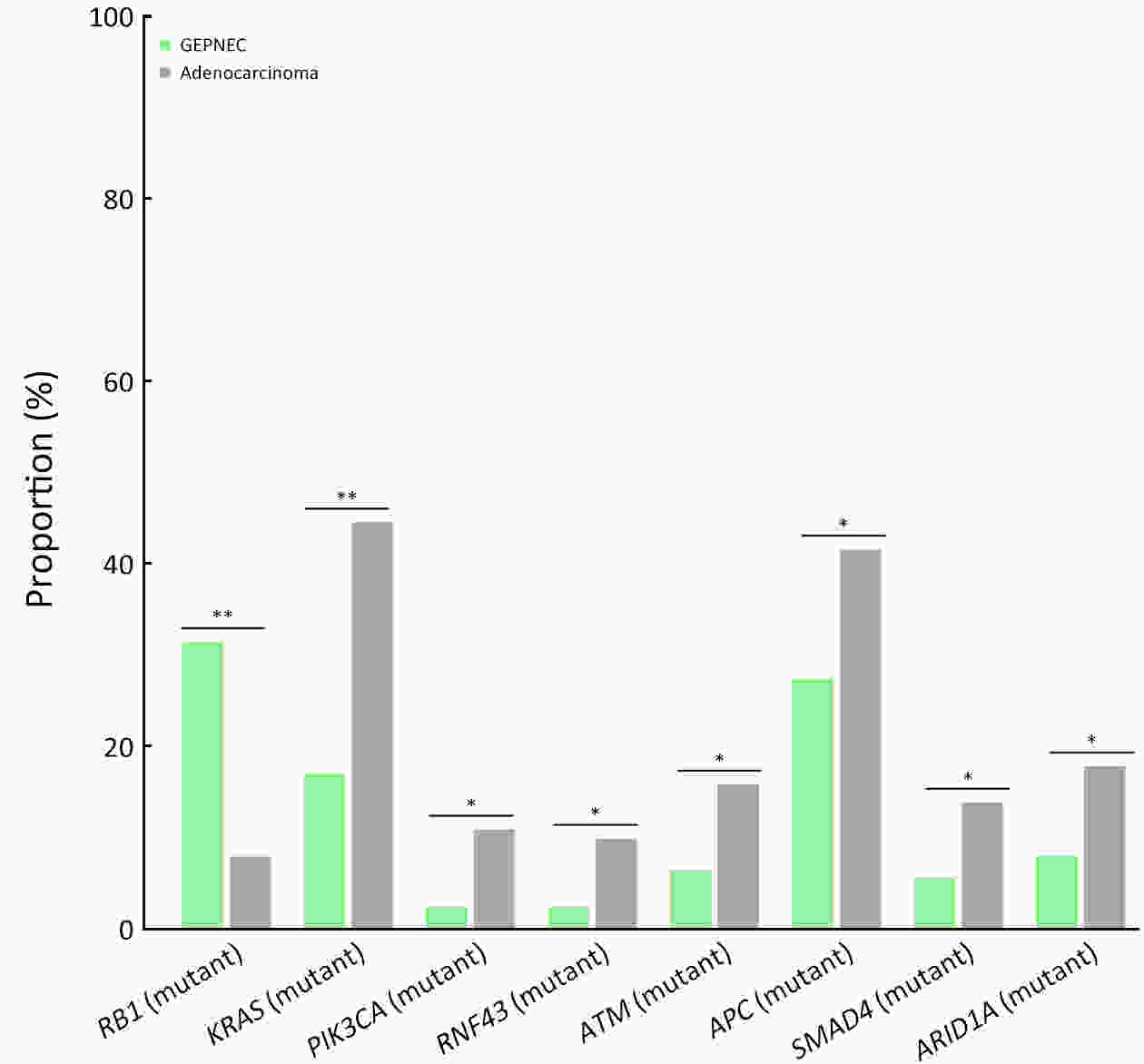
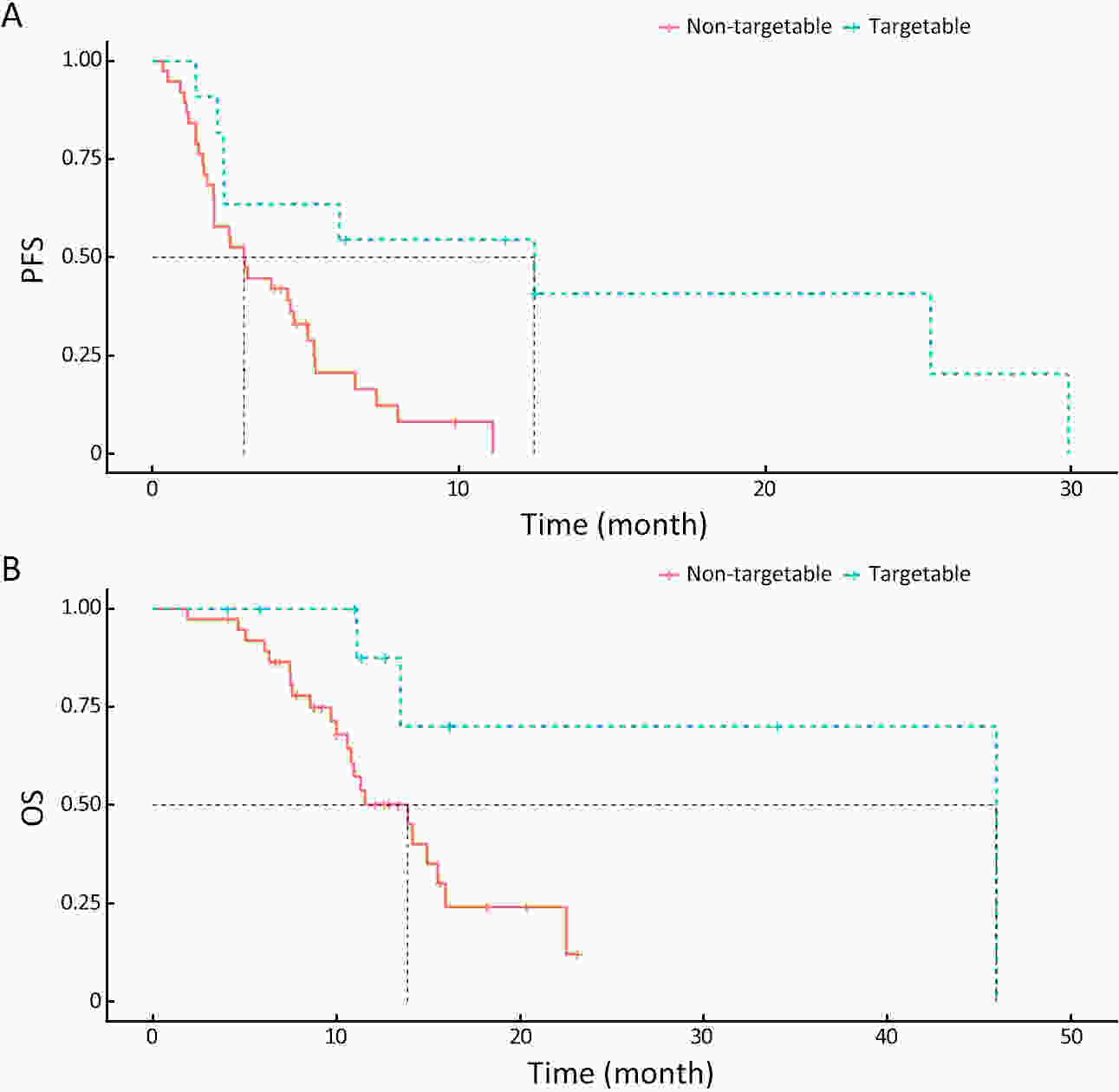
ObjectiveThere is an ongoing debate about whether the management of gastroenteropancreatic (GEP) neuroendocrine carcinoma (NEC) should follow the guidelines of small-cell lung cancer (SCLC). We aim to identify the genetic differences of GEPNEC and its counterpart. MethodsWe recruited GEPNEC patients as the main cohort, with lung NEC and digestive adenocarcinomas as comparative cohorts. All patients undergone next-generation sequencing (NGS). Different gene alterations were compared and analyzed between GEPNEC and lung NEC (LNEC), GEPNEC and adenocarcinoma to yield the remarkable genes. ResultsWe recruited 257 patients, including 99 GEPNEC, 57 LNEC, and 101 digestive adenocarcinomas. Among the mutations, KRAS, RB1, TERT, IL7R, and CTNNB1 were found to have different gene alterations between GEPNEC and LNEC samples. Specific genes for each site were revealed: gastric NEC (TERT amplification), colorectal NEC (KRAS mutation), and bile tract NEC (ARID1A mutation). The gene disparities between small-cell NEC (SCNEC) and large-cell NEC (LCNEC) were KEAP1 and CDH1. Digestive adenocarcinoma was also compared with GEPNEC and suggested RB1, APC, and KRAS as significant genes. The TP53/RB1 mutation pattern was associated with first-line effectiveness. Putative targetable genes and biomarkers in GEPNEC were identified in 22.2% of the patients, and they had longer progression-free survival (PFS) upon targetable treatment [12.5 months vs. 3.0 months, HR=0.40 (0.21−0.75), P=0.006]. ConclusionsThis work demonstrated striking gene distinctions in GEPNEC compared with LNEC and adenocarcinoma and their clinical utility.

 Abstract
Abstract FullText HTML
FullText HTML PDF 1947KB
PDF 1947KB