2024 Vol.36(2)
Display Mode: |
2024, 36(2): 103-113.
doi: 10.21147/j.issn.1000-9604.2024.02.01
Abstract:
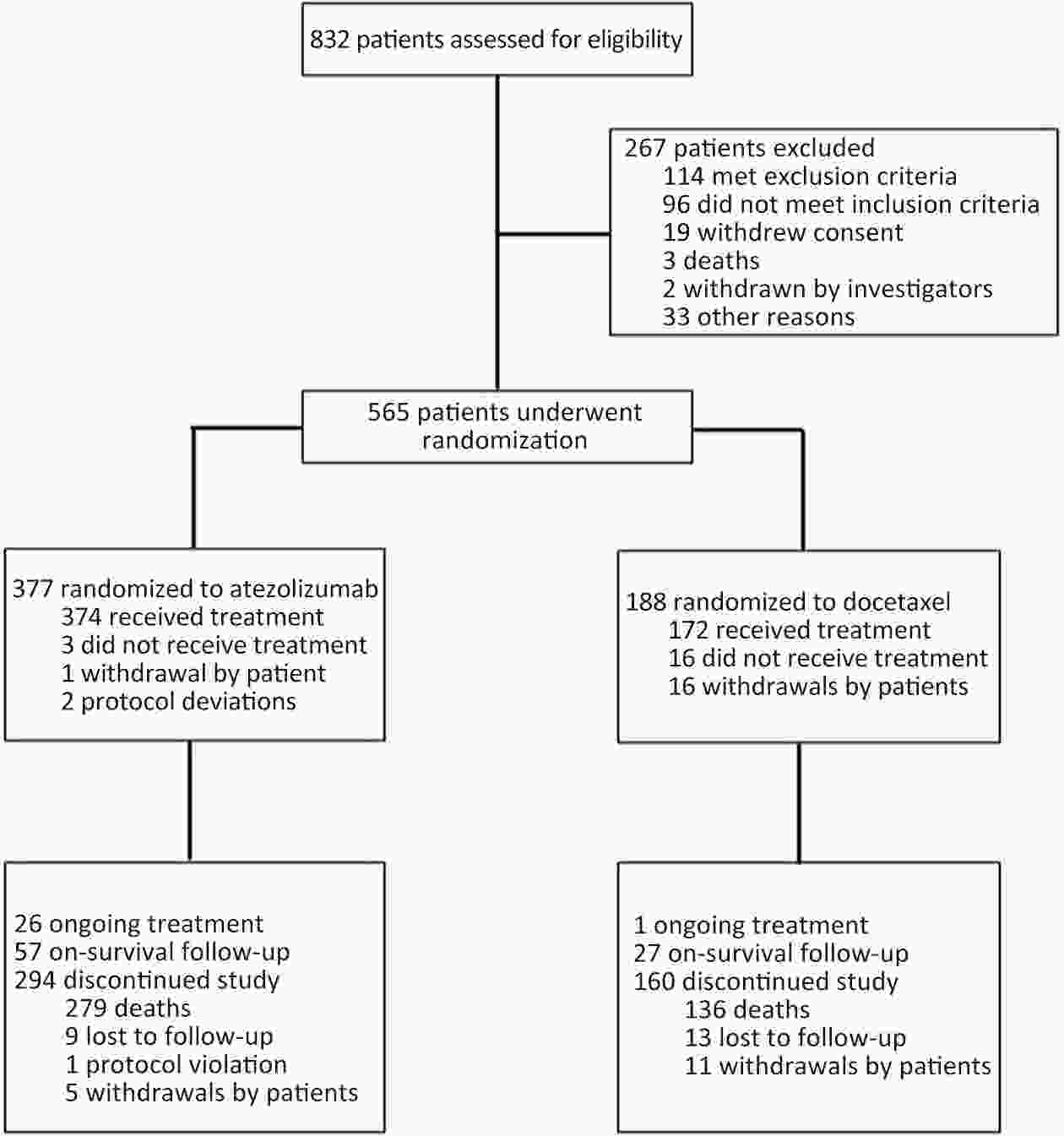
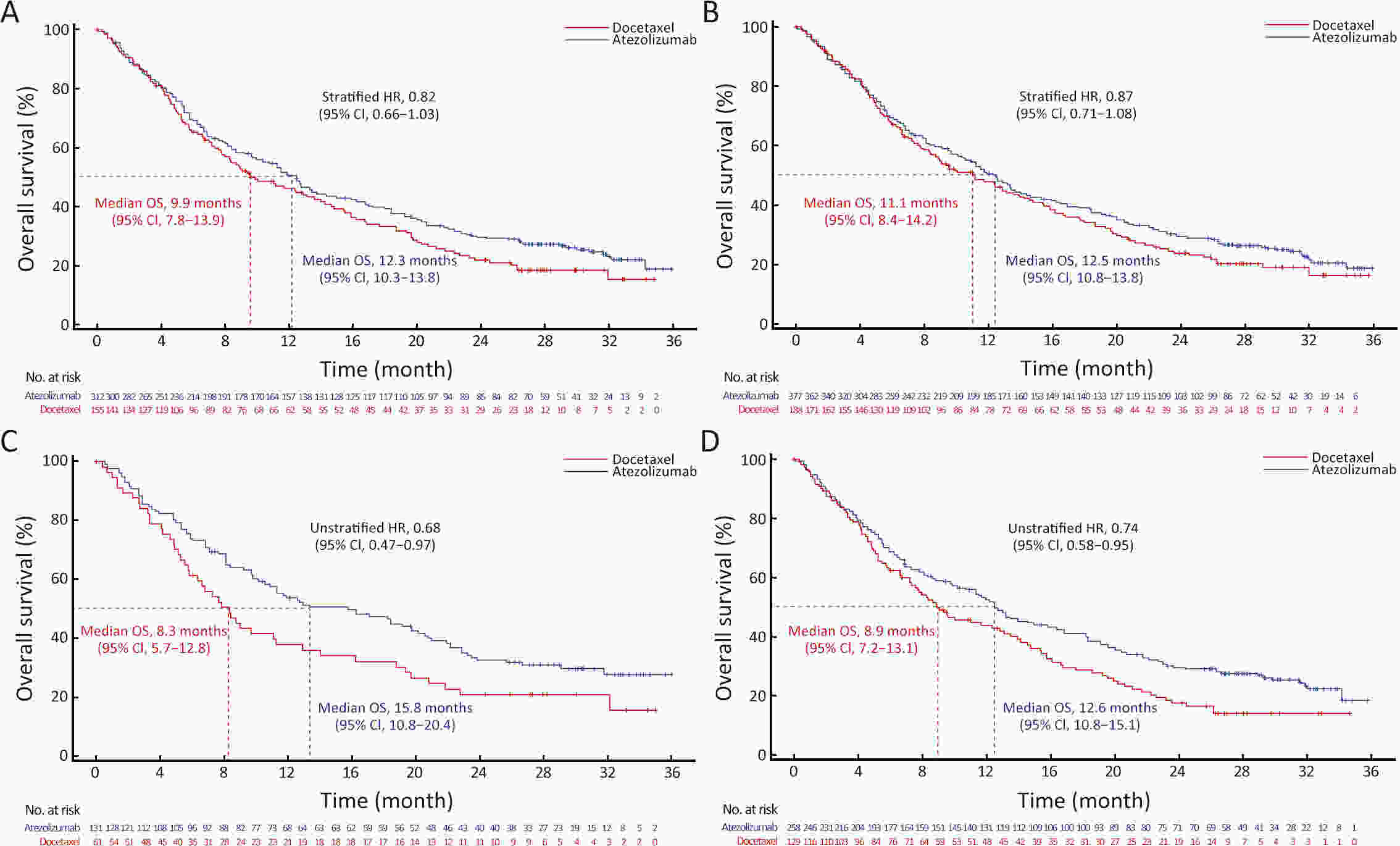
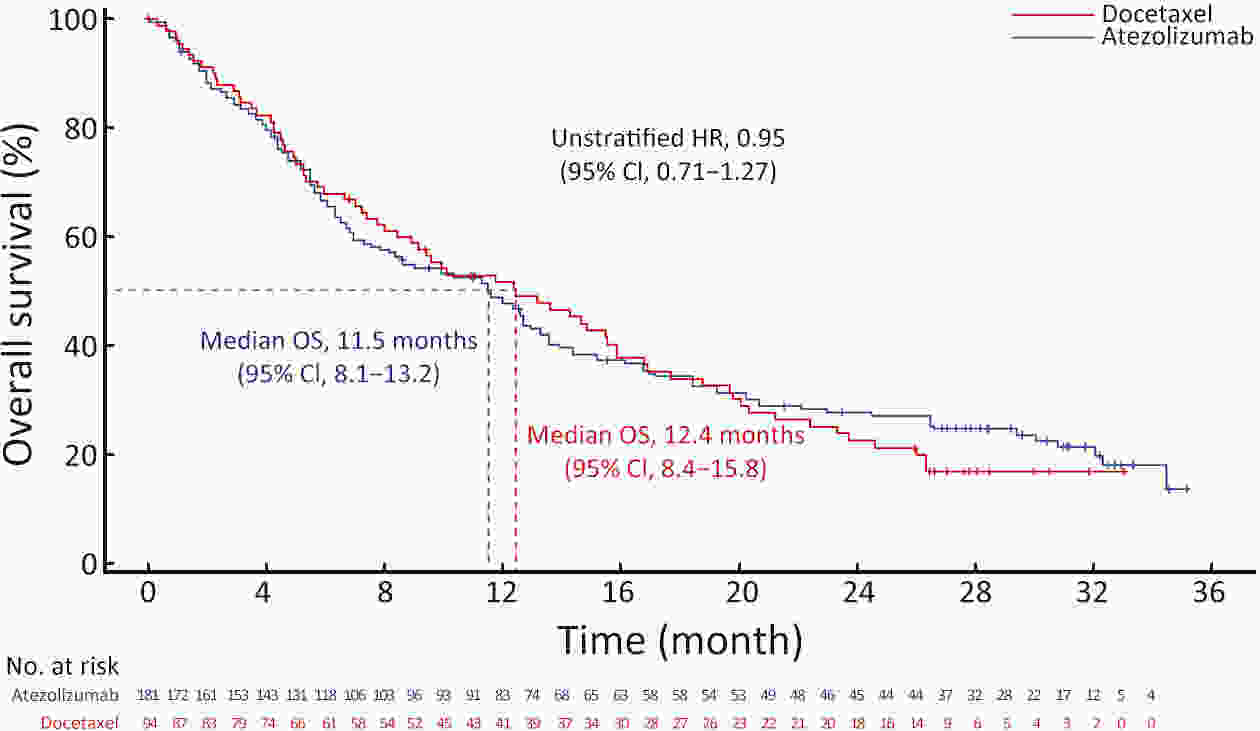
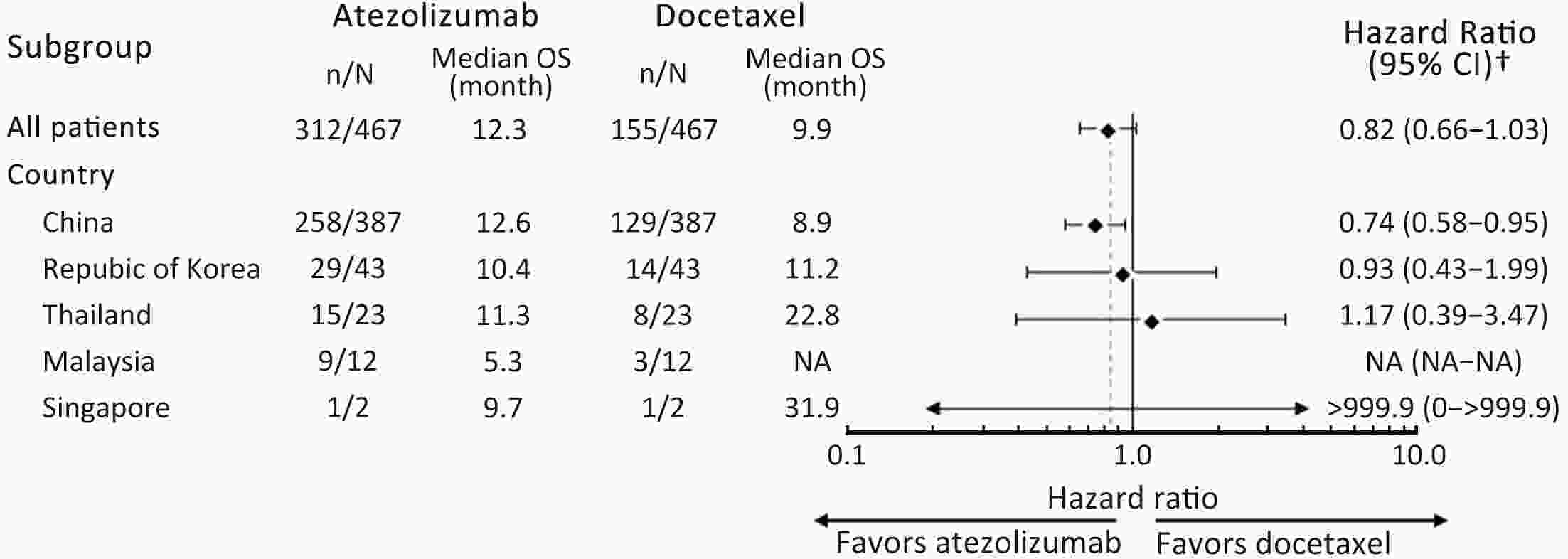
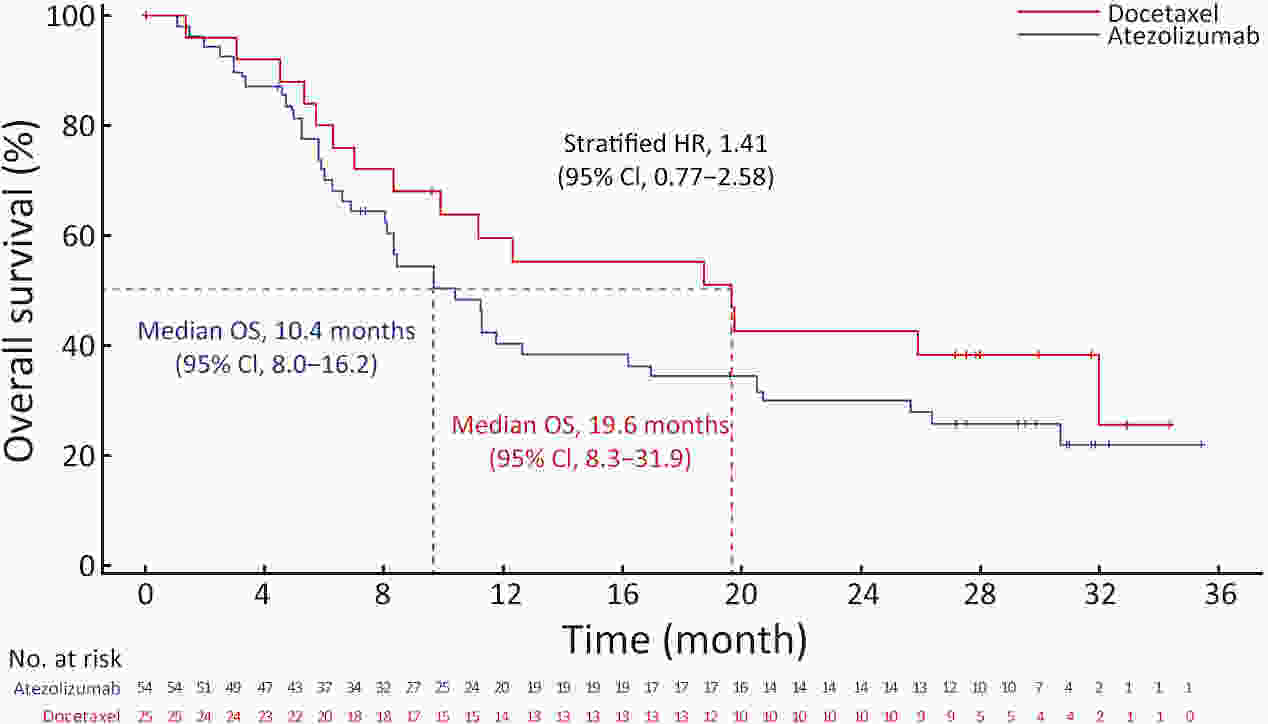
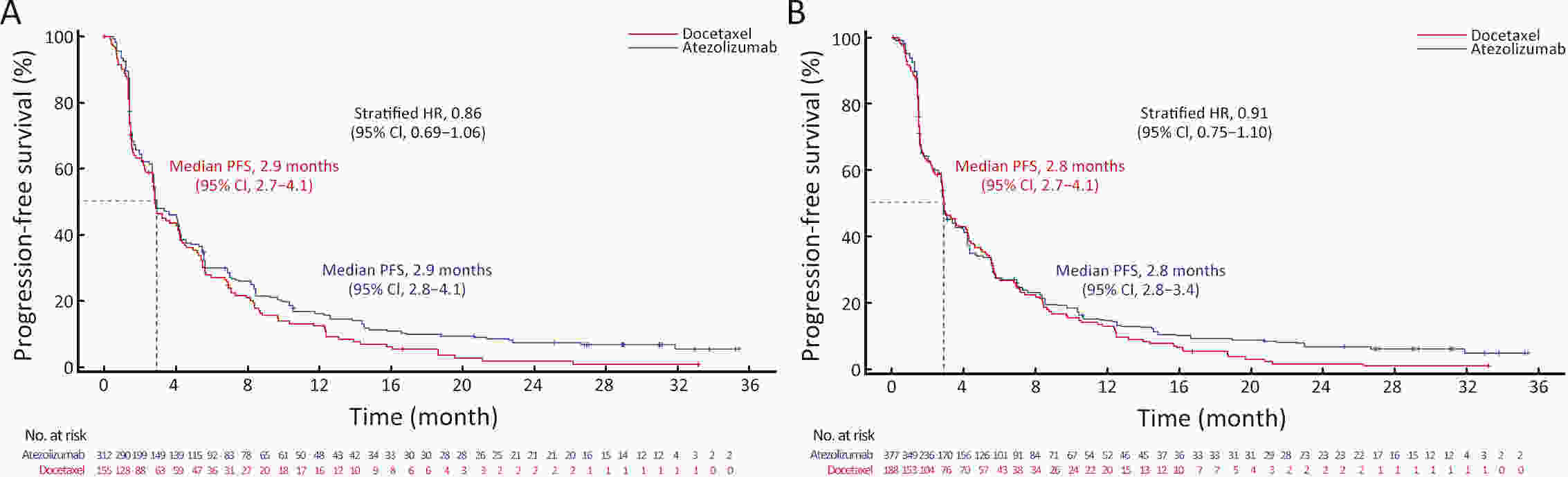
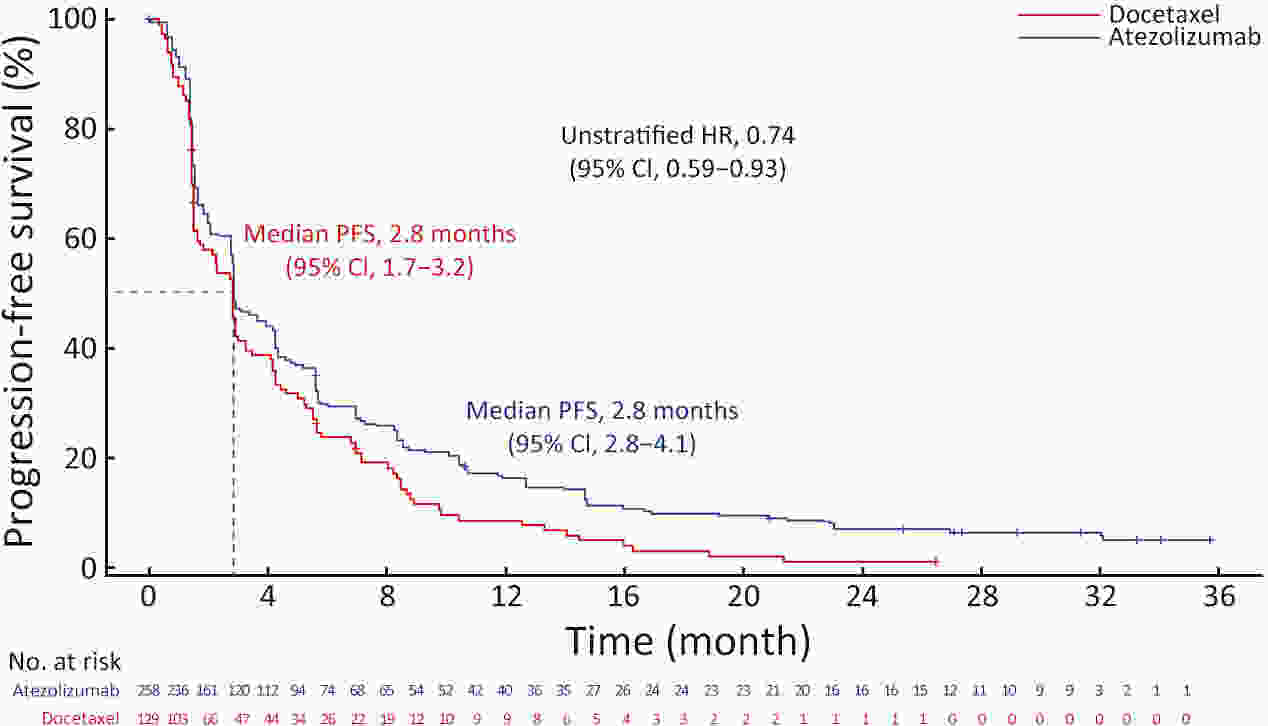
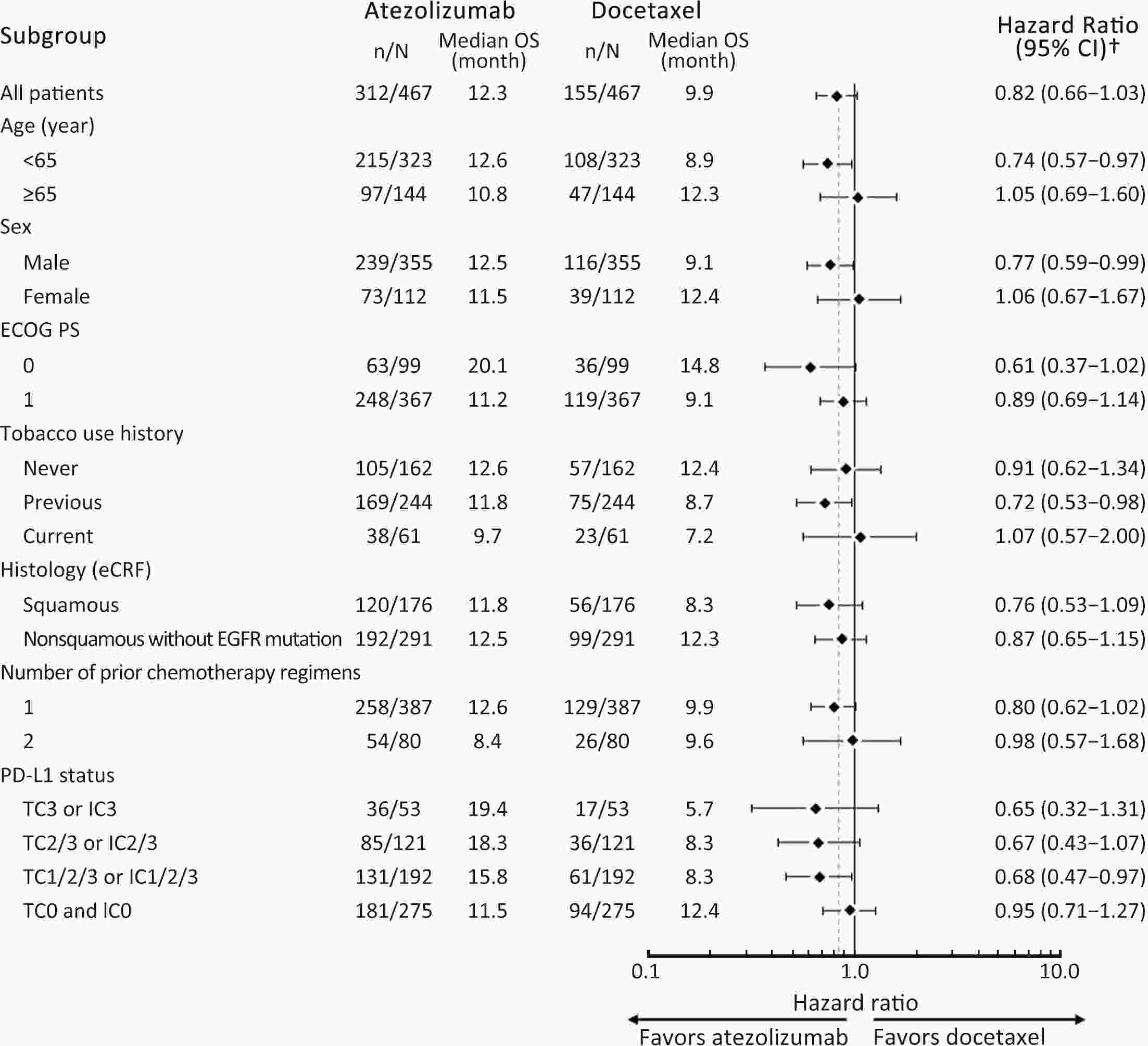
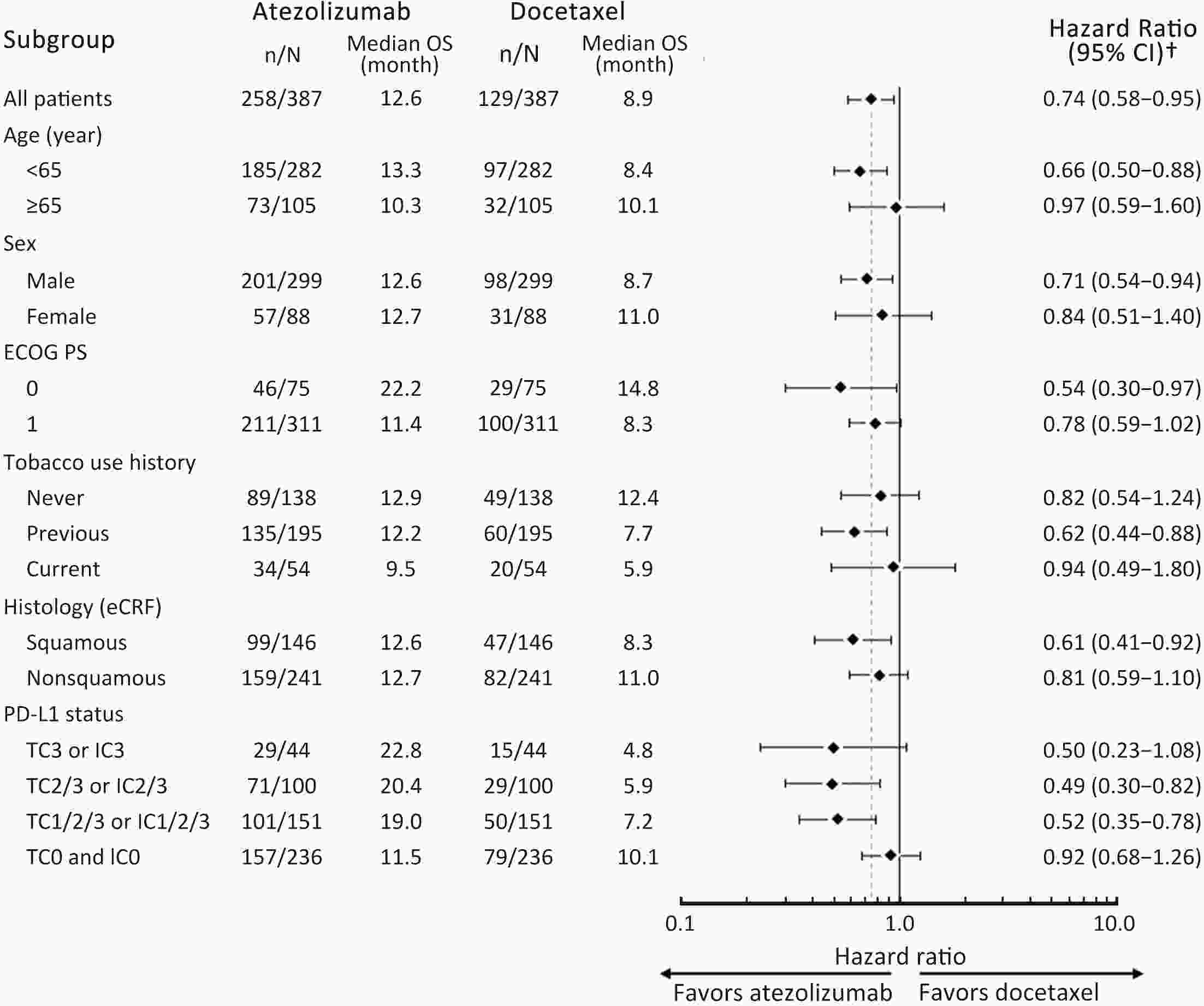
ObjectiveIMpower210 (NCT02813785) explored the efficacy and safety of single-agent atezolizumab vs. docetaxel as second-line treatment for advanced non-small cell lung cancer (NSCLC) in East Asian patients. MethodsKey eligibility criteria for this phase III, open-label, randomized study included age ≥18 years; histologically documented advanced NSCLC per the Union for International Cancer Control/American Joint Committee on Cancer staging system (7th edition); Eastern Cooperative Oncology Group performance status of 0 or 1; and disease progression following platinum-based chemotherapy for advanced or metastatic NSCLC. Patients were randomized 2:1 