2024 Vol.36(3)
Display Mode: |
2024, 36(3): 233-239.
doi: 10.21147/j.issn.1000-9604.2024.03.01
Abstract:
2024, 36(3): 240-256.
doi: 10.21147/j.issn.1000-9604.2024.03.02
Abstract:
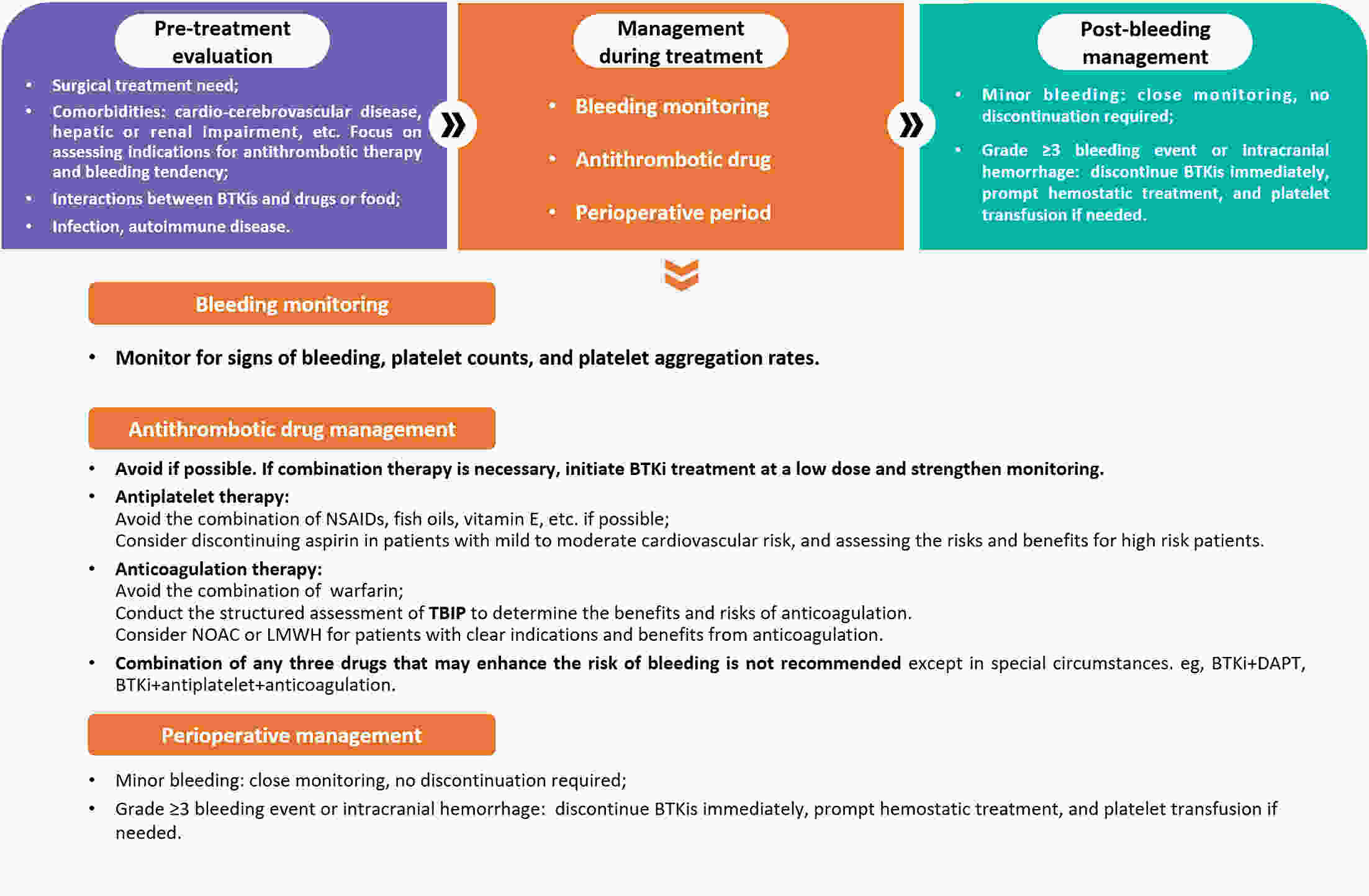
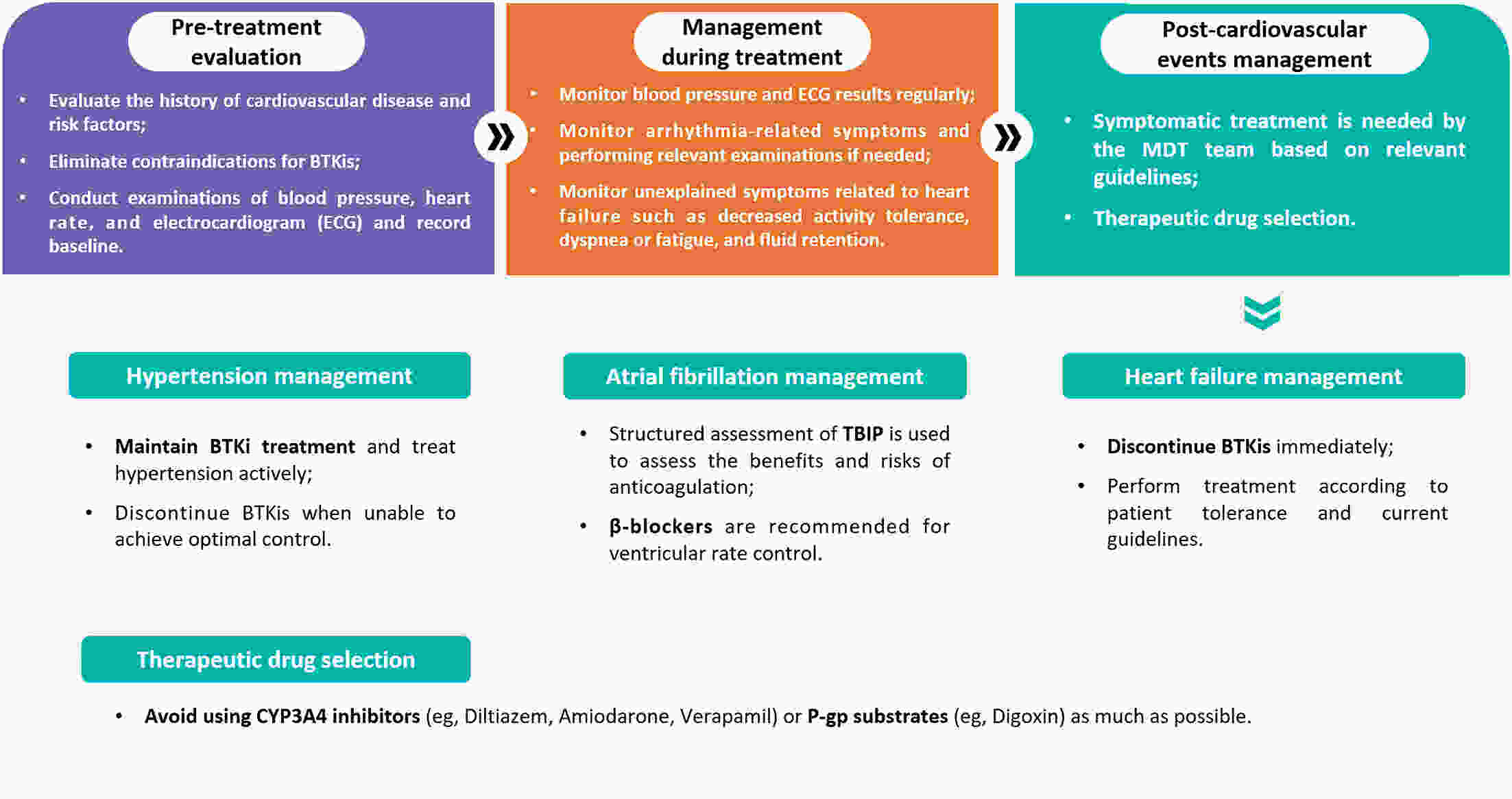
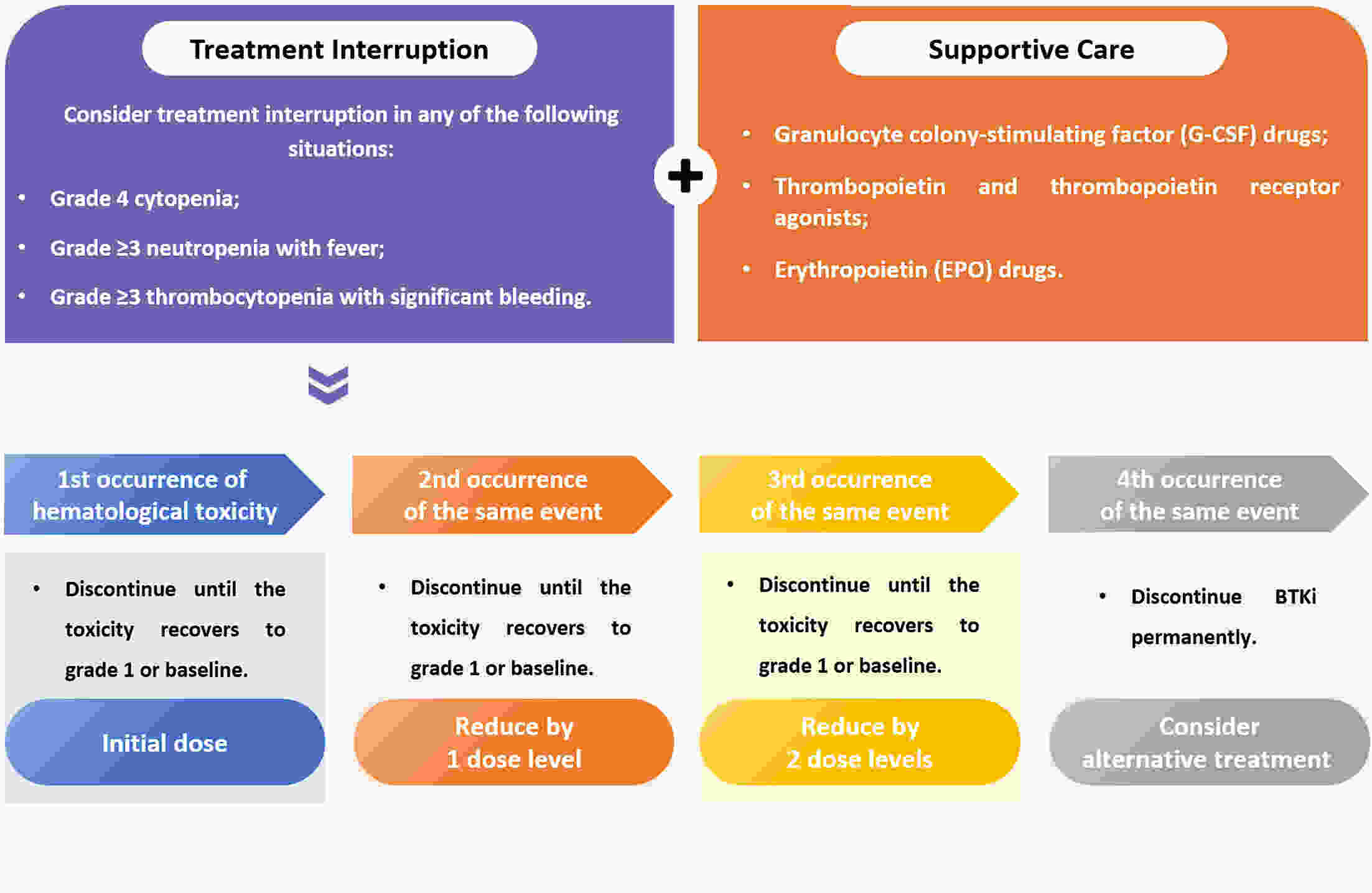
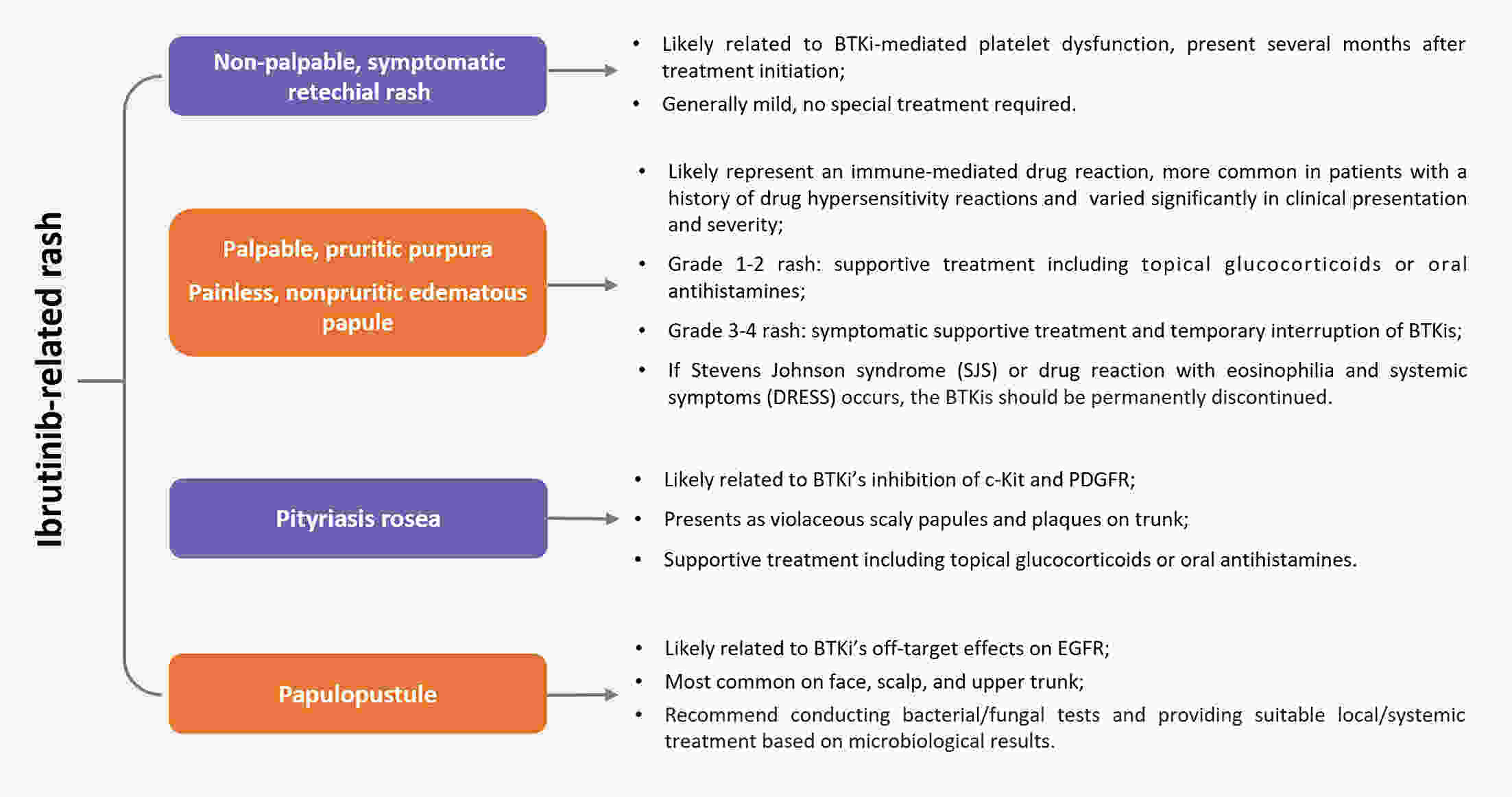
Bruton’s tyrosine kinase inhibitors (BTKis) have revolutionized the treatment of B-cell lymphomas. However, safety issues related to the use of BTKis may hinder treatment continuity and further affect clinical efficacy. A comprehensive and systematic expert consensus from a pharmacological perspective is lacking for safety issues associated with BTKi treatment. A multidisciplinary consensus working group was established, comprising 35 members from the fields of hematology, cardiovascular disease, cardio-oncology, clinical pharmacy, and evidence-based medicine. This evidence-based expert consensus was formulated using an evidence-based approach and the Delphi method. The Joanna Briggs Institute Critical Appraisal (JBI) tool and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach were used to rate the quality of evidence and grade the strength of recommendations, respectively. This consensus provides practical recommendations for BTKis medication based on nine aspects within three domains, including the management of common adverse drug events such as bleeding, cardiovascular events, and hematological toxicity, as well as the management of drug-drug interactions and guidance for special populations. This multidisciplinary expert consensus could contribute to promoting a multi-dimensional, comprehensive and standardized management of BTKis.
Bruton’s tyrosine kinase inhibitors (BTKis) have revolutionized the treatment of B-cell lymphomas. However, safety issues related to the use of BTKis may hinder treatment continuity and further affect clinical efficacy. A comprehensive and systematic expert consensus from a pharmacological perspective is lacking for safety issues associated with BTKi treatment. A multidisciplinary consensus working group was established, comprising 35 members from the fields of hematology, cardiovascular disease, cardio-oncology, clinical pharmacy, and evidence-based medicine. This evidence-based expert consensus was formulated using an evidence-based approach and the Delphi method. The Joanna Briggs Institute Critical Appraisal (JBI) tool and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach were used to rate the quality of evidence and grade the strength of recommendations, respectively. This consensus provides practical recommendations for BTKis medication based on nine aspects within three domains, including the management of common adverse drug events such as bleeding, cardiovascular events, and hematological toxicity, as well as the management of drug-drug interactions and guidance for special populations. This multidisciplinary expert consensus could contribute to promoting a multi-dimensional, comprehensive and standardized management of BTKis.
2024, 36(3): 257-269.
doi: 10.21147/j.issn.1000-9604.2024.03.03
Abstract:
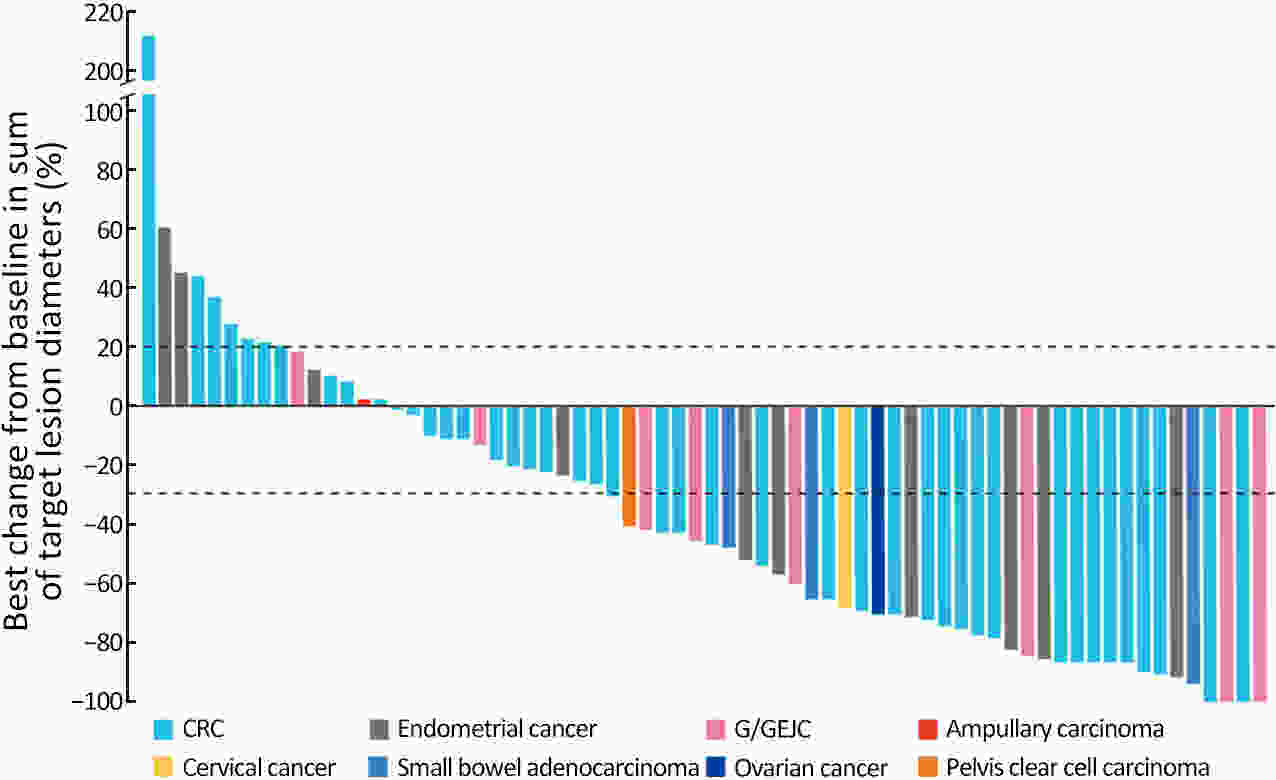
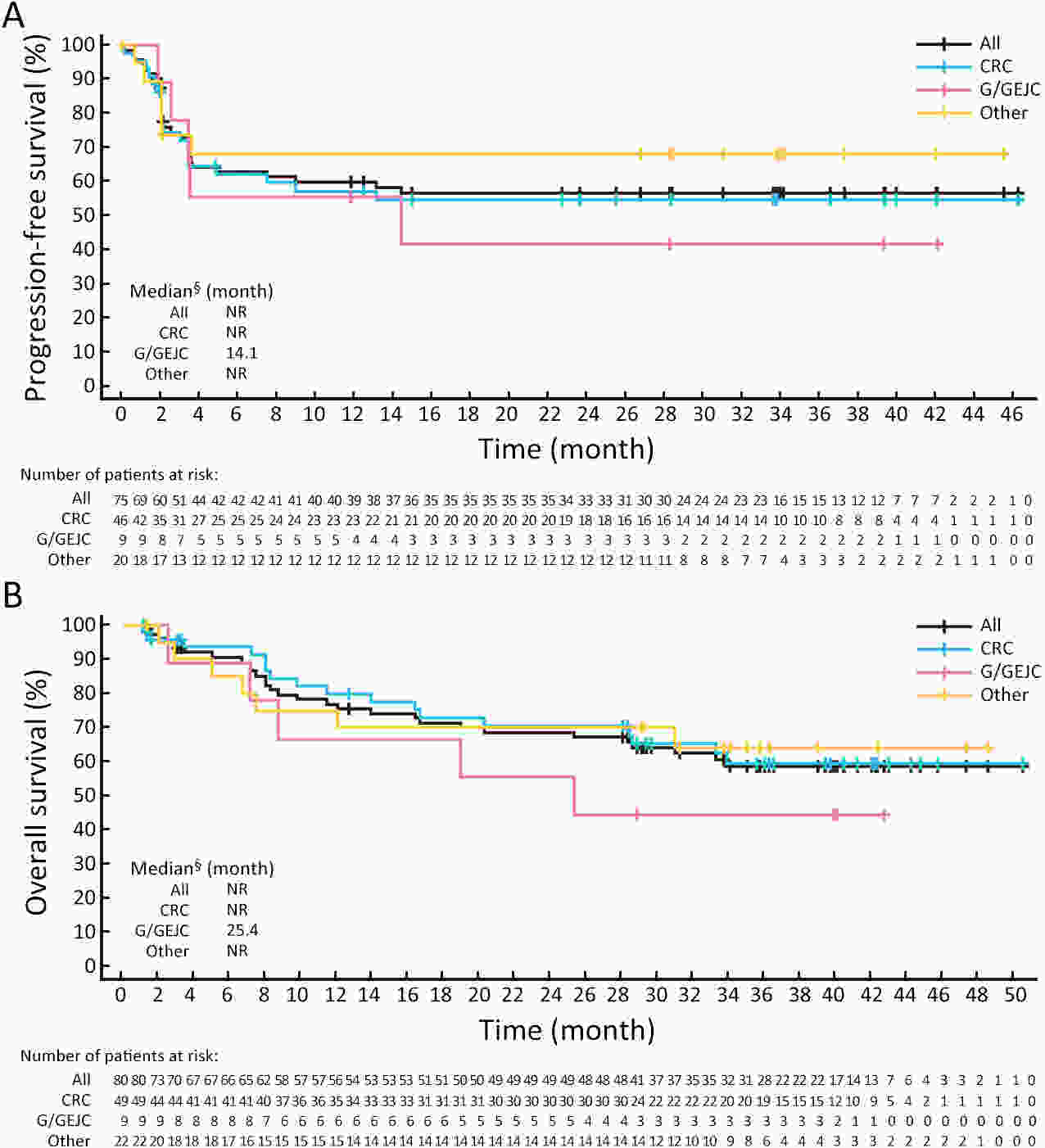
ObjectiveThe open-label, phase II RATIONALE-209 study evaluated tislelizumab (anti-programmed cell death protein 1 antibody) as a tissue-agnostic monotherapy for microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR) tumors. MethodsAdults with previously treated, locally advanced unresectable or metastatic MSI-H/dMMR solid tumors were enrolled. Patients received tislelizumab 200 mg intravenously every 3 weeks. Objective response rate (ORR; primary endpoint), duration of response (DoR), and progression-free survival (PFS) were assessed by independent review committee (Response Evaluation Criteria in Solid Tumors v1.1). ResultsEighty patients were enrolled and treated; 75 (93.8%) patients had measurable disease at baseline. Most had metastatic disease and received at least one prior therapy for advanced/metastatic disease (n=79; 98.8%). At primary analysis (data cutoff July 8, 2021; median follow-up 15.2 months), overall ORR [46.7%; 95% confidence interval (95% CI), 35.1−58.6; one-sided P<0.0001] and ORR across tumor-specific subgroups [colorectal (n=46): 39.1% (95% CI, 25.1–54.6); gastric/gastroesophageal junction (n=9): 55.6% (95% CI, 21.2−86.3); others (n=20): 60.0% (95% CI, 36.1−80.9)] were significantly greater with tislelizumab vs. a prespecified historical control ORR of 10%; five (6.7%) patients had complete responses. Median DoR, PFS, and overall survival were not reached with long-term follow-up (data cutoff December 5, 2022; median follow-up 28.9 months). Tislelizumab was well tolerated with no unexpected safety signals. Treatment-related adverse events (TRAEs) of grade ≥3 occurred in 53.8% of patients; 7.5% of patients discontinued treatment due to TRAEs. ConclusionsTislelizumab demonstrated a significant ORR improvement in patients with previously treated, locally advanced unresectable or metastatic MSI-H/dMMR tumors and was generally well tolerated.
2024, 36(3): 270-281.
doi: 10.21147/j.issn.1000-9604.2024.03.04
Abstract:
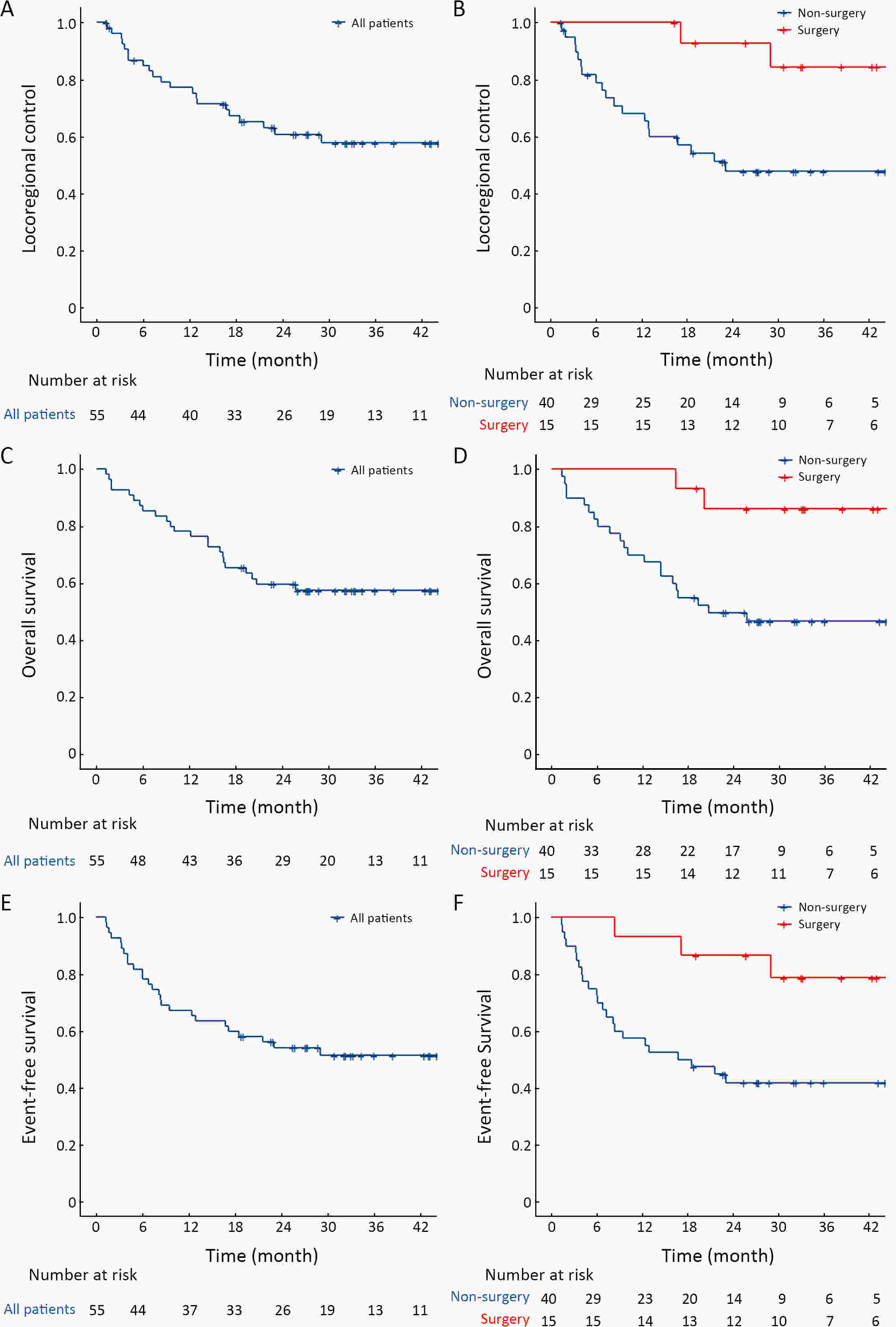
ObjectiveDefinitive chemoradiotherapy (dCRT) is the standard treatment for unresectable locally advanced esophageal cancer. However, this treatment is associated with substantial toxicity, and most malnourished or elderly patients are unable to complete this therapy. Therefore, there is a need for a more suitable radiotherapy combination regimen for this population. This study was aimed to evaluate the efficacy and safety of a combination regimen comprising chemotherapy with nimotuzumab and S-1 and concurrent radiotherapy for patients with fragile locally advanced esophageal cancer with a high Nutritional Risk Screening 2002 (NRS-2002) score. MethodsEligible patients with unresectable esophageal carcinoma who had an NRS-2002 score of 2 or higher were enrolled. They were treated with S-1 and nimotuzumab with concurrent radiotherapy, followed by surgery or definitive radiotherapy. The primary endpoint was the locoregional control (LRC) rate. ResultsA total of 55 patients who met the study criteria were enrolled. After completion of treatment, surgery was performed in 15 patients and radiotherapy was continued in 40 patients. The median follow-up period was 33.3 [95% confidence interval (95% CI), 31.4−35.1)] months. The LRC rate was 77.2% (95% CI, 66.6%−89.4%) at 1 year in the entire population. The overall survival (OS) rate and event-free survival (EFS) rate were 57.5% and 51.5% at 3 years, respectively. Surgery was associated with better LRC [hazard ratio (HR)=0.16; 95% CI, 0.04−0.70; P=0.015], OS (HR=0.19; 95% CI, 0.04−0.80; P=0.024), and EFS (HR=0.25; 95% CI, 0.08−0.75; P=0.013). Most adverse events were of grade 1 or 2, and no severe adverse events occurred. ConclusionsFor malnourished or elderly patients with locally advanced esophageal cancer, radiotherapy combined with nimotuzumab and S-1 is effective and has a good safety profile.