to receive either atezolizumab (1,200 mg) or docetaxel (75 mg/m2). The primary study endpoint was overall survival (OS) in the intention-to-treat (ITT) population with wild-type epidermal growth factor receptor expression (ITT EGFR-WT) and in the overall ITT population. ResultsMedian OS in the ITT EGFR-WT population (n=467) was 12.3 [95% confidence interval (95% CI), 10.3−13.8] months in the atezolizumab arm (n=312) and 9.9 (95% CI, 7.8−13.9) months in the docetaxel arm [n=155; stratified hazard ratio (HR), 0.82; 95% CI, 0.66−1.03]. Median OS in the overall ITT population was 12.5 (95% CI, 10.8−13.8) months with atezolizumab treatment and 11.1 (95% CI, 8.4−14.2) months (n=377) with docetaxel treatment (n=188; stratified HR, 0.87; 95% CI, 0.71−1.08). Grade 3/4 treatment-related adverse events (TRAEs) occurred in 18.4% of patients in the atezolizumab arm and 50.0% of patients in the docetaxel arm. ConclusionsIMpower210 did not meet its primary efficacy endpoint of OS in the ITT EGFR-WT or overall ITT populations. Atezolizumab was comparatively more tolerable than docetaxel, with a lower incidence of grade 3/4 TRAEs.