2024, 36(3): 282-297.
doi: 10.21147/j.issn.1000-9604.2024.03.05
Abstract:
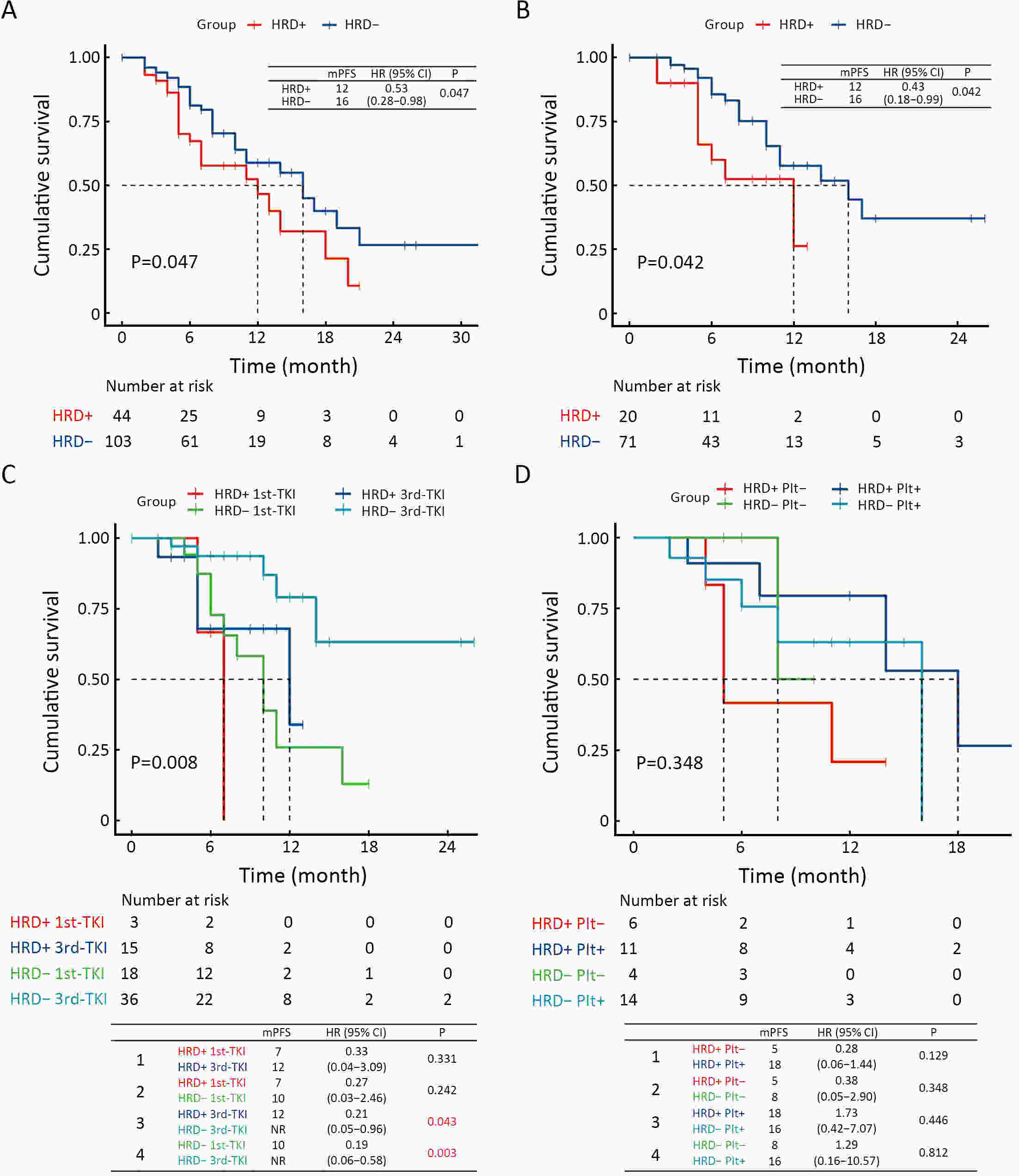
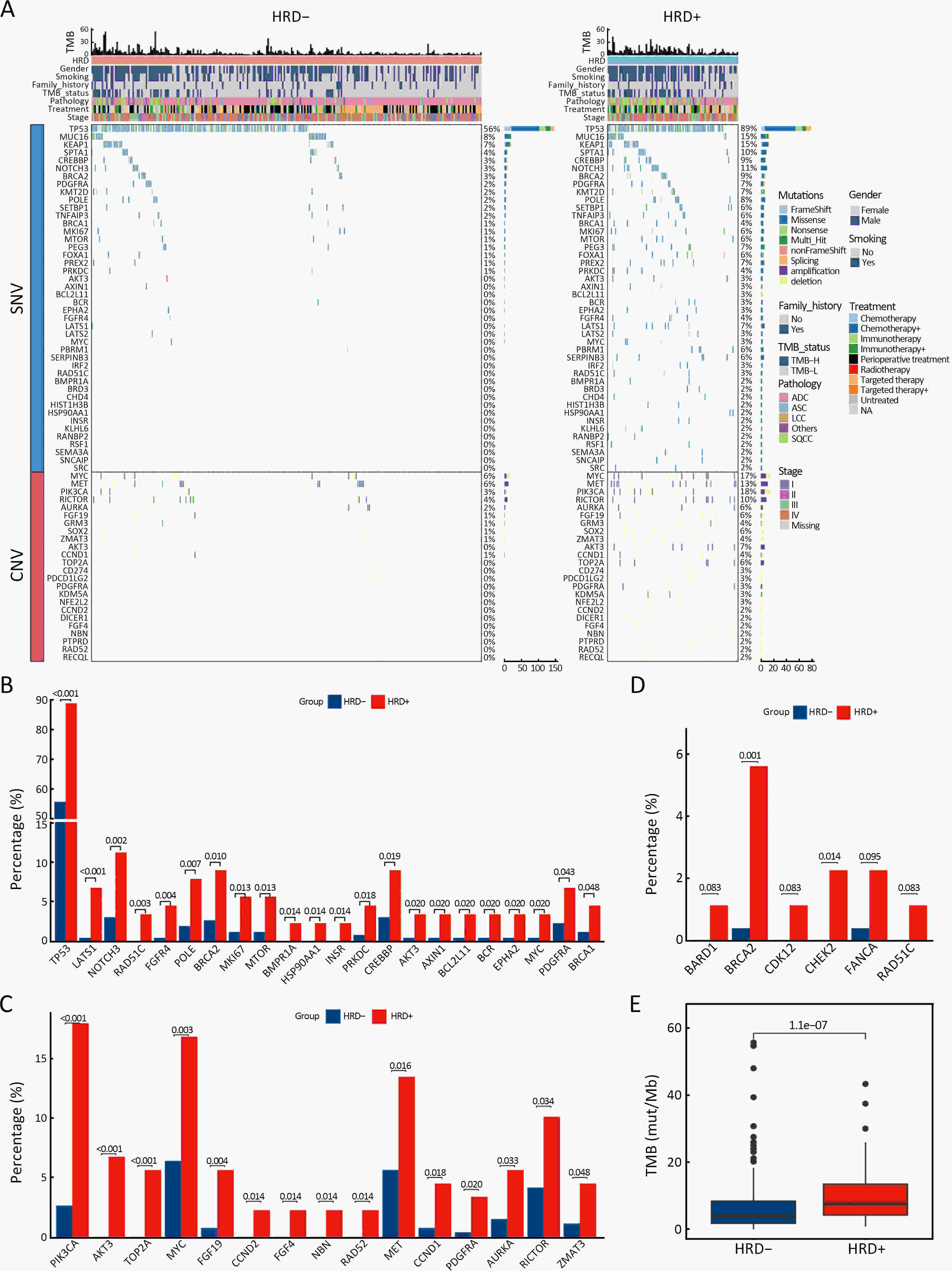
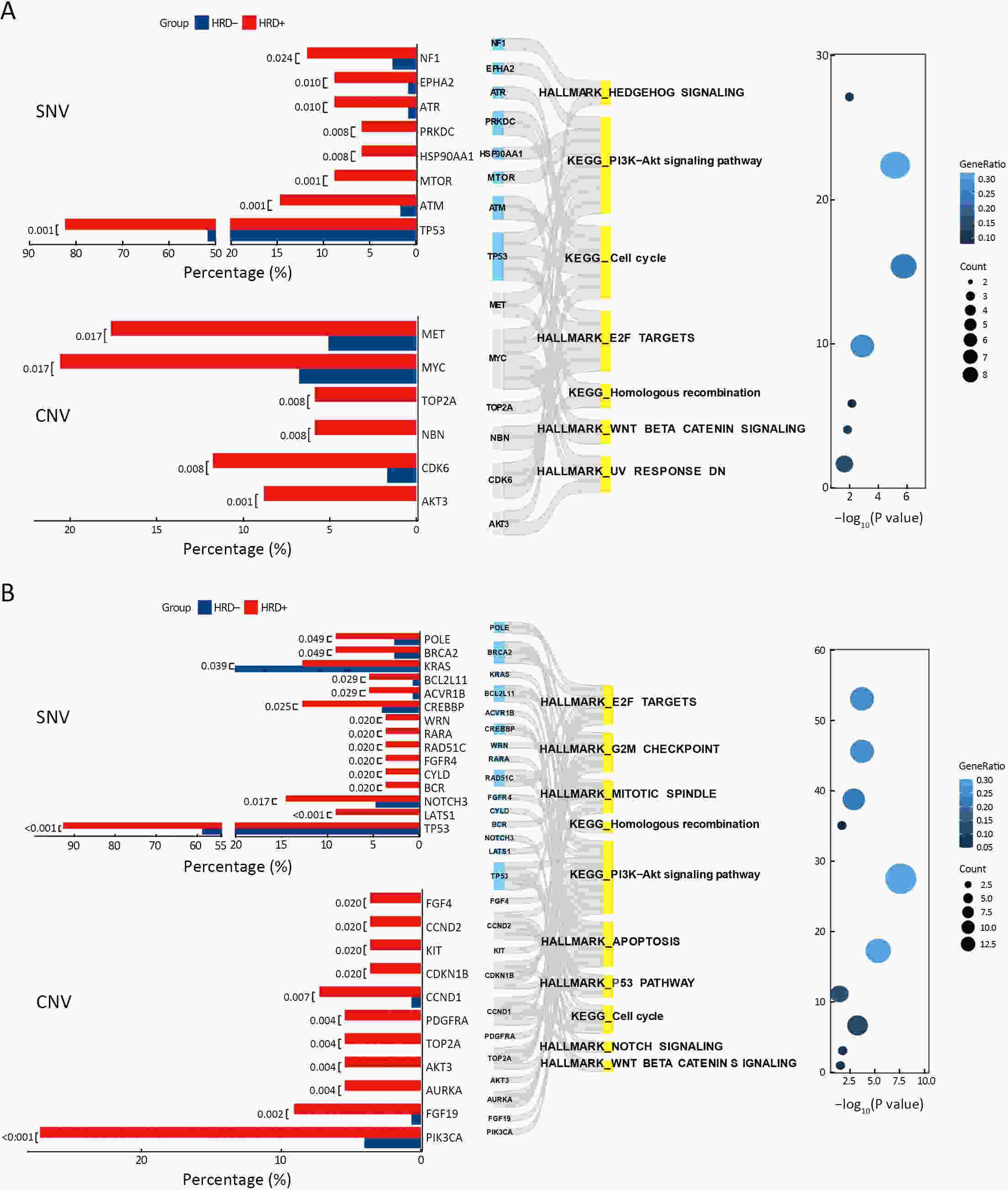
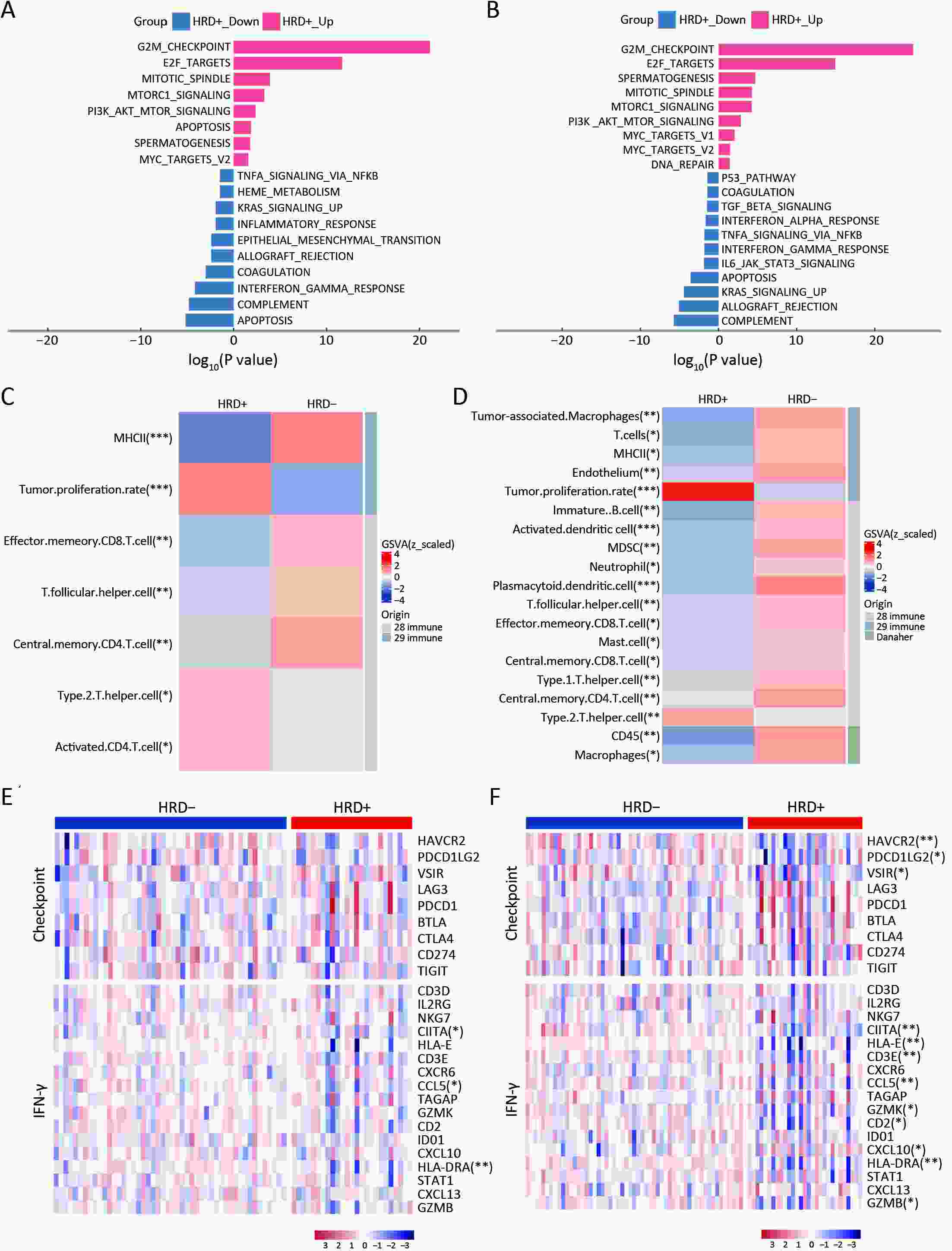
ObjectiveThe clinical significance of homologous recombination deficiency (HRD) in breast cancer, ovarian cancer, and prostate cancer has been established, but the value of HRD in non-small cell lung cancer (NSCLC) has not been fully investigated. This study aimed to systematically analyze the HRD status of untreated NSCLC and its relationship with patient prognosis to further guide clinical care. MethodsA total of 355 treatment-naïve NSCLC patients were retrospectively enrolled. HRD status was assessed using the AmoyDx Genomic Scar Score (GSS), with a score of ≥50 considered HRD-positive. Genomic, transcriptomic, tumor microenvironmental characteristics and prognosis between HRD-positive and HRD-negative patients were analyzed. ResultsOf the patients, 25.1% (89/355) were HRD-positive. Compared to HRD-negative patients, HRD-positive patients had more somatic pathogenic homologous recombination repair (HRR) mutations, higher tumor mutation burden (TMB) (P<0.001), and fewer driver gene mutations (P<0.001). Furthermore, HRD-positive NSCLC had more amplifications in PI3K pathway and cell cycle genes, MET and MYC in epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) mutant NSCLC, and more PIK3CA and AURKA in EGFR/ALK wild-type NSCLC. HRD-positive NSCLC displayed higher tumor proliferation and immunosuppression activity. HRD-negative NSCLC showed activated signatures of major histocompatibility complex (MHC)-II, interferon (IFN)-γ and effector memory CD8+ T cells. HRD-positive patients had a worse prognosis and shorter progression-free survival (PFS) to targeted therapy (first- and third-generation EGFR-TKIs) (P=0.042). Additionally, HRD-positive, EGFR/ALK wild-type patients showed a numerically lower response to platinum-free immunotherapy regimens. ConclusionsUnique genomic and transcriptional characteristics were found in HRD-positive NSCLC. Poor prognosis and poor response to EGFR-TKIs and immunotherapy were observed in HRD-positive NSCLC. This study highlights potential actionable alterations in HRD-positive NSCLC, suggesting possible combinational therapeutic strategies for these patients.
2024, 36(3): 298-305.