2024, 36(2): 114-123.
doi: 10.21147/j.issn.1000-9604.2024.02.02
Abstract:
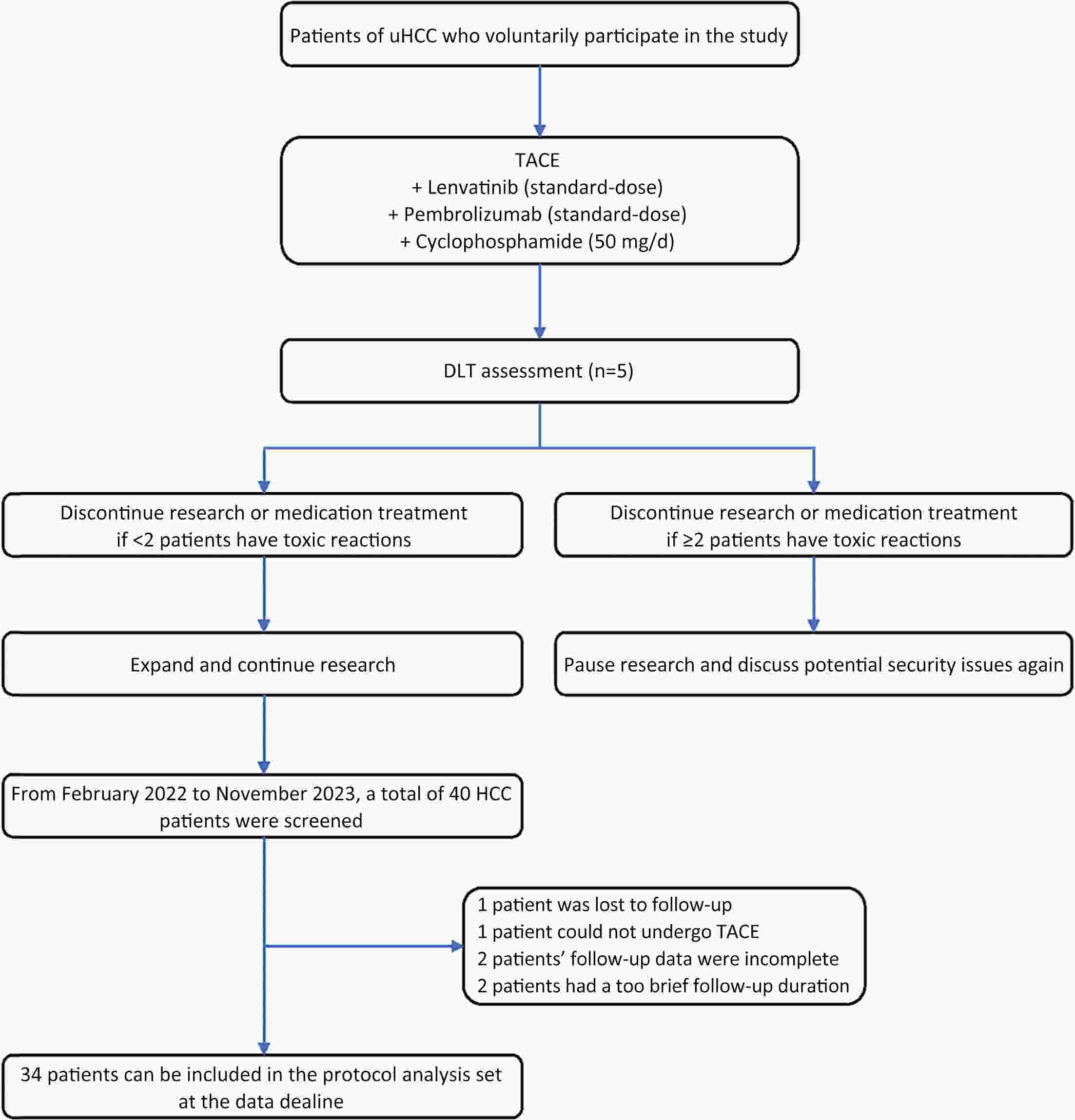
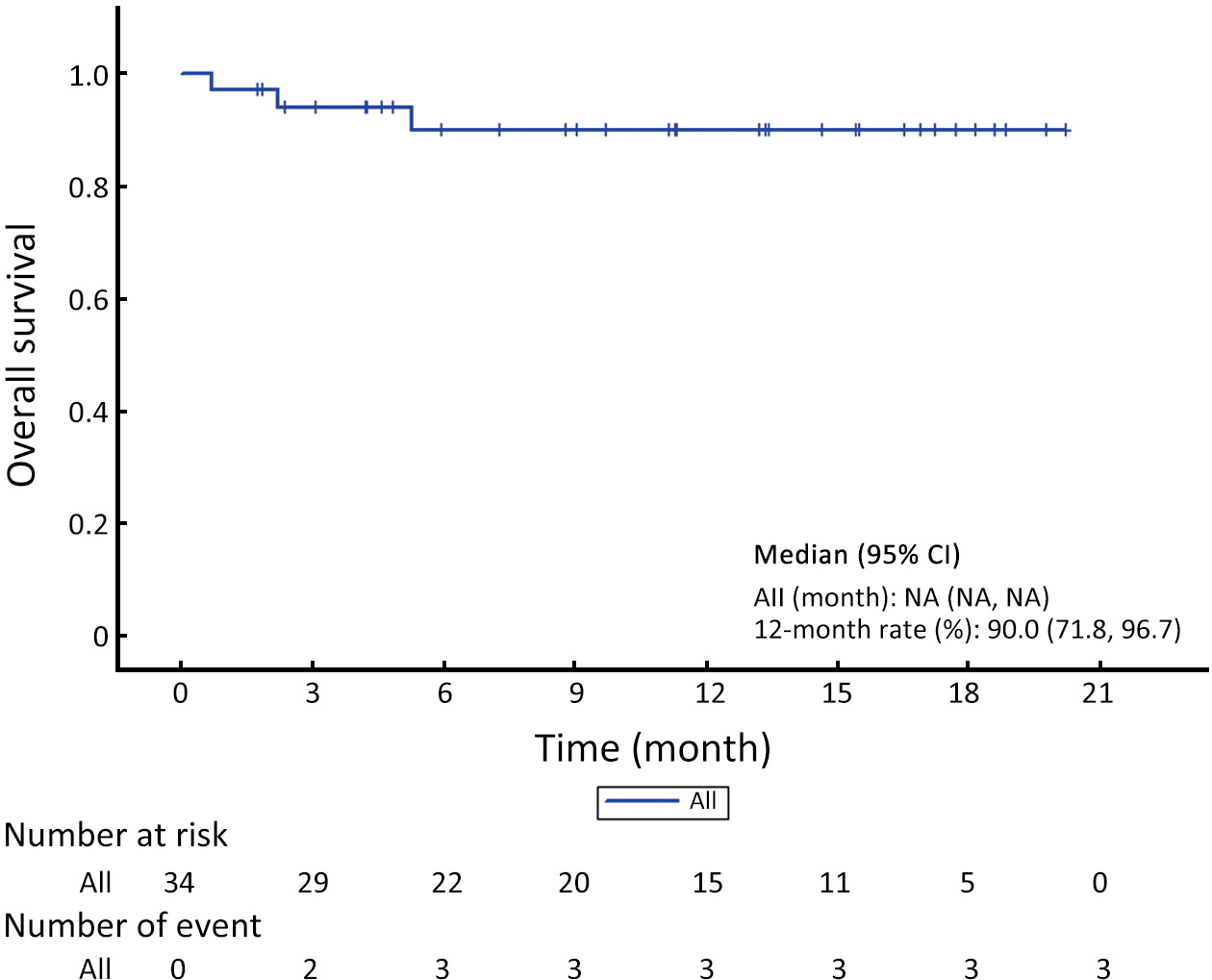
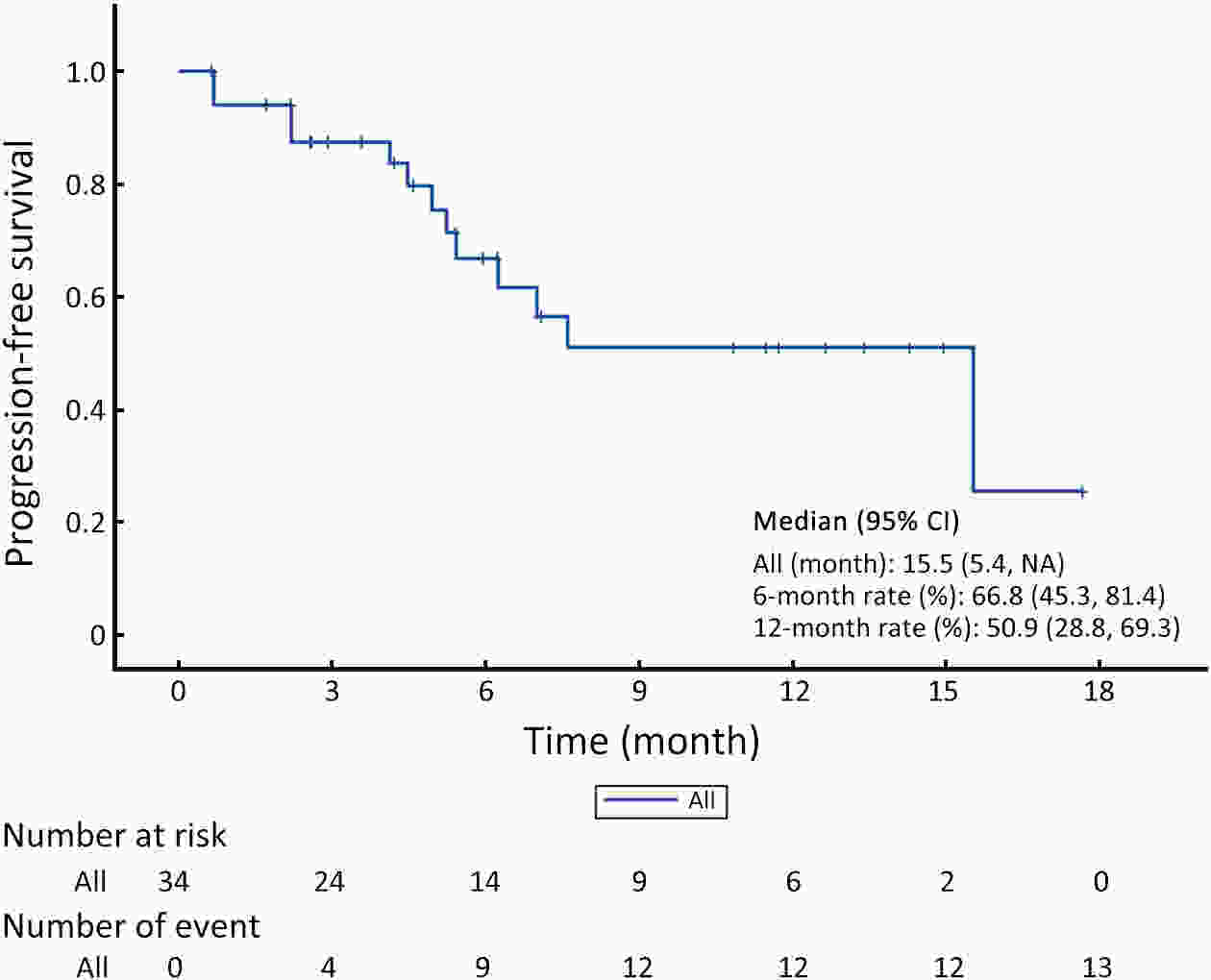
ObjectiveUnresectable hepatocellular carcinoma (uHCC) continues to pose effective treatment options. The objective of this study was to assess the efficacy and safety of combining low-dose cyclophosphamide with lenvatinib, pembrolizumab and transarterial chemoembolization (TACE) for the treatment of uHCC. MethodsFrom February 2022 to November 2023, a total of 40 patients diagnosed with uHCC were enrolled in this small-dose, single-center, single-arm, prospective study. They received a combined treatment of low-dose cyclophosphamide with lenvatinib, pembrolizumab, and TACE. Study endpoints included progression-free survival (PFS), objective response rate (ORR), and safety assessment. Tumor response was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST), while survival analysis was conducted through Kaplan-Meier curve analysis for overall survival (OS) and PFS. Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). ResultsA total of 34 patients were included in the study. The median follow-up duration was 11.2 [95% confidence interval (95% CI), 5.3−14.6] months, and the median PFS (mPFS) was 15.5 (95% CI, 5.4−NA) months. Median OS (mOS) was not attained during the study period. The ORR was 55.9%, and the disease control rate (DCR) was 70.6%. AEs were reported in 27 (79.4%) patients. The most frequently reported AEs (with an incidence rate >10%) included abnormal liver function (52.9%), abdominal pain (44.1%), abdominal distension and constipation (29.4%), hypertension (20.6%), leukopenia (17.6%), constipation (17.6%), ascites (14.7%), and insomnia (14.7%). Abnormal liver function (14.7%) had the most common grade 3 or higher AEs. ConclusionsA combination of low-dose cyclophosphamide with lenvatinib, pembrolizumab, and TACE is safe and effective for uHCC, showcasing a promising therapeutic strategy for managing uHCC.
2024, 36(2): 124-137.
doi: 10.21147/j.issn.1000-9604.2024.02.03
Abstract:
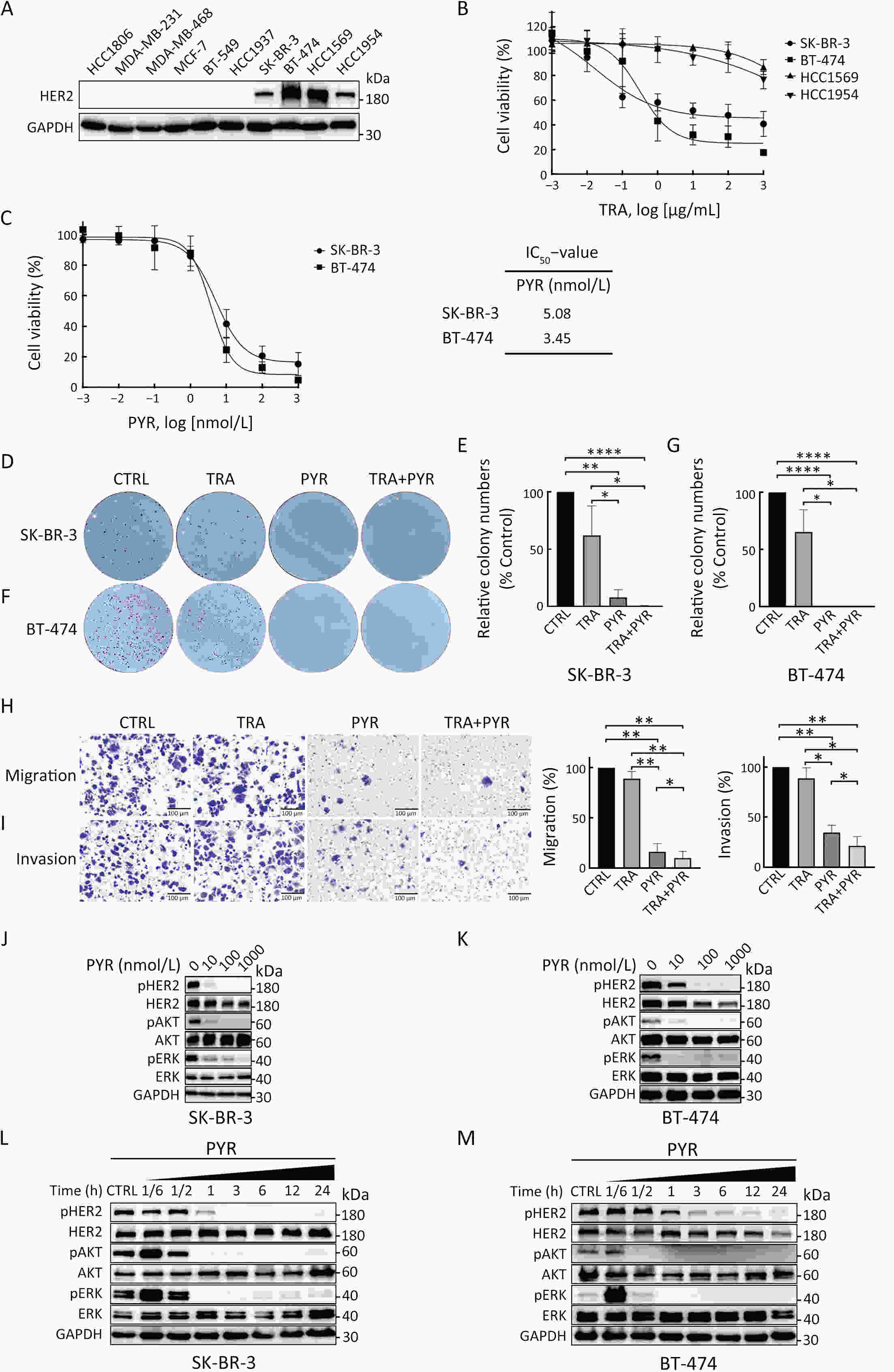
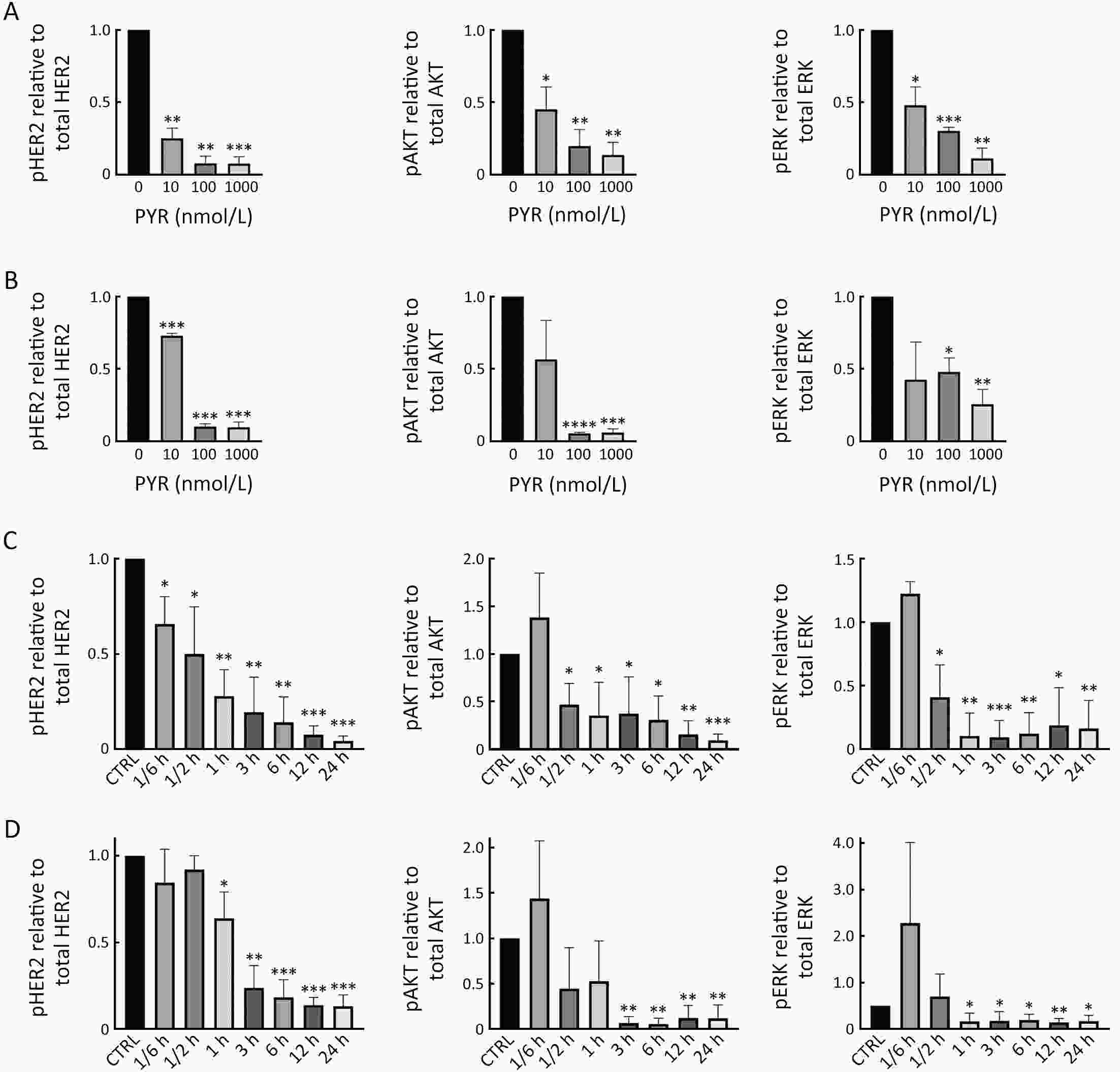
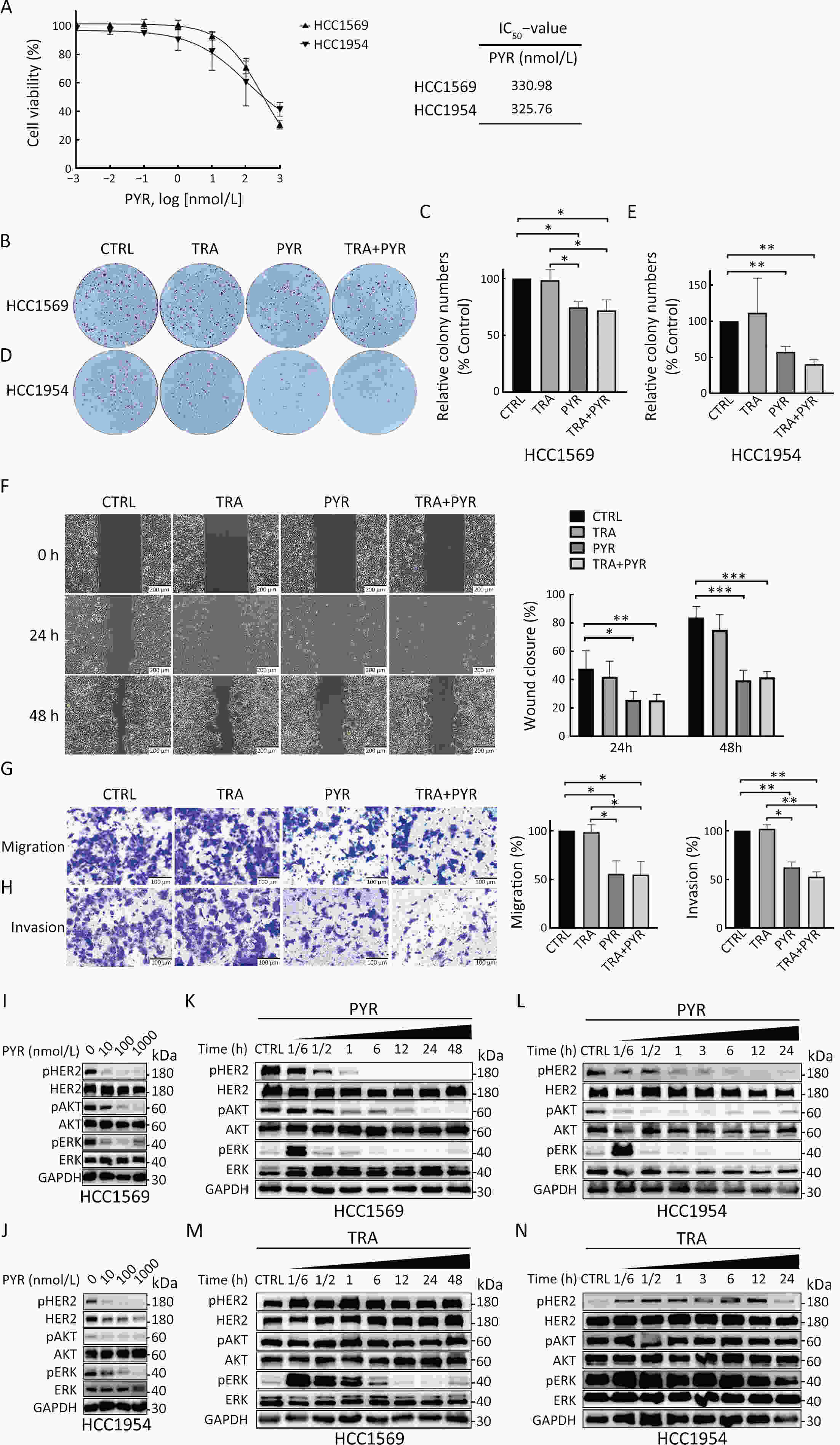
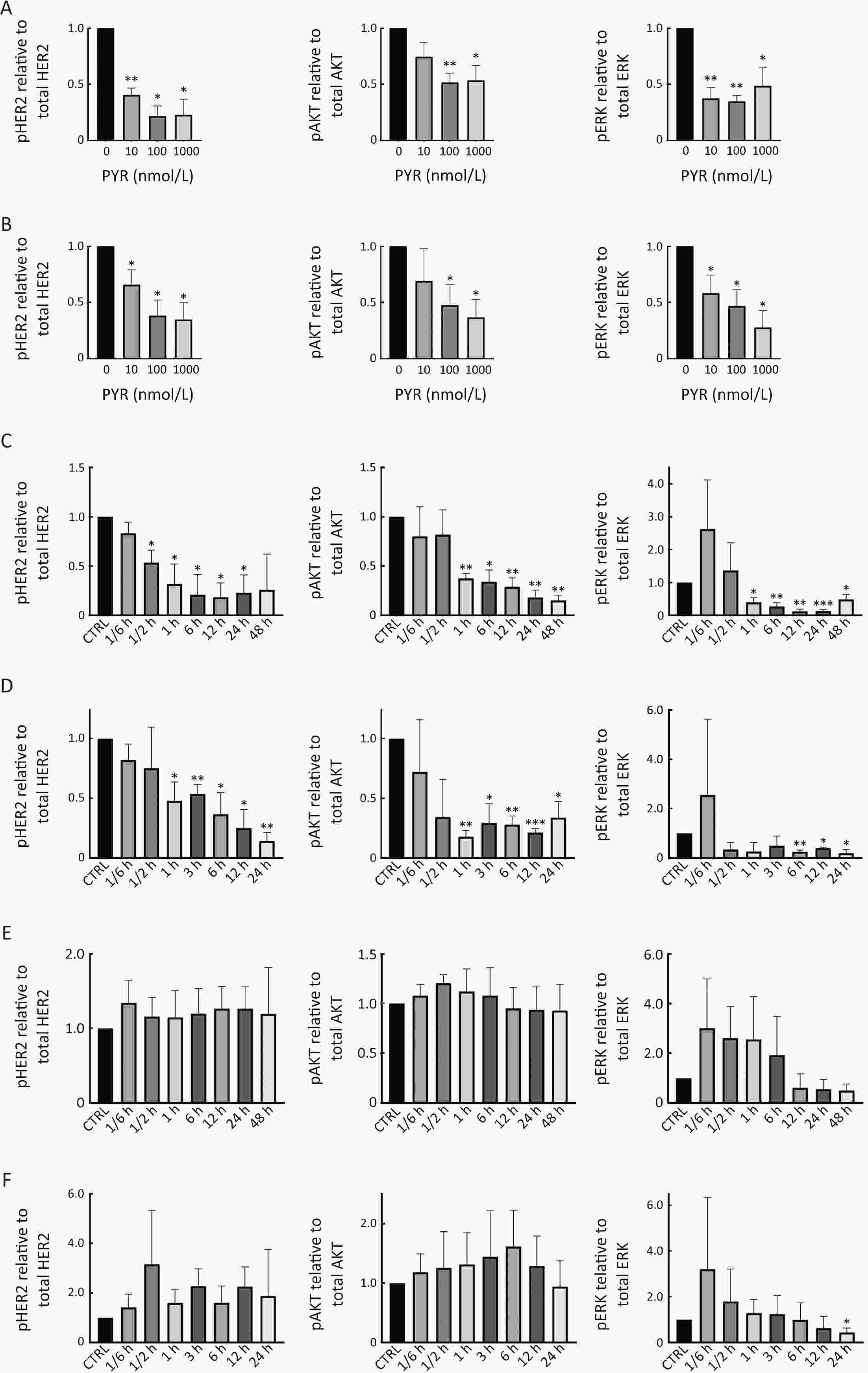
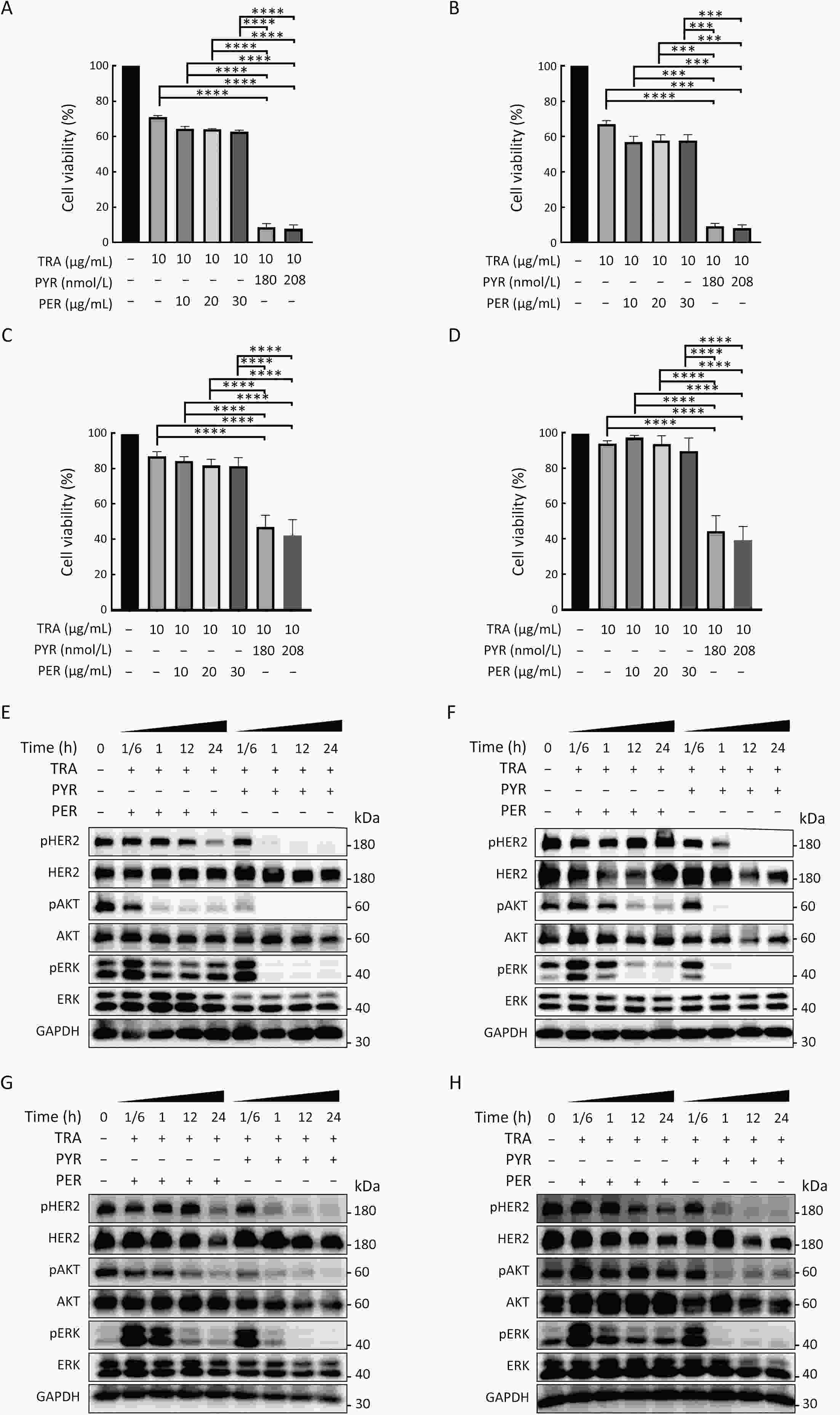
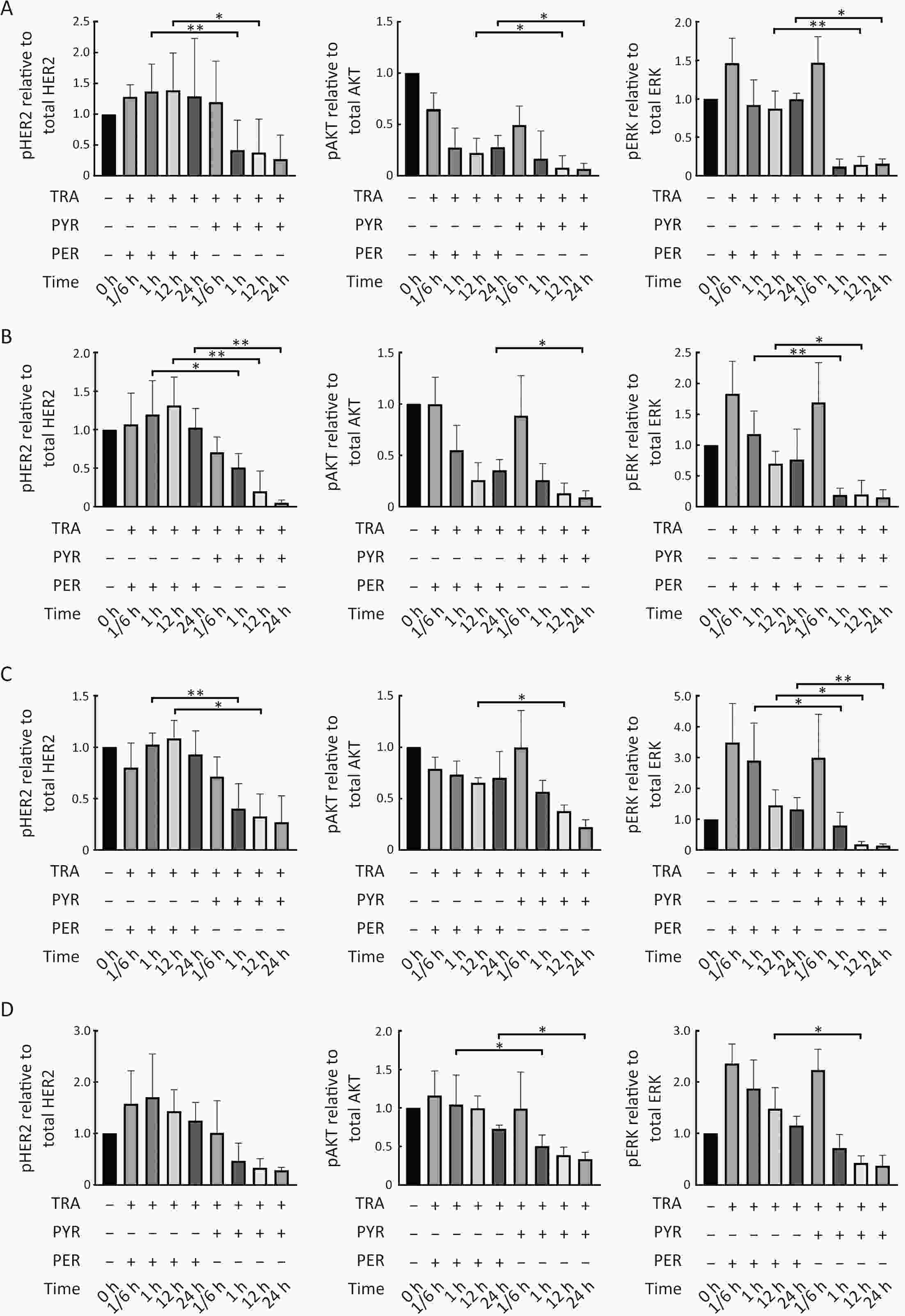
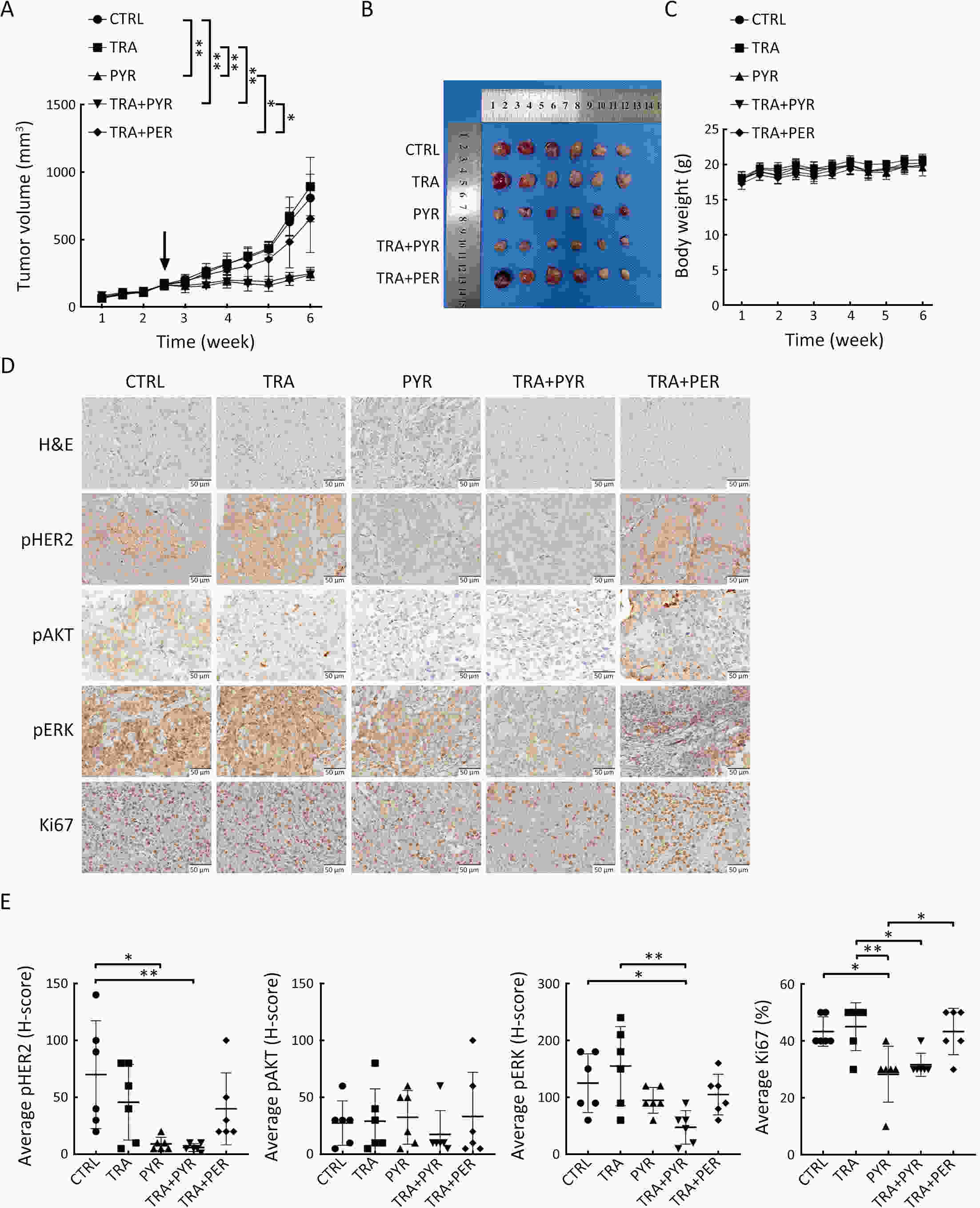
ObjectivePrimary resistance to trastuzumab frequently occurs in human epidermal growth factor receptor 2 (HER2)-positive (+) breast cancer patients and remains a clinical challenge. Pyrotinib is a novel tyrosine kinase inhibitor that has shown efficacy in the treatment of HER2+ breast cancer. However, the efficacy of pyrotinib in HER2+ breast cancer with primary trastuzumab resistance is unknown. MethodsHER2+ breast cancer cells sensitive or primarily resistant to trastuzumab were treated with trastuzumab, pyrotinib, or the combination. Cell proliferation, migration, invasion, and HER2 downstream signal pathways were analyzed. The effects of pyrotinib plus trastuzumab and pertuzumab plus trastuzumab were compared in breast cancer cells in vitro and a xenograft mouse model with primary resistance to trastuzumab. ResultsPyrotinib had a therapeutic effect on trastuzumab-sensitive HER2+ breast cancer cells by inhibiting phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and rat sarcoma virus (RAS)/rapidly accelerated fibrosarcoma (RAF)/mitogen-activated protein kinase (MAPK)/extracellular-signal regulated kinase (ERK) pathways. In primary trastuzumab-resistant cells, pyrotinib inhibited cell growth, migration, invasion, and HER2 downstream pathways, whereas trastuzumab had no effects. The combination with trastuzumab did not show increased effects compared with pyrotinib alone. Compared