doi: 10.21147/j.issn.1000-9604.2024.03.06
Abstract:
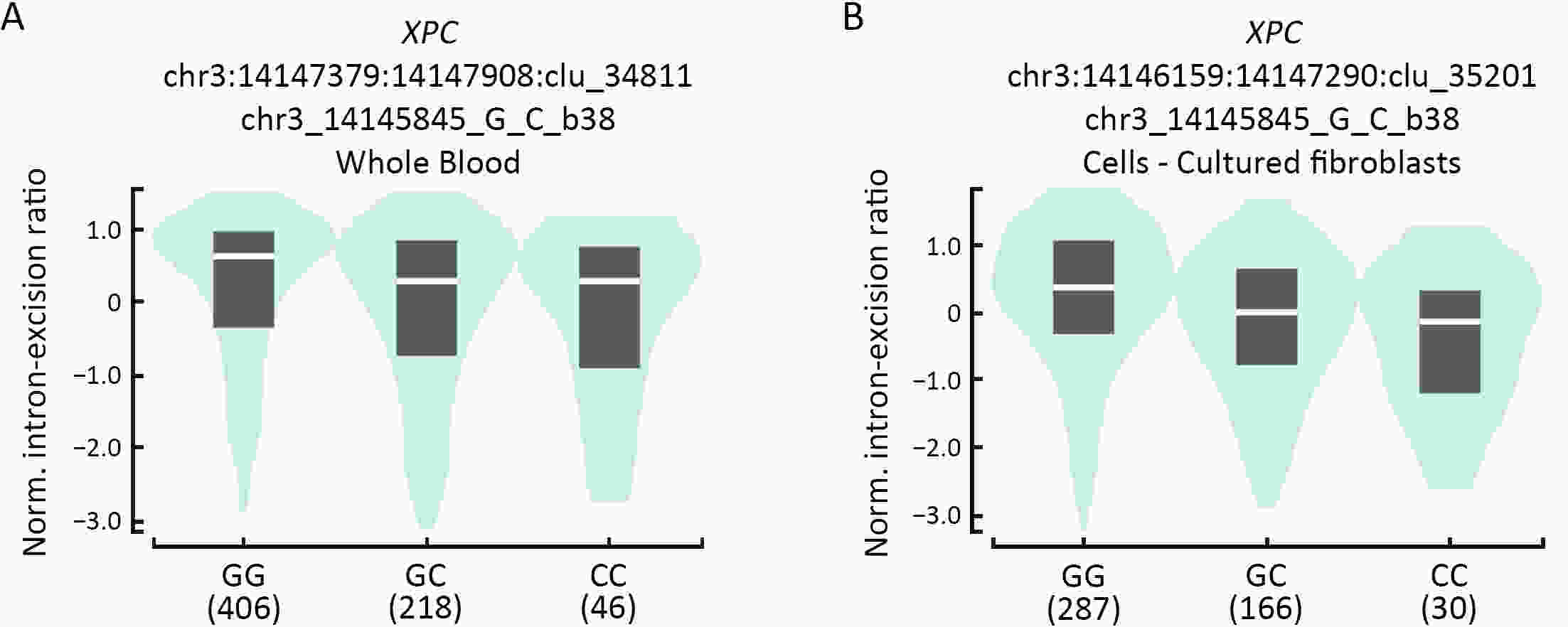
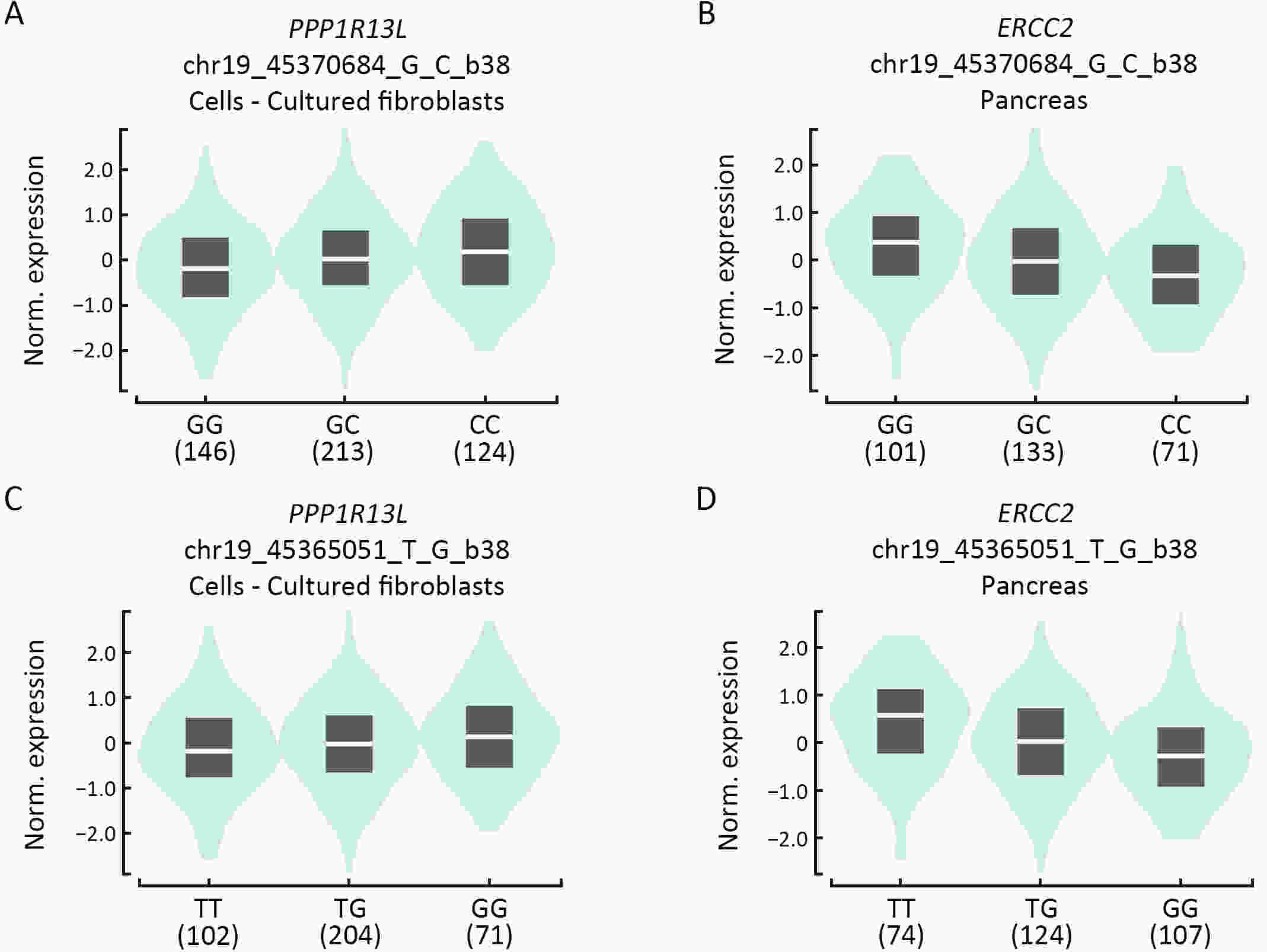
ObjectiveNucleotide excision repair (NER) plays a vital role in maintaining genome stability, and the effect of NER gene polymorphisms on hepatoblastoma susceptibility is still under investigation. This study aimed to evaluate the relationship between NER gene polymorphisms and the risk of hepatoblastoma in Eastern Chinese Han children. MethodsIn this five-center case-control study, we enrolled 966 subjects from East China (193 hepatoblastoma patients and 773 healthy controls). The TaqMan method was used to genotype 19 single nucleotide polymorphisms (SNPs) in NER pathway genes, including ERCC1, XPA, XPC, XPD, XPF, and XPG. Then, multivariate logistic regression analysis was performed, and odds ratios (ORs) and 95% confidence intervals (95% CIs) were utilized to assess the strength of associations. ResultsThree SNPs were related to hepatoblastoma risk. XPC rs2229090 and XPD rs3810366 significantly contributed to hepatoblastoma risk according to the dominant model (adjusted OR=1.49, 95% CI=1.07−2.08, P=0.019; adjusted OR=1.66, 95% CI=1.12−2.45, P=0.012, respectively). However, XPD rs238406 conferred a significantly decreased risk of hepatoblastoma under the dominant model (adjusted OR=0.68, 95% CI=0.49−0.95; P=0.024). Stratified analysis demonstrated that these significant associations were more prominent in certain subgroups. Moreover, there was evidence of functional implications of these significant SNPs suggested by online expression quantitative trait loci (eQTLs) and splicing quantitative trait loci (sQTLs) analysis. ConclusionsIn summary, NER pathway gene polymorphisms (XPC rs2229090, XPD rs3810366, and XPD rs238406) are significantly associated with hepatoblastoma risk, and further research is required to verify these findings.
2024, 36(3): 306-321.
doi: 10.21147/j.issn.1000-9604.2024.03.07
Abstract:
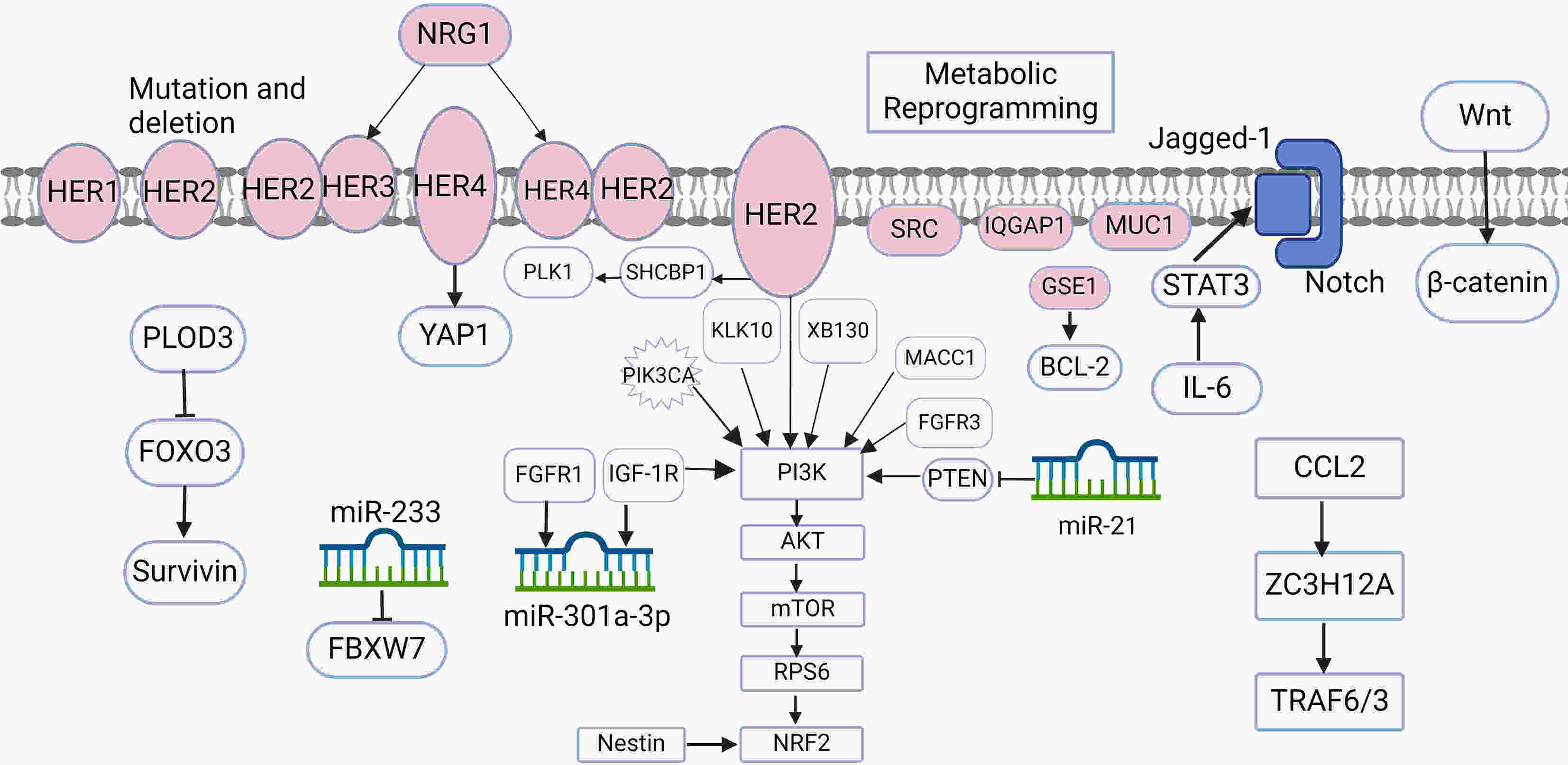
Gastric cancer is one of the most prevalent cancers worldwide, and human epidermal growth factor receptor 2 (HER2)-positive cases account for approximately 20% of the total cases. Currently, trastuzumab + chemotherapy is the recommended first-line treatment for patients with HER2-positive advanced gastric cancer, and the combination has exhibited definite efficacy in HER2-targeted therapy. However, the emergence of drug resistance during treatment considerably reduces its effectiveness; thus, it is imperative to investigate the potential mechanisms underlying resistance. In the present review article, we comprehensively introduce multiple mechanisms underlying resistance to trastuzumab in HER2-positive gastric cancer cases, aiming to provide insights for rectifying issues associated with resistance to trastuzumab and devising subsequent treatment strategies.