with pertuzumab plus trastuzumab, pyrotinib plus trastuzumab was more effective in inhibiting cell proliferation and HER2 downstream pathways in breast cancer cells and tumor growth in a trastuzumab-resistant HER2+ breast cancer xenograft model. ConclusionsPyrotinib-containing treatments exhibited anti-cancer effects in HER2+ breast cancer cells sensitive and with primary resistance to trastuzumab. Notably, pyrotinib plus trastuzumab was more effective than trastuzumab plus pertuzumab in inhibiting tumor growth and HER2 downstream pathways in HER2+ breast cancer with primary resistance to trastuzumab. These findings support clinical testing of the therapeutic efficacy of dual anti-HER2 treatment combining an intracellular small molecule with an extracellular antibody.
2024, 36(2): 138-150.
doi: 10.21147/j.issn.1000-9604.2024.02.04
Abstract:
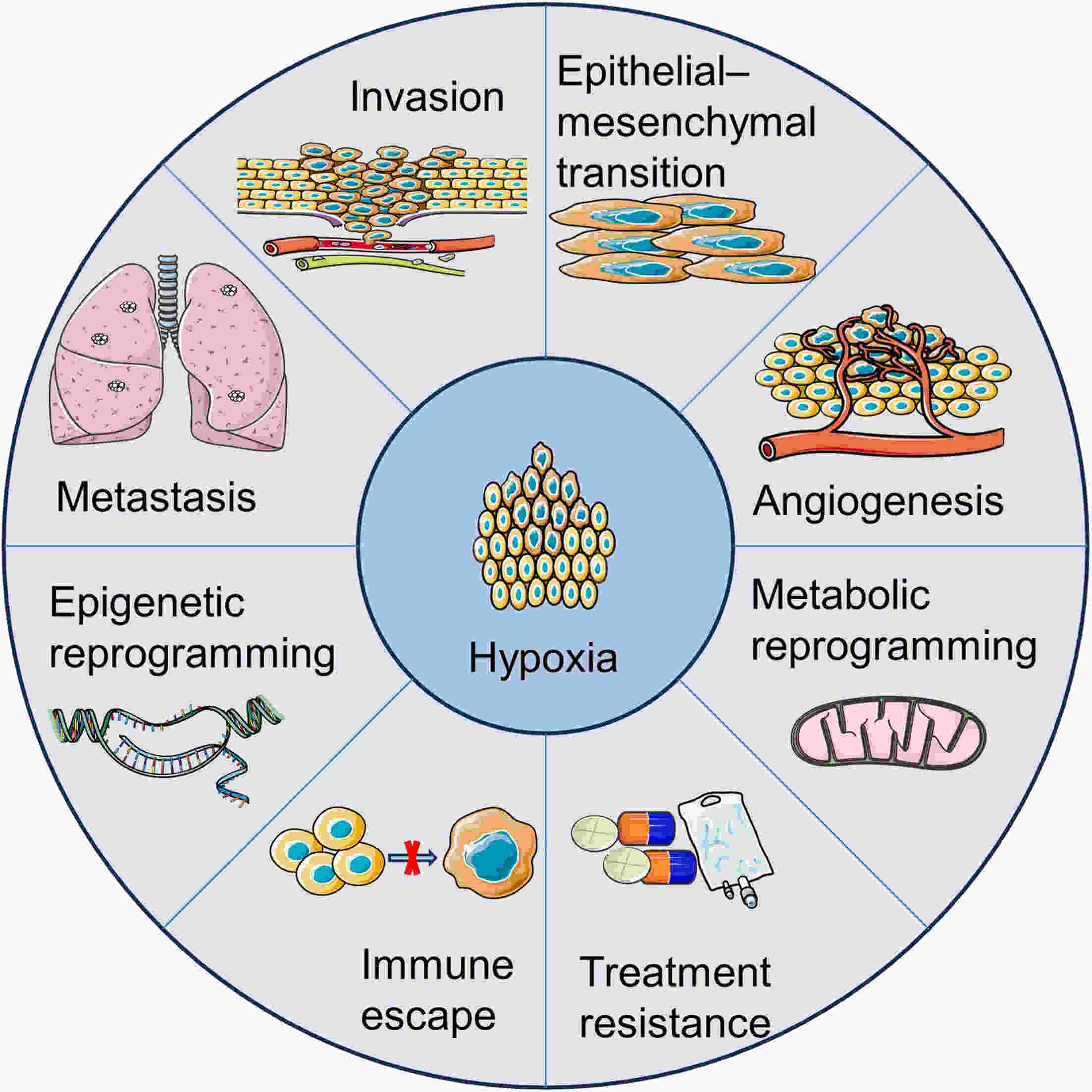
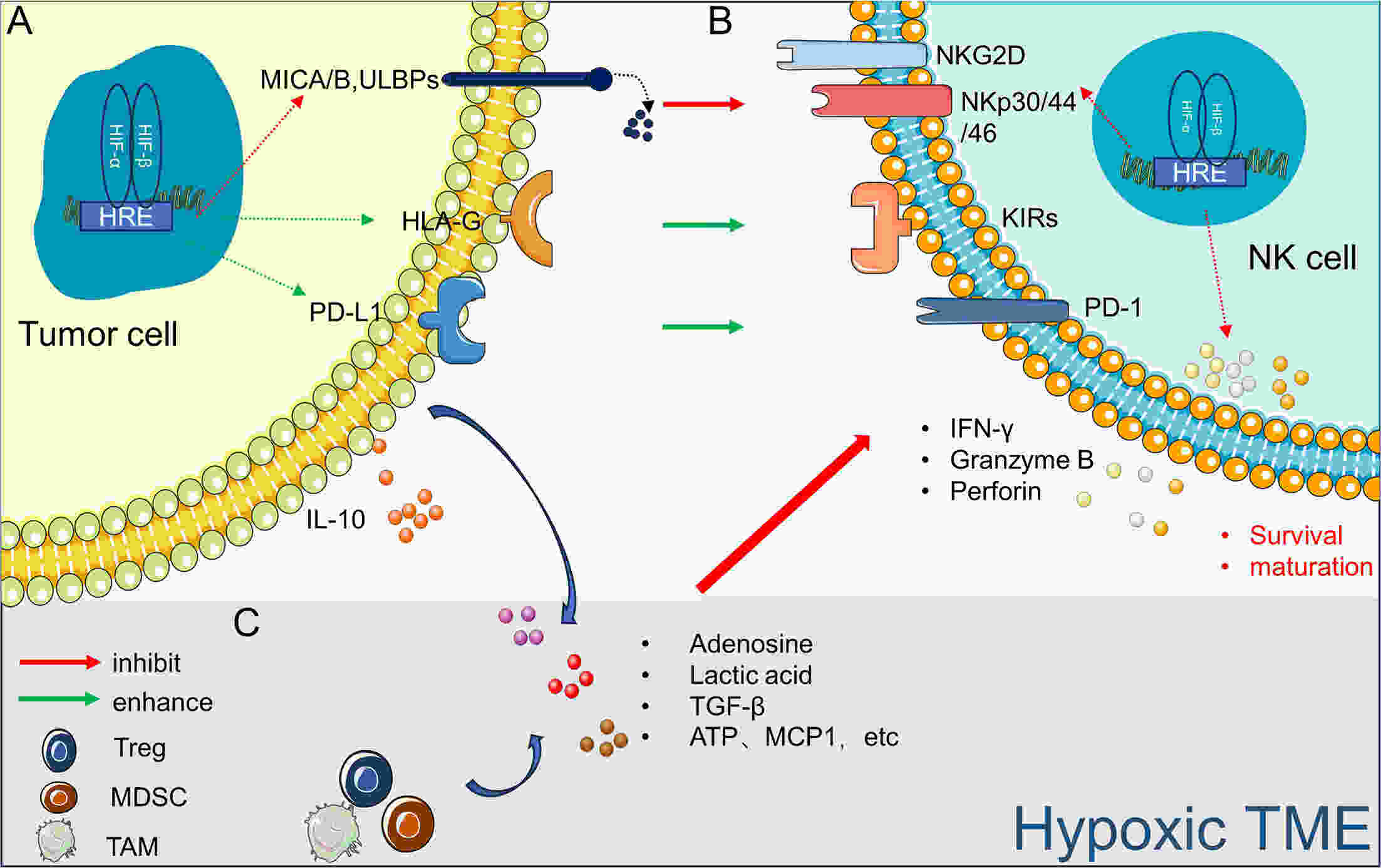
In recent years, immunotherapy has made remarkable progress in treating certain tumors and hematological malignancies. However, the efficacy of natural killer (NK) cells, which are an important subset of innate lymphocytes used in anticancer immunotherapy, remains limited. Hypoxia, a critical characteristic of the tumor microenvironment (TME), is involved in tumor development and resistance to radiotherapy, chemotherapy, and immunotherapy. Moreover, hypoxia contributes to the impairment of NK cell function and may be a significant factor that limits their therapeutic effects. Targeted hypoxia therapy has emerged as a promising research area for enhancing the efficacy of NK cell therapy. Therefore, understanding how the hypoxic TME influences NK cell function is crucial for improving antitumor treatment outcomes.