Gastric cancer is one of the most prevalent cancers worldwide, and human epidermal growth factor receptor 2 (HER2)-positive cases account for approximately 20% of the total cases. Currently, trastuzumab + chemotherapy is the recommended first-line treatment for patients with HER2-positive advanced gastric cancer, and the combination has exhibited definite efficacy in HER2-targeted therapy. However, the emergence of drug resistance during treatment considerably reduces its effectiveness; thus, it is imperative to investigate the potential mechanisms underlying resistance. In the present review article, we comprehensively introduce multiple mechanisms underlying resistance to trastuzumab in HER2-positive gastric cancer cases, aiming to provide insights for rectifying issues associated with resistance to trastuzumab and devising subsequent treatment strategies.
2024, 36(3): 322-340.
doi: 10.21147/j.issn.1000-9604.2024.03.08
Abstract:
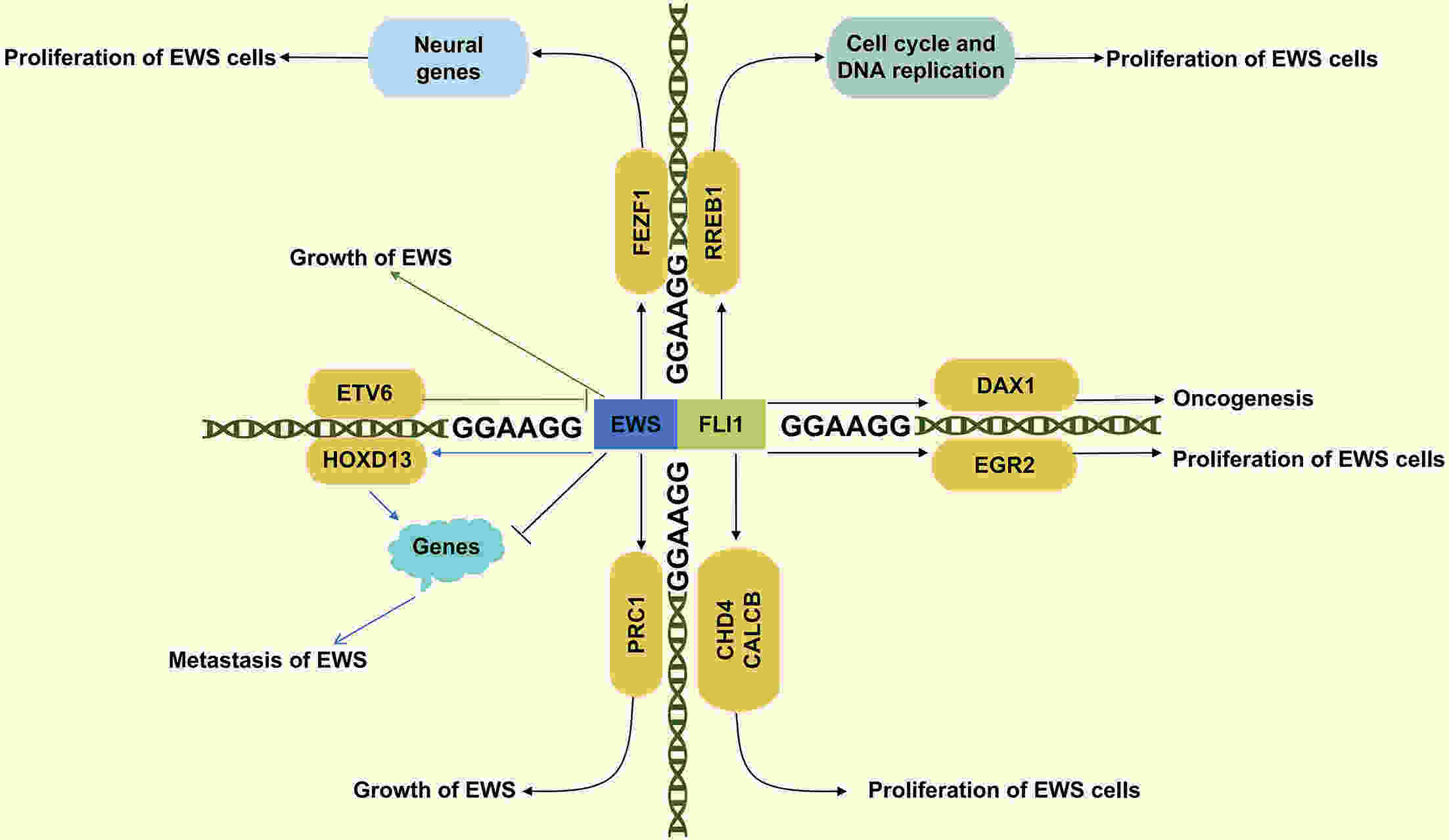
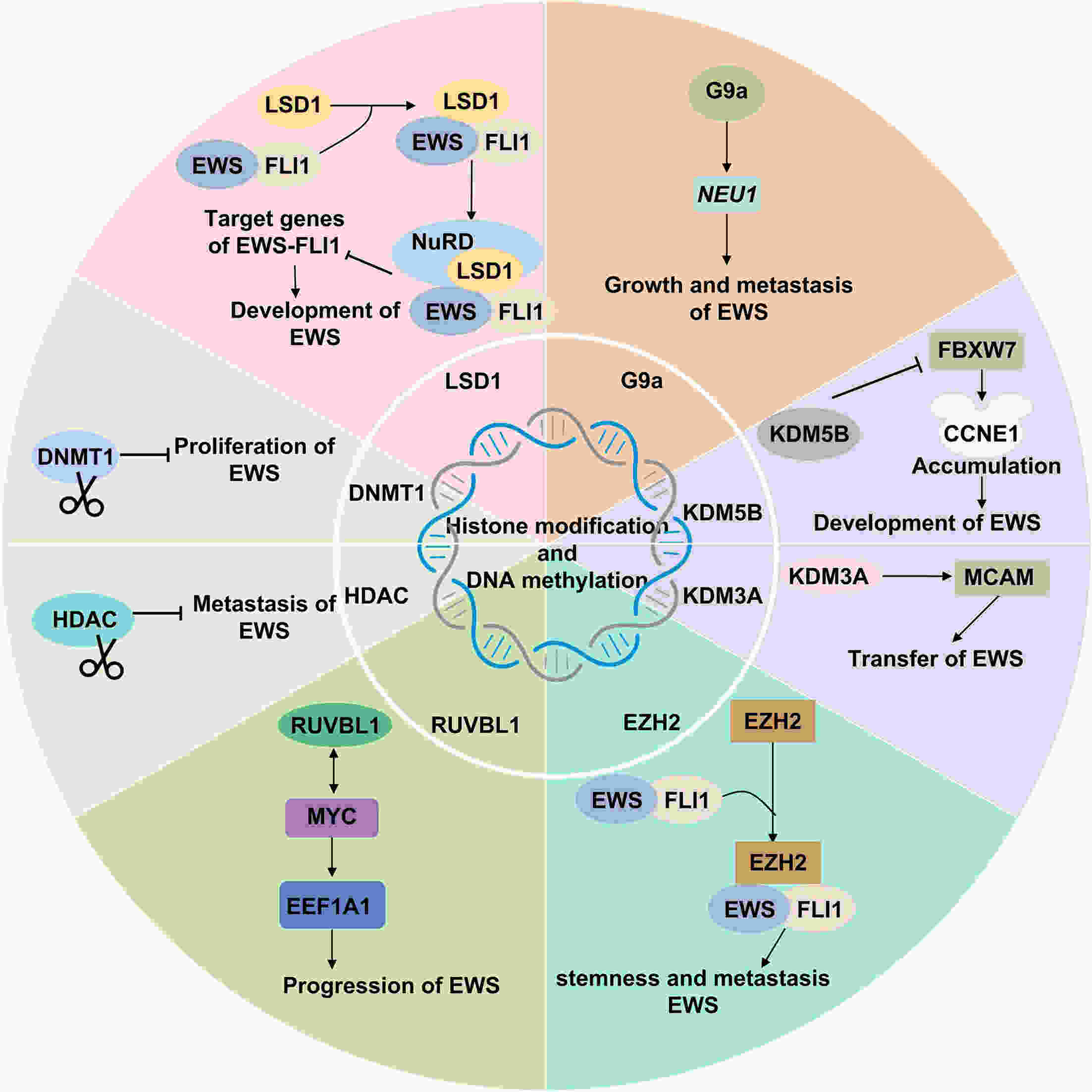
Ewing’s sarcoma (EWS) is a highly aggressive malignant bone tumor primarily affecting adolescents and young adults. Despite the efficacy of chemoradiotherapy in some cases, the cure rate for patients with metastatic and recurrent disease remains low. Therefore, there is an urgent need for innovative therapeutic approaches to address the challenges associated with EWS treatment. Epigenetic regulation, a crucial factor in physiological processes, plays a significant role in controlling cell proliferation, maintaining gene integrity, and regulating transcription. Recent studies highlight the importance of abnormal epigenetic regulation in the initiation and progression of EWS. A comprehensive understanding of the intricate interactions between EWS and aberrant epigenetic regulation is essential for advancing clinical drug development. This review aims to provide a comprehensive overview of both epigenetic targets implicated in EWS, integrating various therapeutic modalities to offer innovative perspectives for the clinical diagnosis and treatment of EWS.