In recent years, immunotherapy has made remarkable progress in treating certain tumors and hematological malignancies. However, the efficacy of natural killer (NK) cells, which are an important subset of innate lymphocytes used in anticancer immunotherapy, remains limited. Hypoxia, a critical characteristic of the tumor microenvironment (TME), is involved in tumor development and resistance to radiotherapy, chemotherapy, and immunotherapy. Moreover, hypoxia contributes to the impairment of NK cell function and may be a significant factor that limits their therapeutic effects. Targeted hypoxia therapy has emerged as a promising research area for enhancing the efficacy of NK cell therapy. Therefore, understanding how the hypoxic TME influences NK cell function is crucial for improving antitumor treatment outcomes.
2024, 36(2): 151-166.
doi: 10.21147/j.issn.1000-9604.2024.02.05
Abstract:
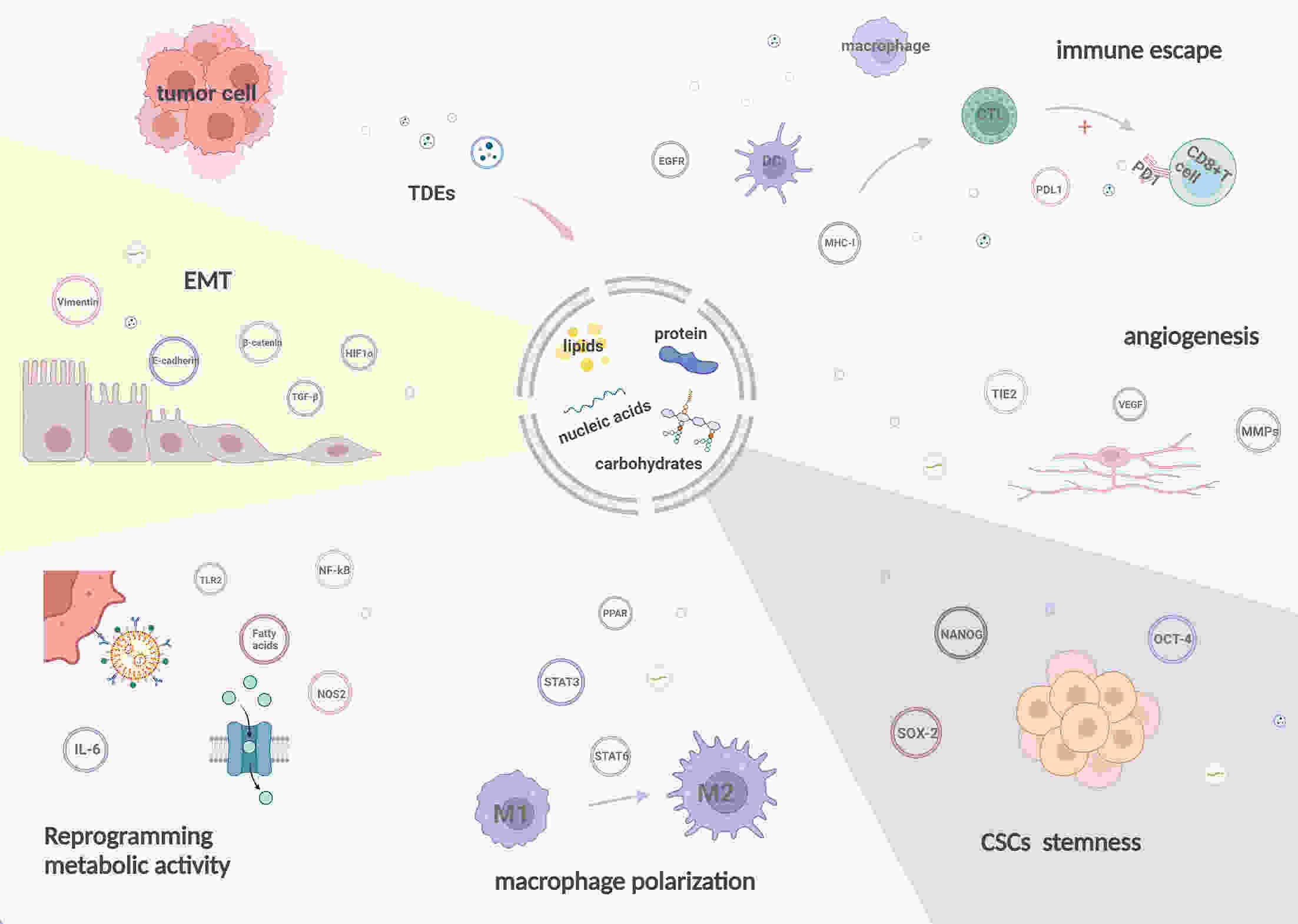
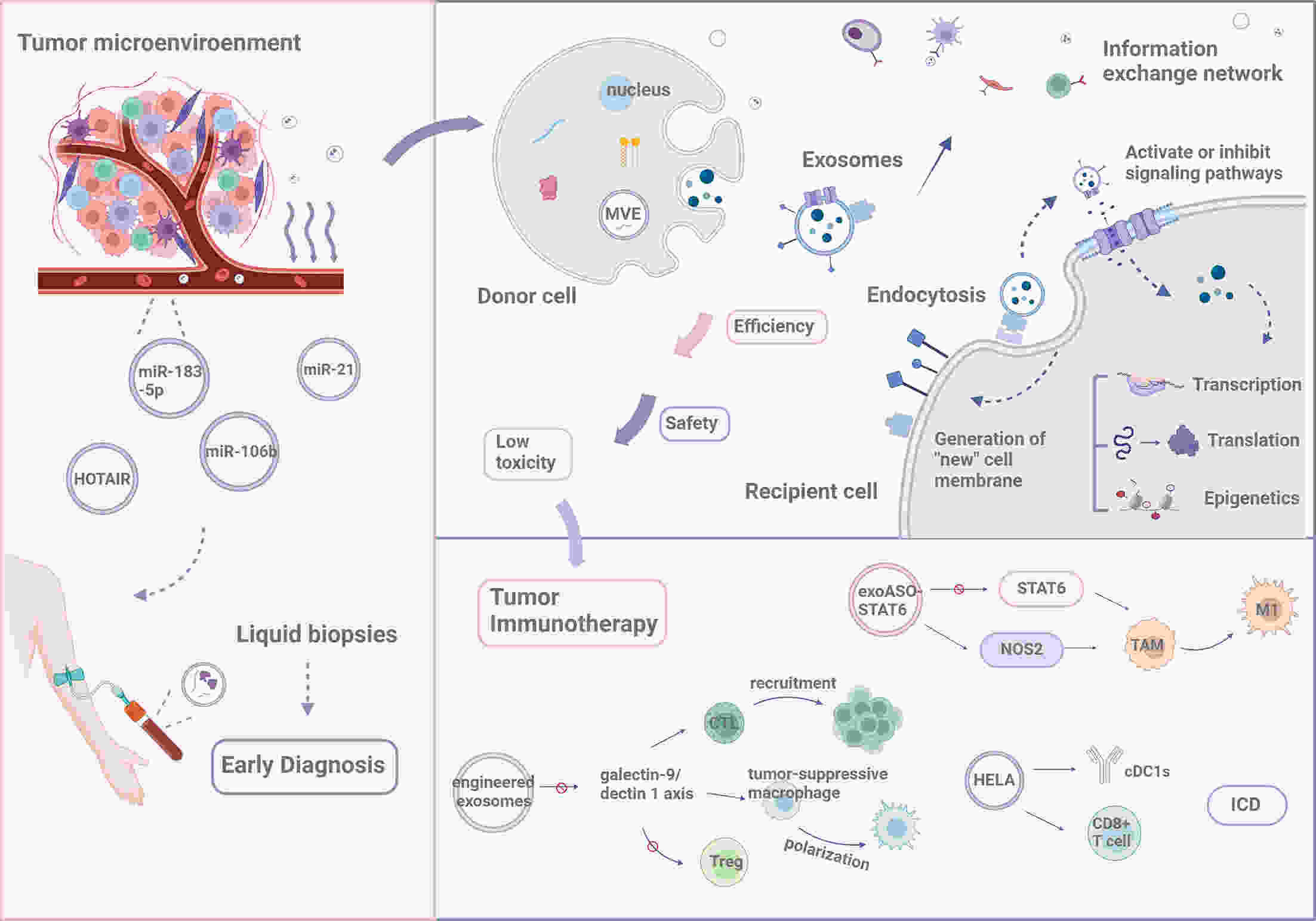
Throughout tumorigenesis, the co-evolution of tumor cells and their surrounding microenvironment leads to the development of malignant phenotypes. Cellular communication within the tumor microenvironment (TME) plays a critical role in influencing various aspects of tumor progression, including invasion and metastasis. The release of exosomes, a type of extracellular vesicle, by most cell types in the body, is an essential mediator of intercellular communication. A growing body of research indicates that tumor-derived exosomes (TDEs) significantly expedite tumor progression through multiple mechanisms, inducing epithelial-mesenchymal transition and macrophage polarization, enhancing angiogenesis, and aiding in the immune evasion of tumor cells. Herein, we describe the formation and characteristics of the TME, and summarize the contents of TDEs and their diverse functions in modulating tumor development. Furthermore, we explore potential applications of TDEs in tumor diagnosis and treatment.
Throughout tumorigenesis, the co-evolution of tumor cells and their surrounding microenvironment leads to the development of malignant phenotypes. Cellular communication within the tumor microenvironment (TME) plays a critical role in influencing various aspects of tumor progression, including invasion and metastasis. The release of exosomes, a type of extracellular vesicle, by most cell types in the body, is an essential mediator of intercellular communication. A growing body of research indicates that tumor-derived exosomes (TDEs) significantly expedite tumor progression through multiple mechanisms, inducing epithelial-mesenchymal transition and macrophage polarization, enhancing angiogenesis, and aiding in the immune evasion of tumor cells. Herein, we describe the formation and characteristics of the TME, and summarize the contents of TDEs and their diverse functions in modulating tumor development. Furthermore, we explore potential applications of TDEs in tumor diagnosis and treatment.