Ewing’s sarcoma (EWS) is a highly aggressive malignant bone tumor primarily affecting adolescents and young adults. Despite the efficacy of chemoradiotherapy in some cases, the cure rate for patients with metastatic and recurrent disease remains low. Therefore, there is an urgent need for innovative therapeutic approaches to address the challenges associated with EWS treatment. Epigenetic regulation, a crucial factor in physiological processes, plays a significant role in controlling cell proliferation, maintaining gene integrity, and regulating transcription. Recent studies highlight the importance of abnormal epigenetic regulation in the initiation and progression of EWS. A comprehensive understanding of the intricate interactions between EWS and aberrant epigenetic regulation is essential for advancing clinical drug development. This review aims to provide a comprehensive overview of both epigenetic targets implicated in EWS, integrating various therapeutic modalities to offer innovative perspectives for the clinical diagnosis and treatment of EWS.
2024, 36(3): 341-350.
doi: 10.21147/j.issn.1000-9604.2024.03.09
Abstract:
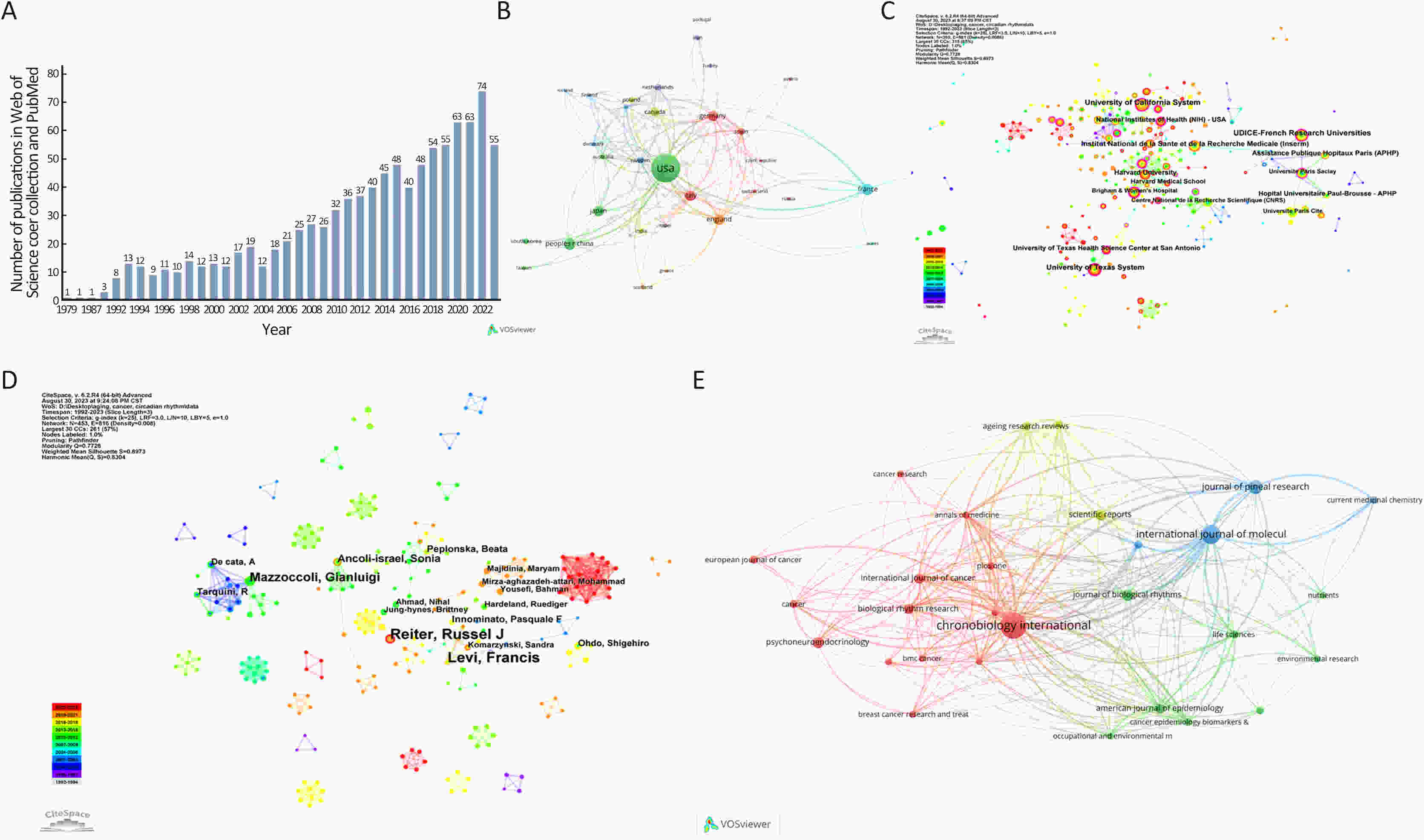
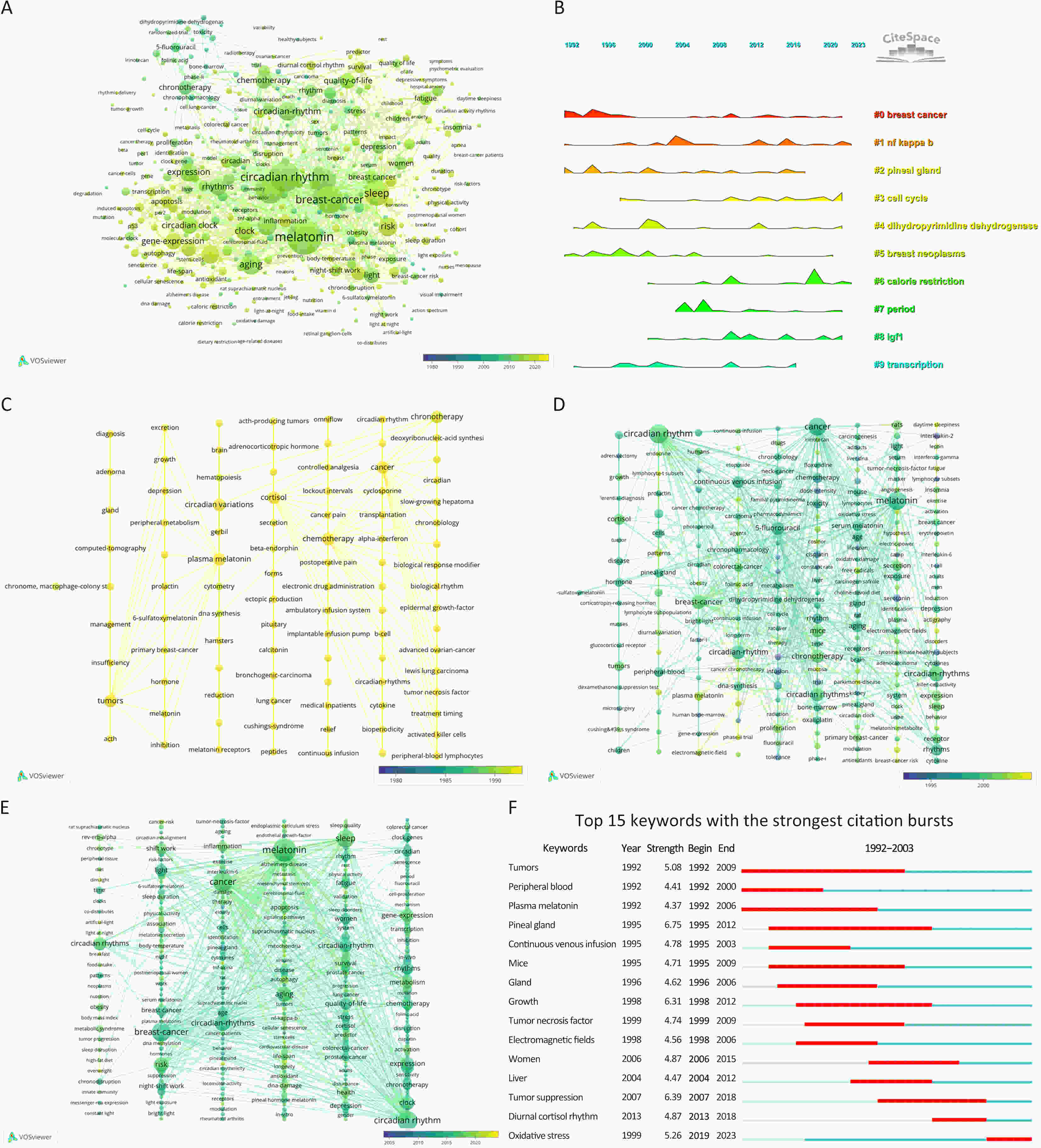
Aging and circadian rhythms have been connected for decades, but their molecular interaction has remained unknown, especially for cancers. In this situation, we summarized the current research actuality and problems in this field using the bibliometric analysis. Publications in the PubMed and Web of Science databases were retrieved. Overall, there is a rising trend in the publication volume regarding aging and circadian rhythms in the field of cancer. Researchers from USA, Germany, Italy, China and England have greater studies than others. Top three publication institutions are University of California System, UDICE-French Research Universities and University of Texas System. Current research hotspots include oxidative stress, breast cancer, melatonin, cell cycle, calorie restriction, prostate cancer and NF-KB. In conclusion, results generated by bibliometric analysis indicate that many approaches involve in the complex interactions between aging and circadian rhythm in cancer. These established and emerging research directions guide our exploration of the regulatory mechanisms of aging and circadian rhythms in cancer and provide a reference for developing new research avenues.
Aging and circadian rhythms have been connected for decades, but their molecular interaction has remained unknown, especially for cancers. In this situation, we summarized the current research actuality and problems in this field using the bibliometric analysis. Publications in the PubMed and Web of Science databases were retrieved. Overall, there is a rising trend in the publication volume regarding aging and circadian rhythms in the field of cancer. Researchers from USA, Germany, Italy, China and England have greater studies than others. Top three publication institutions are University of California System, UDICE-French Research Universities and University of Texas System. Current research hotspots include oxidative stress, breast cancer, melatonin, cell cycle, calorie restriction, prostate cancer and NF-KB. In conclusion, results generated by bibliometric analysis indicate that many approaches involve in the complex interactions between aging and circadian rhythm in cancer. These established and emerging research directions guide our exploration of the regulatory mechanisms of aging and circadian rhythms in cancer and provide a reference for developing new research avenues.

 Abstract
Abstract FullText HTML
FullText HTML PDF 886KB
PDF 886KB