2024, 36(2): 167-194.
doi: 10.21147/j.issn.1000-9604.2024.02.06
Abstract:
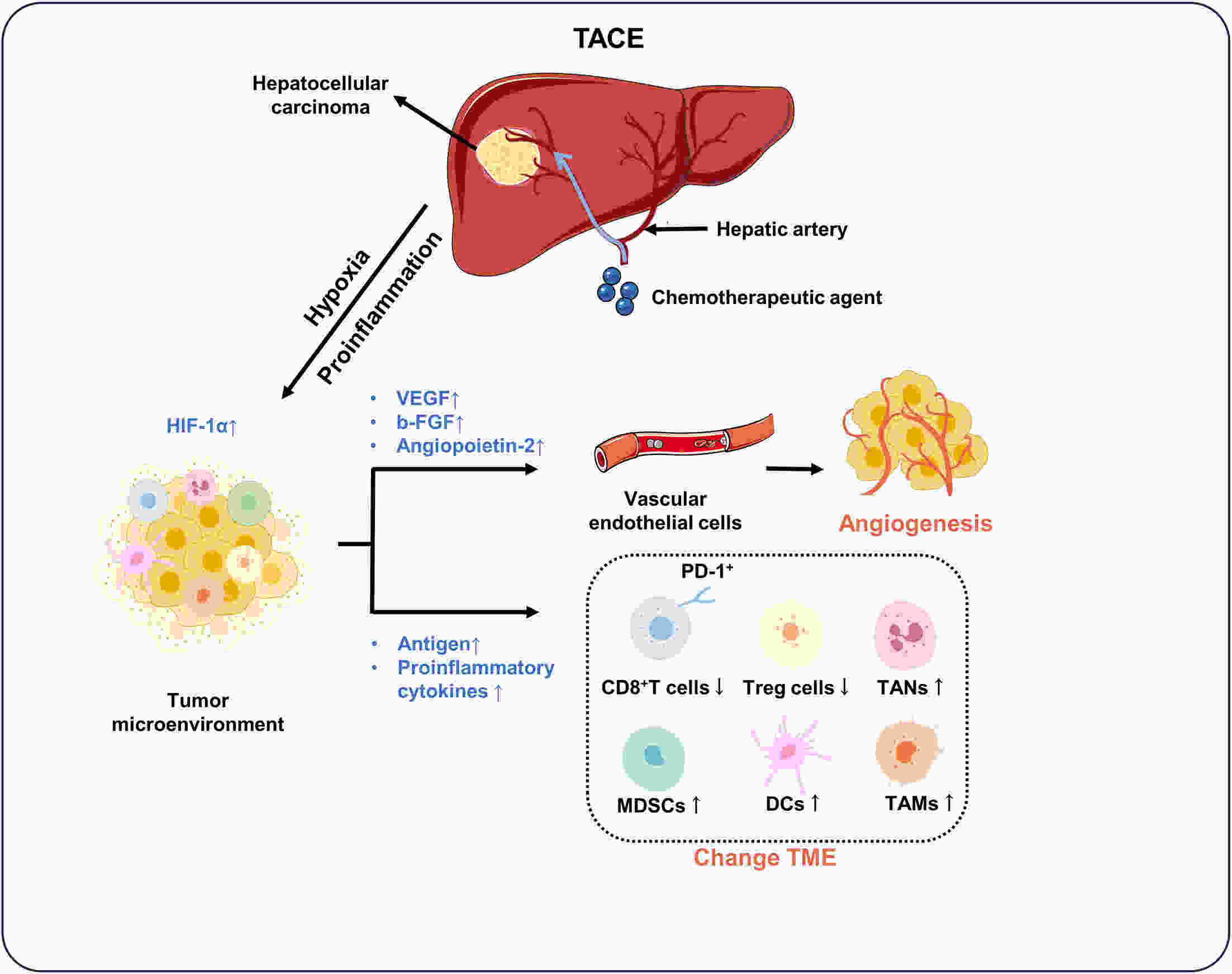
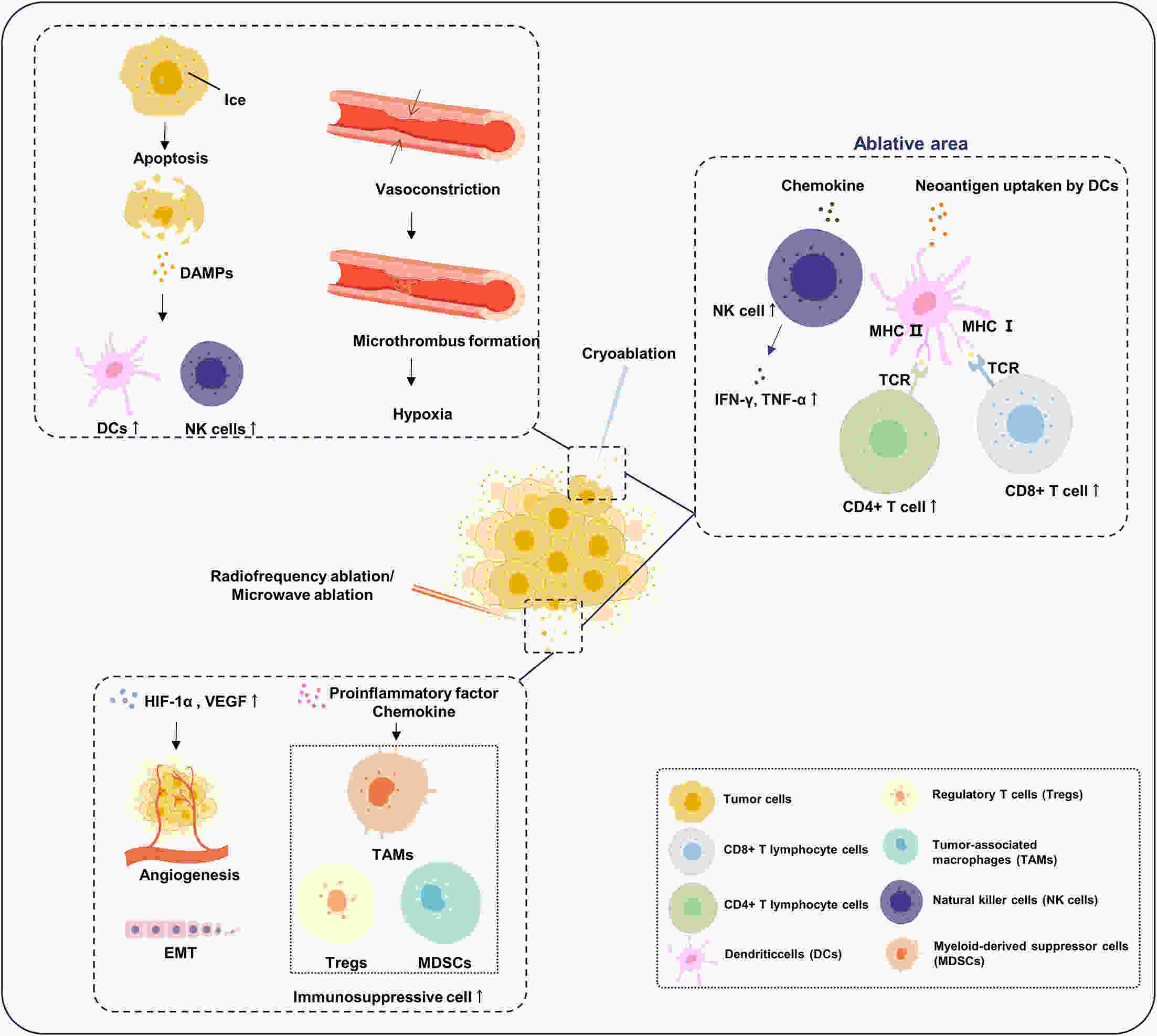
Hepatocellular carcinoma (HCC) is responsible for a significant number of cancer-related deaths worldwide and its incidence is increasing. Locoregional treatments, which are precision procedures guided by imaging to specifically target liver tumors, play a critical role in the management of a substantial portion of HCC cases. These therapies have become an essential element of the HCC treatment landscape, with transarterial chemoembolization (TACE) being the treatment of choice for patients with intermediate to advanced stages of the disease. Other locoregional therapies, like radiofrequency ablation, are highly effective for small, early-stage HCC. Nevertheless, the advent of targeted immunotherapy has challenged these established treatments. Tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) have shown remarkable efficacy in clinical settings. However, their specific uses and the development of resistance in subsequent treatments have led clinicians to reevaluate the future direction of HCC therapy. This review concentrates on the distinct features of both systemic and novel locoregional therapies. We investigate their effects on the tumor microenvironment at the molecular level and discuss how targeted immunotherapy can be effectively integrated with locoregional therapies. We also examine research findings from retrospective studies and randomized controlled trials on various combined treatment regimens, assessing their validity to determine the future evolution of locoregional therapies within the framework of personalized, comprehensive treatment.
Hepatocellular carcinoma (HCC) is responsible for a significant number of cancer-related deaths worldwide and its incidence is increasing. Locoregional treatments, which are precision procedures guided by imaging to specifically target liver tumors, play a critical role in the management of a substantial portion of HCC cases. These therapies have become an essential element of the HCC treatment landscape, with transarterial chemoembolization (TACE) being the treatment of choice for patients with intermediate to advanced stages of the disease. Other locoregional therapies, like radiofrequency ablation, are highly effective for small, early-stage HCC. Nevertheless, the advent of targeted immunotherapy has challenged these established treatments. Tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) have shown remarkable efficacy in clinical settings. However, their specific uses and the development of resistance in subsequent treatments have led clinicians to reevaluate the future direction of HCC therapy. This review concentrates on the distinct features of both systemic and novel locoregional therapies. We investigate their effects on the tumor microenvironment at the molecular level and discuss how targeted immunotherapy can be effectively integrated with locoregional therapies. We also examine research findings from retrospective studies and randomized controlled trials on various combined treatment regimens, assessing their validity to determine the future evolution of locoregional therapies within the framework of personalized, comprehensive treatment.
2024, 36(2): 195-214.
doi: 10.21147/j.issn.1000-9604.2024.02.07
Abstract:
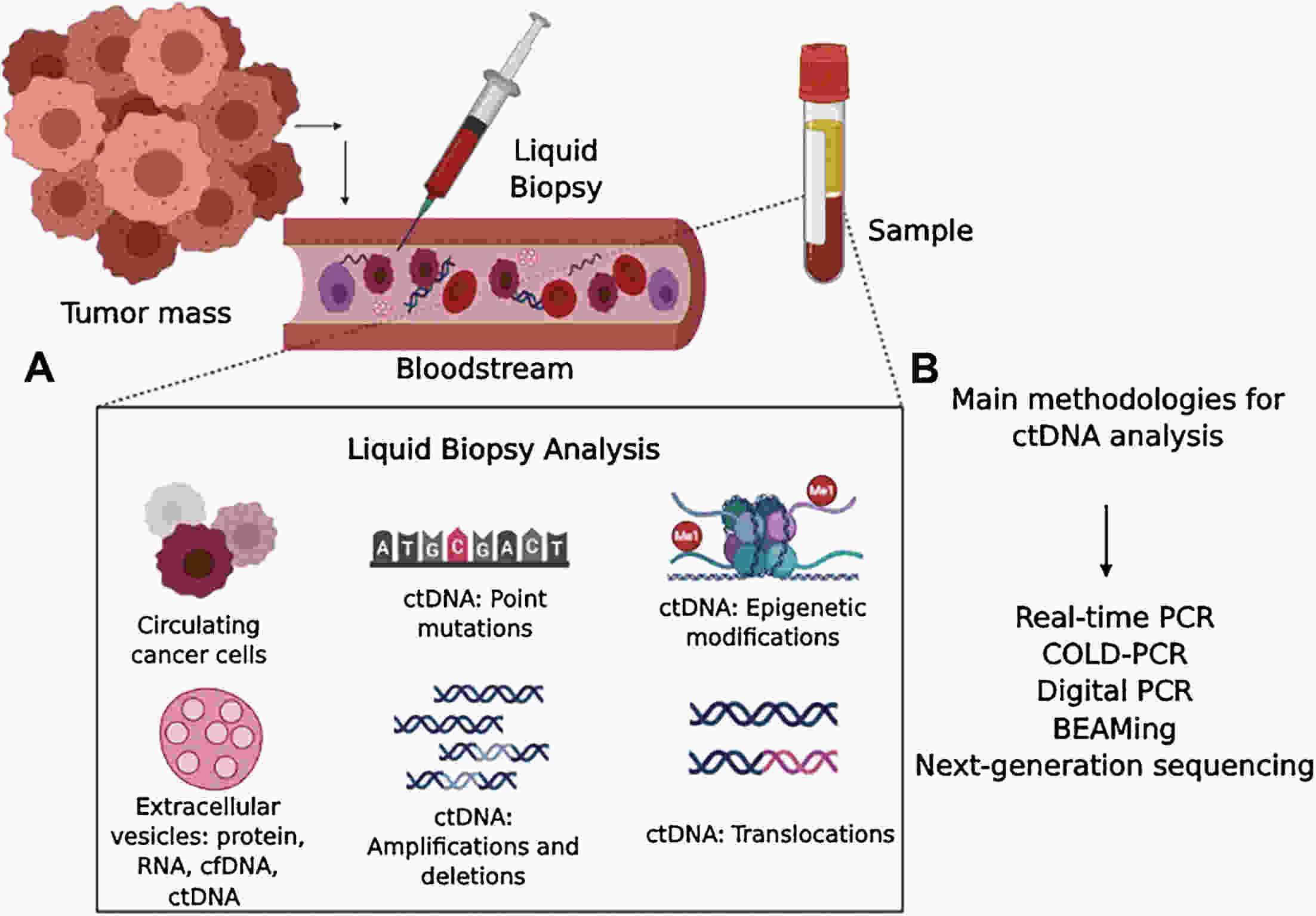
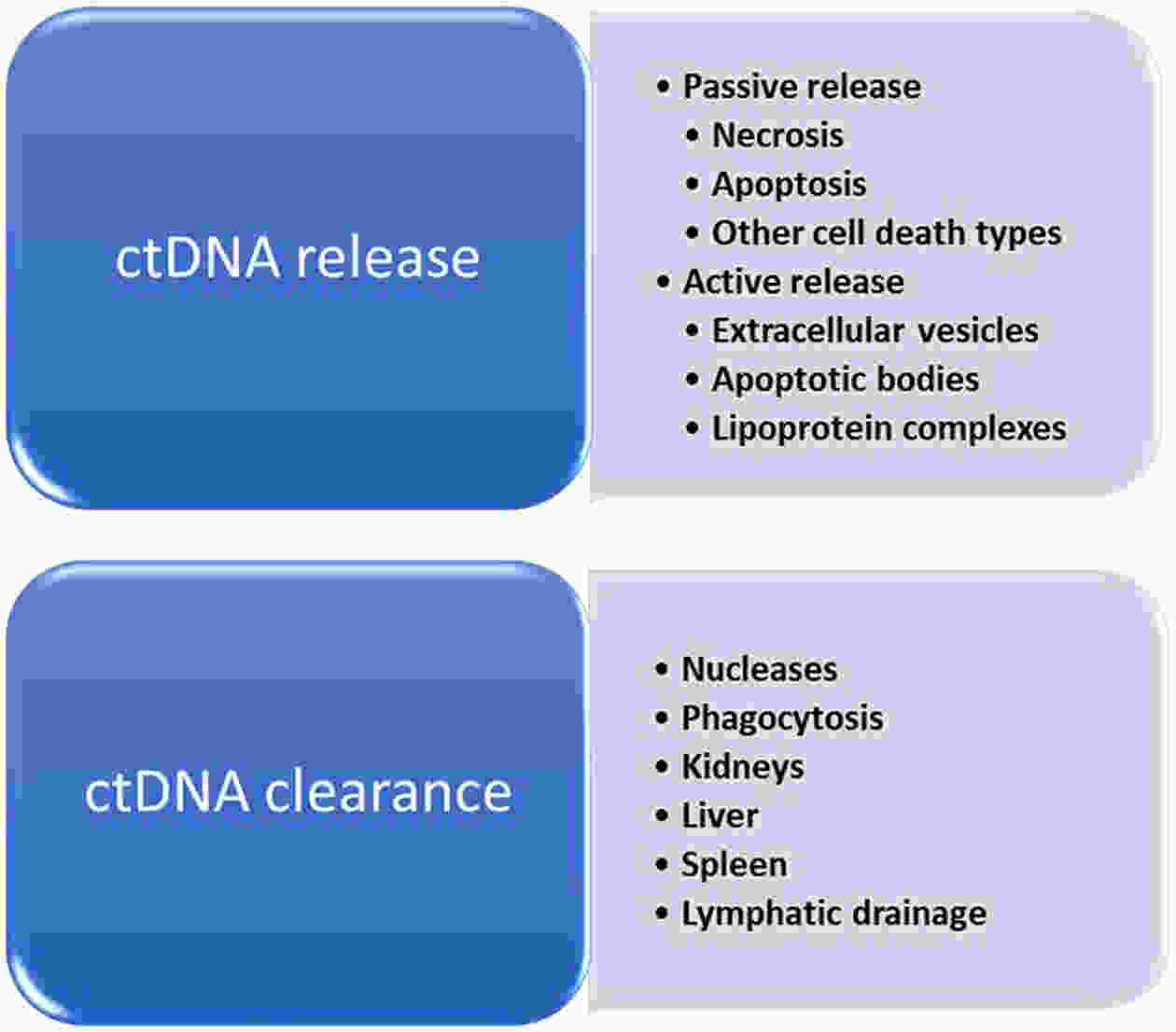
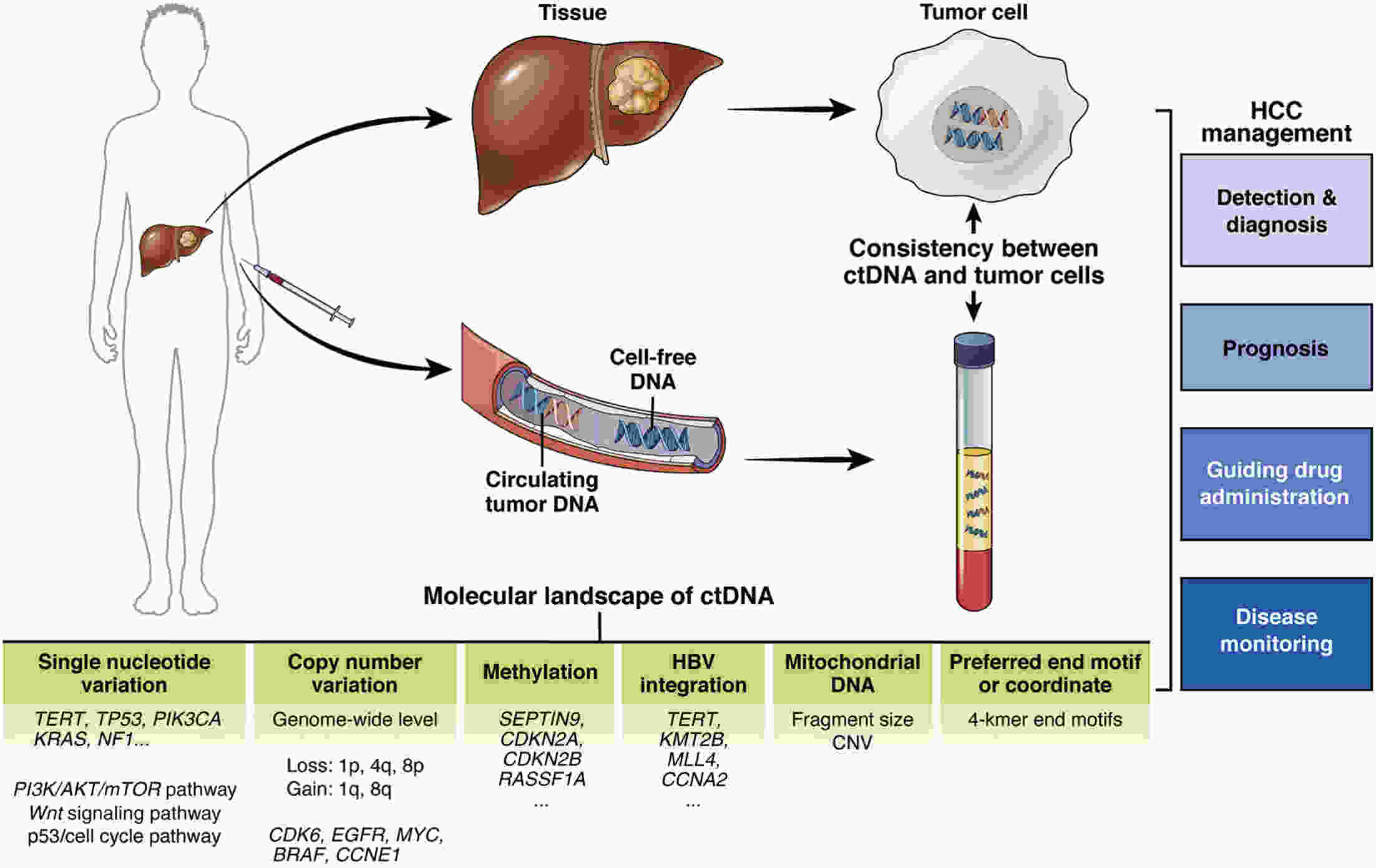
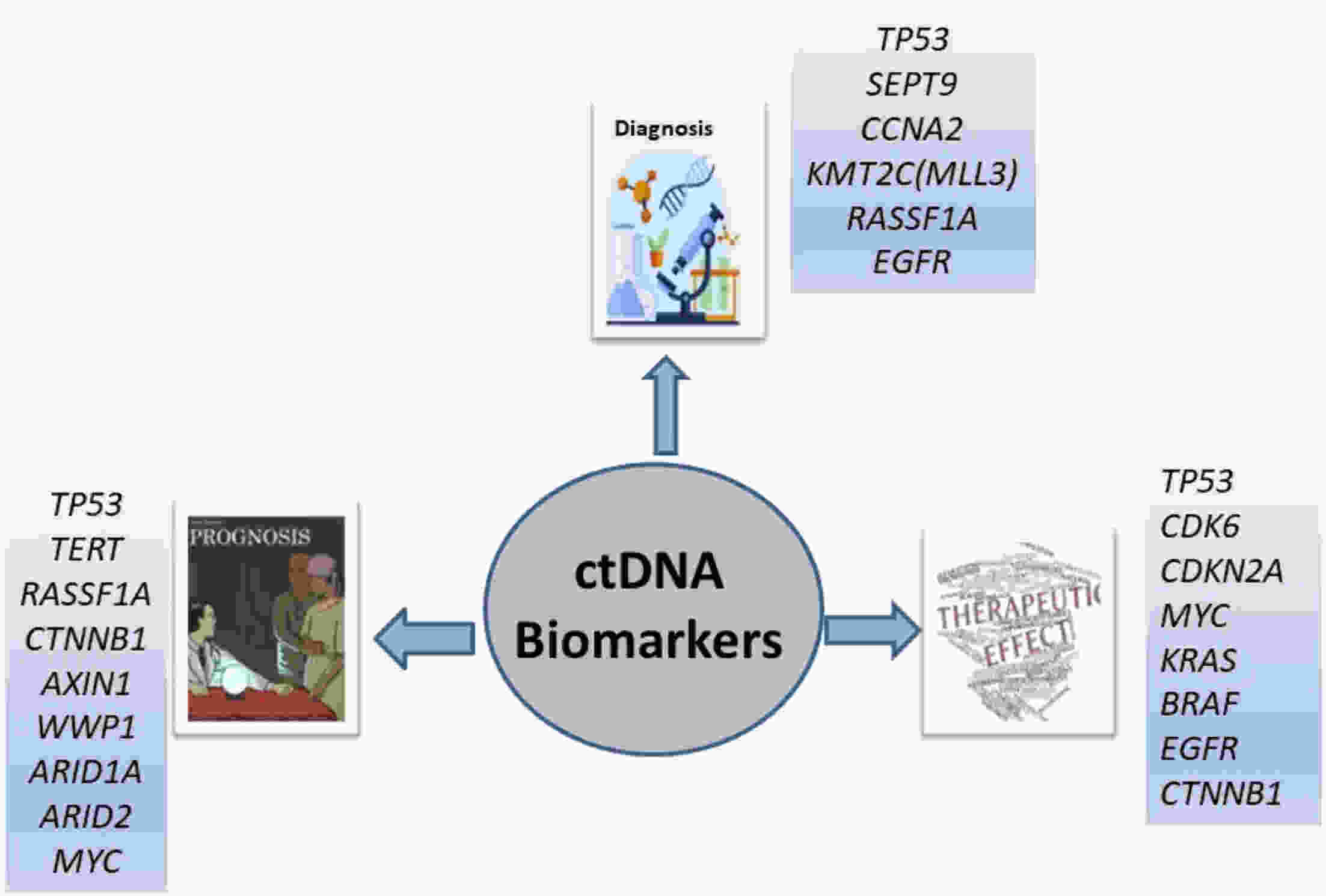
Hepatocellular carcinoma (HCC) is considered the fifth most prevalent cancer among all types of cancers and has the third most morbidity value. It has the most frequent duplication time and a high recurrence rate. Recently, the most unique technique used is liquid biopsies, which carry many markers; the most prominent is circulating tumor DNA (ctDNA). Varied methods are used to investigate ctDNA, including various forms of polymerase chain reaction (PCR) [emulsion PCR (ePCR), digital PCR (dPCR), and bead, emulsion, amplification, magnetic (BEAMing) PCR]. Hence ctDNA is being recognized as a potential biomarker that permits early cancer detection, treatment monitoring, and predictive data on tumor burden are subjective to therapy or surgery. Numerous ctDNA biomarkers have been investigated based on their alterations such as 1) single nucleotide variations (either insertion or deletion of a nucleotide) markers including TP53, KRAS, and CCND1; 2) copy number variations which include markers such as CDK6, EFGR, MYC and BRAF; 3) DNA methylation (RASSF1A, SEPT9, KMT2C and CCNA2); 4) homozygous mutation includes ctDNA markers as CDKN2A, AXIN1; and 5) gain or loss of function of the genes, particularly for HCC. Various researchers have conducted many studies and gotten fruitful results. Still, there are some drawbacks to ctDNA namely low quantity, fragment heterogeneity, less stability, limited mutant copies and standards, and differential sensitivity. However, plenty of investigations demonstrate ctDNA’s significance as a polyvalent biomarker for cancer and can be viewed as a future diagnostic, prognostic and therapeutic agent. This article overviews many conditions in genetic changes linked to the onset and development of HCC, such as dysregulated signaling pathways, somatic mutations, single-nucleotide polymorphisms, and genomic instability. Additionally, efforts are also made to develop treatments for HCC that are molecularly targeted and to unravel some of the genetic pathways that facilitate its early identification.
Hepatocellular carcinoma (HCC) is considered the fifth most prevalent cancer among all types of cancers and has the third most morbidity value. It has the most frequent duplication time and a high recurrence rate. Recently, the most unique technique used is liquid biopsies, which carry many markers; the most prominent is circulating tumor DNA (ctDNA). Varied methods are used to investigate ctDNA, including various forms of polymerase chain reaction (PCR) [emulsion PCR (ePCR), digital PCR (dPCR), and bead, emulsion, amplification, magnetic (BEAMing) PCR]. Hence ctDNA is being recognized as a potential biomarker that permits early cancer detection, treatment monitoring, and predictive data on tumor burden are subjective to therapy or surgery. Numerous ctDNA biomarkers have been investigated based on their alterations such as 1) single nucleotide variations (either insertion or deletion of a nucleotide) markers including TP53, KRAS, and CCND1; 2) copy number variations which include markers such as CDK6, EFGR, MYC and BRAF; 3) DNA methylation (RASSF1A, SEPT9, KMT2C and CCNA2); 4) homozygous mutation includes ctDNA markers as CDKN2A, AXIN1; and 5) gain or loss of function of the genes, particularly for HCC. Various researchers have conducted many studies and gotten fruitful results. Still, there are some drawbacks to ctDNA namely low quantity, fragment heterogeneity, less stability, limited mutant copies and standards, and differential sensitivity. However, plenty of investigations demonstrate ctDNA’s significance as a polyvalent biomarker for cancer and can be viewed as a future diagnostic, prognostic and therapeutic agent. This article overviews many conditions in genetic changes linked to the onset and development of HCC, such as dysregulated signaling pathways, somatic mutations, single-nucleotide polymorphisms, and genomic instability. Additionally, efforts are also made to develop treatments for HCC that are molecularly targeted and to unravel some of the genetic pathways that facilitate its early identification.
2024, 36(2): 215-225.
doi: 10.21147/j.issn.1000-9604.2024.02.08
Abstract:
With the continuous improvement of systemic treatment, reasonable local regional control of early-stage breast cancer can be translated into survival benefits. The optimization of regional nodal management in patients with limited sentinel lymph node (SLN) metastasis needs to be weighed by surgical complications, regional recurrence risk, and lymph node status, as well as other escalating treatment (systemic/radiotherapy) that may result from de-escalating surgery. With the effective support and supplementation of systemic therapy and radiotherapy, the management of axillary surgery is developing in a de-escalating trend. The widespread application of neoadjuvant therapy has contributed to optimizing the management of patients with clinically node-negative/imaging node-positive disease. In clinical practice, it is necessary to consider the residual tumor burden of regional lymph nodes when formulating the optimal irradiation fields in patients with limited positive SLN without axillary lymph node dissection. The combined application of genomic tests and American College of Surgeons Oncology Group Z0011/AMAROS criteria could provide patients with a better strategy of dual de-escalation treatment, which includes the de-escalation of both axillary surgery and systemic treatment. In the era of sentinel lymph node biopsy (SLNB), the regional nodal management of breast cancer should adhere to the concept of “updating ideas, making bold assumptions, and carefully seeking proof”, make full use of the benefits of systemic therapy and radiotherapy to reduce the scope of surgery and complications, and expand the “net benefit” of efficacy and quality of life. This review discusses the optimization of regional nodal management in the era of SLNB, in order to provide reference information for clinicians.
With the continuous improvement of systemic treatment, reasonable local regional control of early-stage breast cancer can be translated into survival benefits. The optimization of regional nodal management in patients with limited sentinel lymph node (SLN) metastasis needs to be weighed by surgical complications, regional recurrence risk, and lymph node status, as well as other escalating treatment (systemic/radiotherapy) that may result from de-escalating surgery. With the effective support and supplementation of systemic therapy and radiotherapy, the management of axillary surgery is developing in a de-escalating trend. The widespread application of neoadjuvant therapy has contributed to optimizing the management of patients with clinically node-negative/imaging node-positive disease. In clinical practice, it is necessary to consider the residual tumor burden of regional lymph nodes when formulating the optimal irradiation fields in patients with limited positive SLN without axillary lymph node dissection. The combined application of genomic tests and American College of Surgeons Oncology Group Z0011/AMAROS criteria could provide patients with a better strategy of dual de-escalation treatment, which includes the de-escalation of both axillary surgery and systemic treatment. In the era of sentinel lymph node biopsy (SLNB), the regional nodal management of breast cancer should adhere to the concept of “updating ideas, making bold assumptions, and carefully seeking proof”, make full use of the benefits of systemic therapy and radiotherapy to reduce the scope of surgery and complications, and expand the “net benefit” of efficacy and quality of life. This review discusses the optimization of regional nodal management in the era of SLNB, in order to provide reference information for clinicians.
2024, 36(2): 226-232.
doi: 10.21147/j.issn.1000-9604.2024.02.09
Abstract:
Colorectal cancer has a high incidence and mortality rate in China, with the majority of cases being middle and low rectal cancer. Surgical intervention is currently the main treatment modality for locally advanced rectal cancer, with the common goal of improving oncological outcomes while preserving function. The controversy regarding the circumferential resection margin distance in rectal cancer surgery has been resolved. With the promotion of neoadjuvant therapy concepts and advancements in technology, treatment strategies have become more diverse. Following tumor downstaging, there is an increasing trend towards extending the safe distance of distal rectal margin. This provides more opportunities for patients with low rectal cancer to preserve their anal function. However, there is currently no consensus on the specific distance of distal resection margin.
Colorectal cancer has a high incidence and mortality rate in China, with the majority of cases being middle and low rectal cancer. Surgical intervention is currently the main treatment modality for locally advanced rectal cancer, with the common goal of improving oncological outcomes while preserving function. The controversy regarding the circumferential resection margin distance in rectal cancer surgery has been resolved. With the promotion of neoadjuvant therapy concepts and advancements in technology, treatment strategies have become more diverse. Following tumor downstaging, there is an increasing trend towards extending the safe distance of distal rectal margin. This provides more opportunities for patients with low rectal cancer to preserve their anal function. However, there is currently no consensus on the specific distance of distal resection margin.

 Abstract
Abstract FullText HTML
FullText HTML PDF 3307KB
PDF 3307KB